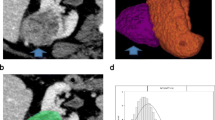
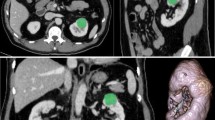
Abstract
Purpose
The purpose of this study was to compare whole-lesion (WL) enhancement parameters to single region of interest (ROI)-based enhancement in discriminating clear cell renal cell carcinoma (ccRCC) from renal oncocytoma.
Materials and Methods
In this IRB-approved retrospective study, the surgical database was queried to derive a cohort of 94 postnephrectomy patients with ccRCC or oncocytoma (68 ccRCC, 26 oncocytoma), who underwent preoperative multiphase contrast-enhanced computed tomography (CECT) between June 2009 and August 2013. CT acquisitions were transferred to a three-dimensional workstation, and WL ROIs were manually segmented. WL enhancement and histogram distribution parameters skewness, kurtosis, standard deviation (SD), and interquartile range (IQR) were calculated. WL enhancement parameters were compared to single ROI-based enhancement using receiver operating characteristic (ROC) analysis.
Results
Oncocytoma had significantly higher WL enhancement than ccRCC in nephrographic (mean, p = 0.02; median, p = 0.03) and excretory phases (mean, p = 0.03; median p < 0.01). ccRCC had significantly higher kurtosis than oncocytoma in corticomedullary (p = 0.03) and excretory phases (p < 0.01), and significantly higher SD and IQR than oncocytoma in all postcontrast phases: corticomedullary (SD, p = 0.02; IQR, p < 0.01), nephrographic (SD, p = 0.01; IQR, p = 0.03), and excretory (SD, p < 0.01; IQR, p < 0.01). When compared to single ROI-based enhancement, WL enhancement alone did not demonstrate a statistical advantage in discriminating between ccRCC and oncocytoma (area under ROC curve of 0.78 and 0.72 respectively), but when combined with histogram distribution parameters (area under ROC curve of 0.86), it did demonstrate a slight improvement.
Conclusion
Our study suggests that voxel-based WL enhancement parameters provide only a slight improvement over single ROI-based enhancement techniques in differentiating between ccRCC and renal oncocytoma.




Similar content being viewed by others
References
Reuter VE (2006) The pathology of renal epithelial neoplasms. Semin Oncol 33(5):534–543
Mai KT, Landry DC, Robertson SJ, et al. (2001) A comparative study of metastatic renal cell carcinoma with correlation to subtype and primary tumor. Pathol Res Pract 197(10):671–675
Trpkov K, Yilmaz A, Uzer D, et al. (2010) Renal oncocytoma revisited: a clinicopathological study of 109 cases with emphasis on problematic diagnostic features. Histopathology 57(6):893–906
Schatz SM, Lieber MM (2003) Update on oncocytoma. Curr Urol Rep 4(1):30–35
Jasinski RW, Amendola MA, Glazer GM, Bree RL, Gikas PW (1985) Computed tomography of renal oncocytomas. Comput Radiol 9:307–314
Tikkakoski T, Paivansalo M, Alanen A, et al. (1991) Radiologic findings in renal oncocytoma. Acta Radiol 32:363–367
Quinn MJ, Hartman DS, Friedman AC, et al. (1994) Renal oncocytoma: new observations. Radiology 153:49–53
Levine E, Huntrakoon M (1983) Computed tomography of renal oncocytoma. Am J Roentgenol 141(4):741–746
Prasad SR, Surabhi VR, Menias CO, Raut AA, Chintapalli KN (2008) Benign renal neoplasms in adults: cross-sectional imaging findings. Am J Roentgenol 190(1):158–164
Kim JI, Cho JY, Moon KC, Lee HJ, Kim SH (2009) Segmental enhancement inversion at biphasic multidetector CT: characteristic finding of small renal oncocytoma. Radiology 252(2):441–448
Schieda N, McInnes MD, Cao L (2014) Diagnostic accuracy of segmental enhancement inversion for diagnosis of renal oncocytoma at biphasic contrast enhanced CT: systematic review. Eur Radiol 24(6):1421–1429
O’Malley ME, Tran P, Hanbidge A, Rogalla P (2012) Small renal oncocytomas: is segmental enhancement inversion a characteristic finding at biphasic MDCT? Am J Roentgenol 199(6):1312–1315
McGahan JP, Lamba R, Fisher J, et al. (2011) Is segmental enhancement inversion on enhanced biphasic MDCT a reliable sign for the noninvasive diagnosis of renal oncocytomas? Am J Roentgenol 197(4):W674–W679
Ishigami K, Jones AR, Dahmoush L, et al. (2015) Imaging spectrum of renal oncocytomas: a pictorial review with pathologic correlation. Insights Imaging 6(1):53–64
Choudhary S, Rajesh A, Mayer NJ, Mulcahy KA, Haroon A (2009) Renal oncocytoma: CT features cannot reliably distinguish oncocytoma from other renal neoplasms. Clin Radiol 64(5):517–522
Bird VG, Kanagarajah P, Morillo G, et al. (2011) Differentiation of oncocytoma and renal cell carcinoma in small renal masses (<4 cm): the role of 4-phase computerized tomography. World J Urol 29(6):787–792
Pierorazio PM, Hyams ES, Tsai S, et al. (2013) Multiphasic enhancement patterns of small renal masses (≤4 cm) on preoperative computed tomography: utility for distinguishing subtypes of renal cell carcinoma, angiomyolipoma, and oncocytoma. Urology 81(6):1265–1271
Millet I, Doyon FC, Hoa D, et al. (2011) Characterization of small solid renal lesions: can benign and malignant tumors be differentiated with CT? Am J Roentgenol 197(4):887–896
Zhang J, Lefkowitz RA, Ishill NM, et al. (2007) Solid renal cortical tumors: differentiation with CT. Radiology 244(2):494–504
Gakis G, Kramer U, Schilling D, et al. (2011) Small renal oncocytomas: differentiation with multiphase CT. Eur J Radiol 80(2):274–278
Young JR, Margolis D, Sauk S, et al. (2013) Clear cell renal cell carcinoma: discrimination from other renal cell carcinoma subtypes and oncocytoma at multiphasic multidetector CT. Radiology 267(2):444–453
Lee-Felker SA, Felker ER, Tan N, et al. (2014) Qualitative and quantitative MDCT features for differentiating clear cell renal cell carcinoma from other solid renal cortical masses. Am J Roentgenol 203:W516–W524
Eble J, Sauter G, Epstein J (2004) World Health Organization classification of tumours: pathology and genetics of tumours of the urinary system and male genital organs. Lyon: IARC Press
Algorithm of Clinical Management of Clinical T1 Renal Mass. 2009. https://www.auanet.org/common/pdf/education/clinical-guidance/Renal-Mass-Algorithm.pdf. Accessed 27 Oct 2015.
Thompson RH, Hill JR, Babayev Y, et al. (2009) Metastatic renal cell carcinoma risk according to tumor size. J Urol 182:41–45
Kunkle DA, Egleston BL, Uzzo RG (2008) Excise, ablate or observe: the small renal mass dilemma-a meta-analysis and review. J Urol 179:1227–1233
Chawla SN, Crispen PL, Hanlon AL, et al. (2006) The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol 175:425–431
Smaldone MC, Kutikov A, Egleston BL, et al. (2012) Small renal masses progressing to metastases under active surveillance: a systematic review and pooled analysis. Cancer 118:997–1006
Mason RJ, Abdolell M, Trottier G, et al. (2011) Growth kinetics of renal masses: analysis of a prospective cohort of patients undergoing active surveillance. Eur Urol 59:863–867
Kouba E, Smith A, McRackan D, et al. (2007) Watchful waiting for solid renal masses: insight into the natural history and results of delayed intervention. J Urol 177:466–470
Pierorazio PM, Hyams ES, Mullins JK, Allaf ME (2012) Active surveillance for small renal masses. Nat Rev Urol 14(1–2):13–19
Volpe A, Kachura JR, Geddie WR, et al. (2007) Techniques, safety and accuracy of sampling of renal tumors by fine needle aspiration and core biopsy. J Urol 178:379
Kümmerlin I, ten Kate F, Smedts F, et al. (2008) Core biopsies of renal tumors: a study on diagnostic accuracy, interobserver, and intraobserver variability. Eur Urol 53:1219
Lebret T, Poulain JE, Molinie V, et al. (2007) Percutaneous core biopsy for renal masses: indications, accuracy and results. J Urol 178(4 Pt 1):1184
Schmidbauer J, Remzi M, Memarsadeghi M, et al. (2008) Diagnostic accuracy of computed tomography-guided percutaneous biopsy of renal masses. Eur Urol 53:1003
Jeon HG, Seo SI, Jeong BC, et al. (2015) Percutaneous kidney biopsy for a small renal mass: a critical appraisal of results. J Urol S0022–5347(15):4881–4888
Chandarana H, Rosenkrantz AB, Mussi TC, et al. (2012) Histogram analysis of whole-lesion enhancement in differentiating clear cell from papillary subtype of renal cell cancer. Radiology 265:790–798
Chen F, Huhdanpaa H, Desai B, et al. (2015) Whole lesion quantitative CT evaluation of renal cell carcinoma: differentiation of clear cell from papillary renal cell carcinoma. Springerplus 4:66
Kim JY, Kim JK, Kim N, Cho KS (2008) CT histogram analysis: differentiation of angiomyolipoma without visible fat from renal cell carcinoma at CT imaging. Radiology 246(2):472–479
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This project has received funding from the Whittier foundation. The project described was supported in part by the Award Number P30CA014089 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Conflict of Interest
None of the authors have any conflict of interest to disclose.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Chen, F., Gulati, M., Hwang, D. et al. Voxel-based whole-lesion enhancement parameters: a study of its clinical value in differentiating clear cell renal cell carcinoma from renal oncocytoma. Abdom Radiol 42, 552–560 (2017). https://doi.org/10.1007/s00261-016-0891-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-016-0891-8