ABSTRACT
Purpose
The aim of the study was to determine in patients undergoing gadoxetate disodium (Gx)-enhanced MR exams whether proton density fat fraction (PDFF) estimation accuracy of magnitude-based multi-gradient-echo MRI (MRI-M) could be improved by using high flip angle (FA) on post-contrast images.
Materials and Methods
Thirty-one adults with known or suspected hepatic steatosis undergoing 3T clinical Gx-enhanced liver MRI were enrolled prospectively. MR spectroscopy (MRS), the reference standard, was performed before Gx to measure MRS-PDFF. Low (10°)- and high (50°)-flip angle (FA) MRI-M sequences were acquired before and during the hepatobiliary phase after Gx administration; MRI-PDFF was estimated in the MRS-PDFF voxel location. Linear regression parameters (slope, intercept, average bias, R 2) were calculated for MRS-PDFF as a function of MRI-PDFF for each MRI-M sequence (pre-Gx low-FA, pre-Gx high-FA, post-Gx low-FA, post-Gx high-FA) for all patients and for patients with MRS-PDFF <10%. Regression parameters were compared (Bonferroni-adjusted bootstrap-based tests).
Results
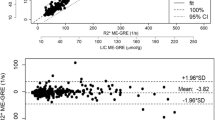
Three of the four MRI-M sequences (pre-Gx low-FA, post-Gx low-FA, post-Gx high-FA) provided relatively unbiased PDFF estimates overall and in the low-PDFF range, with regression slopes close to 1 and intercepts and biases close to zero. Pre-Gx high-FA MRI overestimated PDFF in proportion to MRS-PDFF, with slopes of 0.72 (overall) and 0.63 (low-PDFF range). Based on regression bias closest to 0, the post-Gx high-FA sequence was the most accurate overall and in the low-PDFF range. This sequence provided statistically significant improvements in at least two regression parameters compared to every other sequence.
Conclusion
In patients undergoing Gx-enhanced MR exams, PDFF estimation accuracy of MRI-M can be improved by using high-FA on post-contrast images.





Similar content being viewed by others
References
Permutt Z, Le TA, Peterson MR, et al. (2012) Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease—MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther 36(1):22–29
Tang A, Tan J, Sun M, et al. (2013) Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology 267(2):422–431
Tang A, Desai A, Hamilton G, et al. (2015) Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology 274(2):416–425
Longo R, Pollesello P, Ricci C, et al. (1995) Proton MR spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. J Magn Reson Imaging 5(3):281–285
Thomsen C, Becker U, Winkler K, et al. (1994) Quantification of liver fat using magnetic resonance spectroscopy. Magn Reson Imaging 12(3):487–495
Hamilton G, Middleton MS, Bydder M, et al. (2009) Effect of PRESS and STEAM sequences on magnetic resonance spectroscopic liver fat quantification. J Magn Reson Imaging 30(1):145–152
Yokoo T, Bydder M, Hamilton G, et al. (2009) Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T. Radiology 251(1):67–76
Yokoo T, Shiehmorteza M, Hamilton G, et al. (2011) Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0 T. Radiology 258(3):749–759
Meisamy S, Hines CD, Hamilton G, et al. (2011) Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology 258(3):767–775
Reeder SB, Cruite I, Hamilton G, Sirlin CB (2011) Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging 34(4):729–749
Yu H, McKenzie CA, Shimakawa A, et al. (2007) Multiecho reconstruction for simultaneous water-fat decomposition and T2* estimation. J Magn Reson Imaging 26(4):1153–1161
Liu CY, McKenzie CA, Yu H, Brittain JH, Reeder SB (2007) Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med 58(2):354–364
Bydder M, Yokoo T, Hamilton G, et al. (2008) Relaxation effects in the quantification of fat using gradient echo imaging. Magn Reson Imaging 26(3):347–359
Yokoo T, Collins JM, Hanna RF, et al. (2008) Effects of intravenous gadolinium administration and flip angle on the assessment of liver fat signal fraction with opposed-phase and in-phase imaging. J Magn Reson Imaging 28(1):246–251
Hamilton GM, MS; Cunha, GM; Sirlin, CB.. Effect of gadolinium-based contrast agent on the relaxation properties of water and fat in human liver as measured in vivo by 1H MRS [abstract]. In: Proceedings 21st Scientific Meeting, International Society for Magnetic Resonance in Medicine. Salt Lake City; 2013. p. 1516.
Hamilton G, Middleton MS, Hooker JC, et al. (2015) In vivo breath-hold H MRS simultaneous estimation of liver proton density fat fraction, and T1 and T2 of water and fat, with a multi-TR, multi-TE sequence. J Magn Reson Imaging 42(6):1538–1543
Hussain HK, Chenevert TL, Londy FJ, et al. (2005) Hepatic fat fraction: MR imaging for quantitative measurement and display–early experience. Radiology 237(3):1048–1055
Bydder M, Hamilton G, Yokoo T, Sirlin CB (2008) Optimal phased-array combination for spectroscopy. Magn Reson Imaging 26(6):847–850
Naressi A, Couturier C, Devos JM, et al. (2001) Java-based graphical user interface for the MRUI quantitation package. Magma 12(2–3):141–152
Vanhamme L, van den Boogaart A, Van Huffel S (1997) Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 129(1):35–43
Hamilton G, Yokoo T, Bydder M, et al. (2011) In vivo characterization of the liver fat (1)H MR spectrum. NMR Biomed 24(7):784–790
Stanisz GJ, Odrobina EE, Pun J, et al. (2005) T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn Reson Med 54(3):507–512
Yu H, Shimakawa A, McKenzie CA, et al. (2008) Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med 60(5):1122–1134
de Bazelaire CM, Duhamel GD, Rofsky NM, Alsop DC (2004) GD, NM, DC. MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology 230(3):652–659
Hines CD, Frydrychowicz A, Hamilton G, et al. (2011) T(1) independent, T(2) (*) corrected chemical shift based fat-water separation with multi-peak fat spectral modeling is an accurate and precise measure of hepatic steatosis. J Magn Reson Imaging 33(4):873–881
Reeder SB, Robson PM, Yu H, et al. (2009) Quantification of hepatic steatosis with MRI: the effects of accurate fat spectral modeling. J Magn Reson Imaging 29(6):1332–1339
Reeder SB, McKenzie CA, Pineda AR, et al. (2007) Water-fat separation with IDEAL gradient-echo imaging. J Magn Reson Imaging 25(3):644–652
Hernando D, Wells SA, Vigen KK, Reeder SB (2015) Effect of hepatocyte-specific gadolinium-based contrast agents on hepatic fat-fraction and R2(). Magn Reson Imaging 33(1):43–50
Heba ER, Desai A, Zand KA, et al. (2016) Accuracy and the effect of possible subject-based confounders of magnitude-based MRI for estimating hepatic proton density fat fraction in adults, using MR spectroscopy as reference. J Magn Reson Imaging 43(2):398–406
Zand KA, Shah A, Heba E, et al. (2015) Accuracy of multiecho magnitude-based MRI (M-MRI) for estimation of hepatic proton density fat fraction (PDFF) in children. J Magn Reson Imaging 42(5):1223–1232
Rehm JL, Wolfgram PM, Hernando D, et al. (2015) Proton density fat-fraction is an accurate biomarker of hepatic steatosis in adolescent girls and young women. Eur Radiol 25(10):2921–2930
Marckmann P, Skov L, Rossen K, et al. (2006) Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 17(9):2359–2362
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The project described was partially supported by the National Institutes of Health (Grants R01 DK08892505, R01 DK102853, TL1 TR00098) and Canon U.S.A./RSNA Research Medical Student Grant. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of interest
Dr. Claude B. Sirlin consults, advises, and is on the speakers’ bureau for Bayer. He received grants from GE Healthcare. The other authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Park, C.C., Hamilton, G., Desai, A. et al. Effect of intravenous gadoxetate disodium and flip angle on hepatic proton density fat fraction estimation with six-echo, gradient-recalled-echo, magnitude-based MR imaging at 3T. Abdom Radiol 42, 1189–1198 (2017). https://doi.org/10.1007/s00261-016-0992-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-016-0992-4