Abstract
Purpose
Multidrug resistance-associated protein 1 (MRP1) is a transport protein with a widespread tissue distribution, which has been implicated in the pathophysiology of Alzheimer’s and chronic respiratory disease. PET with 6-bromo-7-[11C]methylpurine ([11C]BMP) has been used to measure MRP1 function in rodents. In this study, [11C]BMP was for the first time characterised in humans to assess the function of MRP1 and other MRP subtypes in different tissues.
Methods
Thirteen healthy volunteers (7 men, 6 women) underwent dynamic whole-body PET scans on a long axial field-of-view (LAFOV) PET/CT system after intravenous injection of [11C]BMP. Three subjects of each sex were scanned a second time to assess reproducibility. Volumes of interest were outlined for MRP-expressing tissues (cerebral cortex, cerebellum, choroid plexus, retina, lungs, myocardium, kidneys, and liver). From the time-activity curves, the elimination rate constant (kE, h− 1) was derived as a parameter for tissue MRP function and its test-retest variability (TRTV, %) was calculated. Radiation dosimetry was calculated using the Medical Internal Radiation Dose (MIRD) methodology.
Results
Mean kE and corresponding TRTV values were: cerebral cortex: 0.055 ± 0.010 h− 1 (− 4 ± 24%), cerebellum: 0.033 ± 0.009 h− 1 (1 ± 39%), choroid plexus: 0.292 ± 0.059 h− 1 (0.1 ± 16%), retina: 0.234 ± 0.045 h− 1 (30 ± 38%), lungs: 0.875 ± 0.095 h− 1 (− 3 ± 11%), myocardium: 0.641 ± 0.105 h− 1 (11 ± 25%), kidneys: 1.378 ± 0.266 h− 1 (14 ± 16%), and liver: 0.685 ± 0.072 h− 1 (7 ± 9%). Significant sex differences were found for kE in the cerebellum, lungs and kidneys. Effective dose was 4.67 ± 0.18 µSv/MBq for men and 4.55 ± 0.18 µSv/MBq for women.
Conclusion
LAFOV PET/CT with [11C]BMP potentially allows for simultaneous assessment of MRP function in multiple human tissues. Mean TRTV of kE in different tissues was in an acceptable range, except for the retina. The radiation dosimetry of [11C]BMP was in the typical range of 11C-tracers. LAFOV PET/CT holds great potential to assess at a whole-body, multi-tissue level molecular targets relevant for drug disposition in humans.
Trial registration
EudraCT 2021-006348-29. Registered 15 December 2021.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multidrug resistance-associated protein 1 (MRP1, encoded in humans by the ABCC1 gene and in rodents by the Abcc1 gene) is an adenosine triphosphate-binding cassette (ABC) transporter with a widespread tissue distribution [1, 2]. MRP1 transports a broad range of endogenous and exogenous compounds, including drugs and drug metabolites. MRP1 is overexpressed in some tumour types and contributes to multidrug resistance by exporting a range of anticancer drugs from tumour cells (e.g., vincristine, doxorubicin, epirubicin, etoposide, daunorubicin, and mitoxantrone) [2]. MRP1 has been further implicated in the pathophysiology of Alzheimer’s disease (AD) [3] and chronic respiratory disease, such as chronic obstructive pulmonary disease [4] and cystic fibrosis [5]. There is evidence that MRP1 contributes to the brain clearance of neurotoxic amyloid-beta (Aβ) peptides across the blood-brain barrier (BBB) and the blood-cerebrospinal fluid barrier [3]. Pharmacological stimulation of MRP1 function has been proposed as a therapeutic strategy to promote brain Aβ clearance in AD patients [3]. To translate such treatment approaches to humans and to assess the role of MRP1 in different diseases, there is a need for methodology to measure MRP1 function in vivo. While several positron emission tomography (PET) radiotracers are available to measure the function of P-glycoprotein (P-gp/ABCB1), another important ABC transporter [6], MRP1 function has so far not been assessed with PET in humans.
6-Bromo-7-[11C]methylpurine ([11C]BMP) has been used to measure MRP1 function in the brain and lungs of mice and rats with PET [7,8,9,10,11,12,13,14]. Following intravenous (i.v.) injection, [11C]BMP is efficiently delivered to different tissues by passive diffusion, where it is rapidly converted by intracellular glutathione-S-transferase enzymes (GSTs) into its glutathione (GSH) conjugate S-(6-(7-[11C]methylpurinyl))glutathione ([11C]MPG) [7, 8]. Due to its high polarity, [11C]MPG cannot diffuse back into blood and is eliminated from tissues by MRP1 and possibly other MRP subtypes [9, 15]. At the same time, [11C]BMP is also rapidly converted in blood into [11C]MPG. The rate constant for radioactivity elimination from tissues (kE), which can be directly derived from the tissue TACs without the need to consider blood radioactivity, has been validated as a parameter for tissue MRP1 function in rodent studies [7,8,9]. In vitro data indicate that MPG is also efficiently transported by human MRP1 [12, 16].
Given the widespread tissue distribution of MRP1 and other MRP subtypes, whole-body dynamic imaging would be desirable to assess MRP function with [11C]BMP and PET. While this is feasible with small-animal PET imaging in mice [9, 10, 13], the limited axial field of view (FOV) of previously available clinical PET scanners has hampered a simultaneous assessment of transporter function in multiple tissues in humans.
In the present study, [11C]BMP was for the first time characterised in humans, employing a long axial field-of-view (LAFOV) PET/CT system [17], for the quantification of MRP function at a whole-body level. We report test-retest variability (TRTV) data in healthy volunteers, sex differences in tissue MRP function and the human dosimetry of [11C]BMP.
Materials and methods
General
This study was conducted in accordance with the ICH-GCP guidelines and the Declaration of Helsinki. The trial was registered in the EudraCT database (2021-006348-29) as a phase 1 first-in-human study and was approved by the Ethics Committee of the Medical University of Vienna and the Austrian Agency for Health and Food Safety. To obtain regulatory approval for the study, an investigational medicinal product dossier (IMPD) containing non-clinical toxicity data and human dosimetry estimates was prepared for [11C]BMP as described before [18]. All subjects gave oral and written informed consent before enrolment in the study. Thirteen healthy subjects (7 men: age: 28 ± 2 years, weight: 80 ± 13 kg and 6 women: age: 26 ± 1 years, weight: 60 ± 10 kg) were included into our study. Subjects were free of any medication for at least 14 days and judged as healthy based on clinical examination and routine blood and urine laboratory assessments.
Radiotracer synthesis
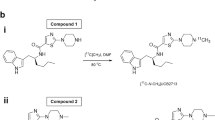
[11C]BMP was automatically synthesised in a TRACERlab™ FX2 C synthesis module (GE Healthcare, Uppsala, Sweden) by regioselective N7-methylation of 6-bromo-7 H-purine with [11C]methyl triflate as described before [18]. [11C]BMP was formulated in 0.9% aqueous sodium chloride solution and ethanol (9/1, v/v). For i.v. injection into humans, an aliquot of the formulated product solution was further diluted with 0.9% aqueous sodium chloride solution to a final injection volume of 10 ml.
PET/CT imaging
All subjects (7 men, 6 women) underwent single dynamic whole-body PET scans after i.v. injection of [11C]BMP on a Biograph Vision Quadra PET/CT system (Siemens Healthineers, Knoxville, TN, USA) (axial FOV: 106 cm) [17]. To assess TRTV, three subjects of each sex were scanned a second time with a mean time interval between the test and the retest scan of 20 ± 10 days (range: 7–28 days). Subjects were scanned in supine position with arms down. First, a low-dose computed tomography (CT) scan (CareDose4D, CarekV setting: Semi, ref. tube voltage: 100 kVp with Sn filter, ref. tube current: 30 mAs) was acquired for attenuation correction. Then, [11C]BMP (368 ± 18 MBq, containing < 50 µg of unlabelled BMP) was administered as an i.v. bolus over 20 s. At the start of the injection, a 90-min list mode PET acquisition was started.
Blood and urine sampling
Venous blood samples were manually collected via a peripheral venous catheter in an antecubital vein at 5, 10, 20, 30, 40, 60, and 90 min after radiotracer injection. The first 2 ml of each blood draw were discarded. Radioactivity in blood and plasma aliquots was measured in a gamma counter (Perkin Elmer 1480 Wizard 3 gamma counter, Meriden, CT, USA), which had been cross-calibrated with the PET/CT scanner. The plasma samples collected at 5, 20, and 40 min after radiotracer injection were analysed with radio-high performance liquid chromatography (radio-HPLC) to assess conversion of [11C]BMP into [11C]MPG as described in the following section. After the imaging session, subjects were asked to empty their urinary bladder. Radioactivity in urine aliquots was measured in the gamma counter. An undiluted 2-ml urine sample was injected into the same radio-HPLC system as used for the plasma analysis to assess the percentage of [11C]MPG in urine. Decay-corrected urinary radioactivity concentrations were multiplied by the collected urine volume to obtain the percentage of the administered activity excreted into the urine.
Plasma metabolite analysis
Plasma samples (830 µl) were mixed with acetonitrile (600 µl) and vortexed to precipitate plasma proteins. After addition of water (600 µl) and phosphate-buffered saline (10-fold concentrate, pH 7.4, 100 µl), samples were centrifuged (4 min, 15,000 × g, 4 °C). For the 5 min sample, the protein pellet and supernatant were separately counted in the gamma counter to determine the recovery of radioactivity. The supernatant (2 ml) was then spiked with unlabelled BMP (0.4 mg/ml in water, 50 µl) and unlabelled MPG (1.0 mg/ml in water, 50 µl) and injected into the radio-HPLC system. An Atlantis T3 OBD HPLC column (250 × 10 mm, 10 μm, Waters, Austria) equipped with a pre-column (Atlantis T3 Prep Guard Cartridge, 10 × 10 mm, 10 μm, Waters, Austria) was eluted with a mixture of 25 mM aqueous ammonium acetate (solvent A) and acetonitrile (solvent B). First, a linear gradient from 5 to 15% of solvent B over 7 min was applied to the column, followed by a step gradient to 35% of solvent B with a total run time of 14 min and a flow rate of 5 ml/min. On this HPLC system, [11C]BMP and [11C]MPG eluted with retention times of approximately 11.2 min and 3.3 min, respectively. HPLC eluates were collected in 1-min fractions, which were counted in the gamma counter. The measured fractions were corrected for radioactive decay to determine the percentages of [11C]BMP and [11C]MPG in plasma at different time points.
MR imaging
On a separate day after completion of the PET/CT examination, a T1-weighted brain magnetic resonance imaging (MRI) scan was acquired in each subject on a Siemens Magnetom Skyra 3T MR system with the following parameters: echo time/repetition time = 2.32/1730 ms, inversion time = 942 ms, flip angle = 10°, 240 × 240 mm field of view, 176 slices, voxel size: 0.47 × 0.47 × 0.9 mm.
Image analysis
The PET list mode data were re-binned into 1 × 15 s, 3 × 5 s, 3 × 10 s, 2 × 30 s, 3 × 60 s, 2 × 150 s, 2 × 300 s, and 7 × 300 s frames and each PET frame was reconstructed into a 440 × 440 × 531 matrix (voxel size: 1.65 × 1.65 × 2 mm3) with an ordinary Poisson ordered subset expectation maximisation algorithm (OP-OSEM, 4 iterations, 5 subsets) with PSF modelling and TOF information. A 2 mm FWHM Gaussian post-reconstruction filter was applied to all images. Scatter correction, CT-attenuation correction and dead-time and randoms correction were applied to the PET data. Volumes of interest (VOIs) were manually outlined on the co-registered PET/CT data for the right lung, myocardium, right kidney cortex, liver, urinary bladder, and gall bladder (including the extrahepatic bile duct) in the PFUS tool in PMOD (version 4.404, PMOD Technologies Ltd., Zürich, Switzerland) (Supplementary Fig. 1). In case of subjects’ motion, the position of the different VOIs was manually adjusted in the individual PET time frames. As the gall bladder was not always clearly visible on the PET images it could not be outlined in all subjects. Three spherical VOIs (diameter: 10 mm) were placed in the right lung (one in each lobe) and averaged to generate a global lung VOI. Three spherical VOIs (diameter: 10 mm) were placed in the liver (two in the right and one in the left liver lobe) and averaged to generate a global liver VOI. One spherical VOI (diameter: 8 mm) was placed in the right kidney cortex. One spherical VOI (diameter: 6 mm) was placed in the myocardium. For the urinary bladder and the gall bladder, the VOIs included all the visible radioactivity.
The brain kinetics of [11C]BMP were analysed using a brain region atlas (N30R83) implemented in the PNEURO tool in PMOD. Brain PET data were automatically motion-corrected in the PNEURO tool. The anatomical MRI was segmented into grey and white matter and spatially normalised to a Montreal Neurological Institute (MNI) T1-MRI template before transferring the atlas regions to the PET data. For a preliminary analysis of the brain distribution of radioactivity, we selected among the 83 available regions from the brain atlas a global cortical grey matter VOI, composed of all cortical sub-regions, and a cerebellar grey matter VOI because of the clearly visible difference in radioactivity concentrations between these two regions (Supplementary Fig. 2a). The choroid plexus and the retina were manually outlined on the MRI data using the PNEURO tool (Supplementary Fig. 2b, c). For the choroid plexus, four spherical VOIs (diameter: 2 mm) were placed in the first and second ventricle (two in each hemisphere) and averaged to generate a global choroid plexus VOI (Supplementary Fig. 2b). From the VOIs, time-activity curves (TACs) in units of standardised uptake value (SUV) were extracted. For selected MRP-expressing tissues (i.e., cerebral cortex, cerebellum, choroid plexus, retina, lungs, myocardium, kidneys and liver), the rate constant (kE) for radioactivity elimination was determined as a parameter for tissue MRP function [7,8,9]. kE is equivalent to the fraction of radioactivity that is eliminated from tissue per time and has a unit of h− 1. kE equals the slope of the linear part of the natural logarithm-transformed tissue TACs from 15 to 90 min after radiotracer injection and was obtained by linear regression analysis using Microsoft® Excel® 2019 MSO. It should be noted that kE is not identical with the efflux rate constant from tissue into plasma k2 determined with compartmental modelling, which requires the measurement of an arterial plasma input function.
Dosimetry
For the dosimetry calculations, VOIs were generated using an Artificial Intelligence segmentation tool, multiple-organ objective segmentation (MOOSE), and validated using visual inspection [19]. The considered organs and tissues were brain, thyroid gland, right lung, myocardium, liver, gall bladder, kidneys, red bone marrow (L3 to L5), muscle (gluteus maximus), and urinary bladder. PET frame images were analysed using an in-house pipeline (built using the Python programming language) to extract TACs for each region. Absorbed doses were calculated using the Medical Internal Radiation Dose (MIRD) methodology [20] with the MIRDcalc software for organ level dosimetry [21, 22]. The standard adult male and female International Commission on Radiological Protection (ICRP) reference phantoms were used for organ masses and source to target organ dose values (S values). Time-integrated activity in the source regions was calculated by integration over the measured time points, while assuming radioactive decay after the 90-min time point. Those were then normalised to each subject’s injected activity to calculate time-integrated-activity coefficients (residence times) and input to MIRDcalc. The sampled organs’ contribution to the total residence time was 37 ± 7% with the remaining being attributed to the rest of the body.
Statistical analysis
Descriptive data are presented as mean ± standard deviation (SD) unless otherwise specified. Reproducibility of kE was assessed using the TRTV (%), which was calculated for each VOI in each subject as ((kE, test − kE, retest) / (kE, test + kE, retest) / 2) × 100%. Differences between test and retest scans were analysed using a two-sided, paired t-test. Sex differences were analysed using a two-sided, unpaired t-test. The level of statistical significance was set to a p value of ≤ 0.05. Statistical analysis was performed using Prism 10.2.3 (Graphpad Software, Dotmatics, Boston, MA, USA).
Results
We included 13 subjects (7 men, 6 women) into our study, who underwent dynamic whole-body PET scans on a LAFOV PET/CT system after i.v. injection of [11C]BMP. Radiotracer administration was well tolerated without occurrence of adverse effects. During the PET scan venous blood samples were collected. Figure 1a shows mean TACs of total radioactivity in venous blood and plasma. Plasma-to-blood ratios of activity gradually increased over the duration of the PET scan (10 min: 1.51 ± 0.09; 90 min: 1.59 ± 0.08, p ≤ 0.001). There were significant sex differences in plasma-to-blood ratios, both at the 10 min time point (men: 1.59 ± 0.06, women: 1.44 ± 0.05, p ≤ 0.001) and at the 90 min time point (men: 1.65 ± 0.06, women: 1.52 ± 0.03, p ≤ 0.001). Plasma samples obtained at 5, 20, and 40 min after radiotracer injection were extracted with acetonitrile and analysed with radio-HPLC to assess conversion of [11C]BMP into its GSH conjugate [11C]MPG (Fig. 2a). The mean recovery for extraction of radioactivity into the acetonitrile fraction was 92.8 ± 1.4% (n = 18) for the 5 min sample. [11C]MPG and unconverted [11C]BMP were the two major radiolabelled species detected in plasma (Fig. 2a). In addition, an unidentified, lipophilic radiolabelled species, which eluted after [11C]BMP, was observed. The percentages of the three radiolabelled species in plasma at different time points are summarised in Supplementary Table 1. The percentage of unconverted [11C]BMP in plasma remained relatively constant (approximately 30% of total radioactivity), while the percentage of the GSH conjugate [11C]MPG declined from 63 ± 5% at 5 min to 38 ± 4% at 40 min and the percentage of the unidentified, lipophilic radiolabelled species increased over time (Supplementary Table 1). There were no sex differences in the percentage of [11C]MPG in plasma at all studied time points (Fig. 1b).
Representative HPLC chromatograms for analysis of plasma collected at 5 min after [11C]BMP injection (a) and urine collected at the end of the PET scan (b). The upper channel represents UV absorption (254 nm) and the lower channel radioactivity detection. Samples were spiked with unlabelled BMP and MPG. Retention times were different between plasma and urine due to matrix differences. [11C]U designates an unidentified radiolabelled species detected in plasma
Figure 3 shows representative whole-body PET/CT images obtained in one female subject at different time points after injection of [11C]BMP. Over the time course of the PET scan, activity was predominantly excreted into the urinary bladder. Figure 4a shows TACs for activity excretion into the urinary bladder. The percentage of the administered activity excreted into the urinary bladder at the end of the PET scan was 55 ± 5% based on the urinary bladder VOI delineated on the PET images and 51 ± 11% based on the gamma counter measurements of urine collected at the end of the PET scan. There were no significant sex differences in the percentage of the administered activity excreted into the urinary bladder (Fig. 4a). Radio-HPLC analysis of urine samples revealed a mixture of several, partly unidentified radiolabelled species with only very low amounts of unconverted [11C]BMP (< 1%) (Fig. 2b). The GSH conjugate [11C]MPG represented 26 ± 7% of total radioactivity in the urine. The amount of activity excreted into the gall bladder was rather low and variable (2.7 ± 2.3% of the administered activity) (Fig. 4b).
We outlined VOIs for different MRP-expressing tissues. The corresponding TACs in male and female subjects are shown in Fig. 5 for central tissues and in Fig. 6 for peripheral tissues. We observed regional differences in the brain distribution of [11C]BMP-derived radioactivity with a significantly higher exposure in the cerebellum than in the cortex (area under the TAC, cerebellum: 265 ± 34 SUV × min, cortex: 192 ± 26 SUV × min, p ≤ 0.0001) (Fig. 5a, b, Supplementary Fig. 2a). As an outcome parameter for tissue MRP function we derived kE from the tissue TACs. Mean kE values for all investigated tissues are given in Table 1. The slowest activity elimination was observed for the cerebellum (kE = 0.033 ± 0.009 h− 1) and the fasted elimination for the kidneys (kE = 1.378 ± 0.266 h− 1). There were significant sex differences in kE for the cerebellum (p ≤ 0.01), the lungs (p ≤ 0.05), and the kidneys (p ≤ 0.05) (Table 1). In the brain, kE was significantly lower for the cerebellum than for the cortex (p ≤ 0.0001).
Three subjects of each sex were scanned a second time to assess the TRTV of kE. In none of the investigated tissues were kE values significantly different between the test and the retest scans (Supplementary Figs. 3 and 4). Mean TRTV values were in the range of − 4 to + 14% in all tissues, except for the retina (Table 1). Mean TRTV values were positive in some tissues (i.e., higher kE in the retest scan) and negative in other tissues (i.e., lower kE in the retest scan).
We performed dosimetry calculations on PET data from all subjects (Table 2). In agreement with the predominantly urinary excretion of activity (Fig. 3), the urinary bladder and the kidneys received the highest absorbed doses for both sexes. Effective doses were 4.67 ± 0.18 µSv/MBq for men and 4.55 ± 0.18 µSv/MBq for women.
Discussion
The use of LAFOV PET/CT [23] enabled us to assess, at a whole-body, multi-tissue level, the function of MRPs, which have a widespread tissue distribution and necessitate quantitative dynamic PET measurements for their assessment. This will allow for human translation of results from previous mouse studies, in which the axial FOV of the employed small-animal PET scanner permitted a whole-body quantification of tissue MRP function [9, 10, 13].
A prerequisite to visualise the function of tissue MRPs with [11C]BMP is its efficient conversion into the corresponding GSH conjugate [11C]MPG. This conversion is mediated by GSTs which have a widespread tissue distribution. Rapid and almost quantitative conversion (i.e., within 5–15 min after radiotracer injection) of [11C]BMP into [11C]MPG has been demonstrated in the mouse brain and lungs [7,8,9] and in the rat lungs [12]. We analysed in our study plasma samples as a surrogate for tissue samples to estimate the extent of [11C]BMP conversion in human tissues (Fig. 2a). We found that 63 ± 5% of total radioactivity in plasma represented the radiolabelled GSH conjugate [11C]MPG at 5 min after radiotracer injection. It appears likely that GSH conjugation of [11C]BMP was already completed at the 5 min time point and that the remaining unconverted [11C]BMP in plasma represented the plasma protein-bound fraction of [11C]BMP, which is not able to distribute into erythrocytes, where GSH conjugation presumably occurs. The decline in the percentage of [11C]MPG in plasma at later time points (Fig. 1b) may be related to its faster plasma clearance relative to the other radiolabelled species. It is not known, however, whether the remaining unconverted [11C]BMP is able to distribute from plasma to tissues, which could affect the measurement of tissue MRP function using the kE parameter. Therefore, in future work attempts will be made to develop a full kinetic model for [11C]BMP, which will benefit from the presence of large blood vessels within the FOV of the LAFOV PET/CT scanner for the measurement of an image-derived arterial input function.
For quantification of tissue MRP function we derived, in analogy to previous rodent studies [7,8,9], the kE parameter from the tissue TACs. Knockout of the Abcc1 gene or pharmacological inhibition of MRPs with MK571 led to significant decreases of kE in the mouse brain and lungs [7,8,9,10,11]. In the brain, MRP1 is predominantly expressed in brain parenchymal cells (e.g., astrocytes and neurons) and in choroid plexus epithelial cells with lower expression levels in brain capillary endothelial cells (BCECs) [24]. Okamura et al. demonstrated, by intracranial injection of [11C]MPG into wild-type and Abcc1(−/−) mice, that MRP1 function is not the rate limiting step in the elimination of [11C]MPG from the mouse brain [15]. Experiments in mice lacking organic anion transporter 3 (OAT3, encoded in rodents by the Slc22a8 gene) or multidrug resistance-associated protein 4 (MRP4, encoded in rodents by the Abcc4 gene) showed that the elimination of [11C]MPG across the mouse BBB is mediated by OAT3 and MRP4, which are localised in the basolateral (brain-facing) and luminal (blood-facing) membranes of BCECs, respectively [15]. An important finding of our study is that, in contrast to the rapid elimination of [11C]BMP-derived radioactivity from the mouse brain (kE: 1.48 ± 0.06 h− 1) [9], radioactivity was very slowly eliminated from the human brain with 30–45 times lower kE values in humans (Table 1). This strongly suggests pronounced species differences in the expression of membrane transporters that can efflux GSH conjugates such as [11C]MPG across the BBB. While OAT3 and MRP4 could be quantified in mouse BCECs with targeted proteomics [25], they were below the limit of detection in human BCECs [26]. It is not certain whether species differences in MRP1 expression contributed to the slow elimination of [11C]BMP-derived radioactivity from the human brain, since available immunohistochemistry data indicate that MRP1 is expressed in the human brain [24]. Our observation is in good agreement with results for the brain perfusion single photon emission computed tomography (SPECT) tracer [99mTc]Tc-ECD (i.e., 99mTc complex with N,N’-1,2-ethylenediylbis-L-cysteine) [27]. [99mTc]Tc-ECD is converted inside the brain into an acid metabolite, which is a substrate of OAT3 and trapped in the human brain while being quickly eliminated from the mouse brain. We also analysed the choroid plexus, in which MRP1 is expressed in the basolateral (blood-facing) membrane of epithelial cells [28], and the retina, in which MRP1 is expressed in the cell membrane of the retinal pigment epithelium forming the outer blood-retina barrier [29]. Interestingly, both tissues showed a considerably faster radioactivity elimination as compared to the brain (Table 1), supporting the presence of some efflux mechanism for [11C]BMP-derived radioactivity. It remains to be determined in future studies involving administration of an MRP1 inhibitor whether the kinetics of [11C]BMP-derived radioactivity in the human brain are dependent on MRP1 function and whether the observed regional and sex differences are caused by differences in MRP1 function or by other factors, such as differences in GST activity or GSH content.
Apart from central tissues, MRP1 is abundantly expressed in the basolateral membrane of pulmonary epithelial cells [30]. It has been shown that [11C]BMP PET can measure MRP1 function in the rodent lungs, both after i.v. administration [8,9,10, 13] and after intratracheal aerosolisation [12, 14]. As opposed to the pronounced species differences observed in the brain kinetics of [11C]BMP-derived radioactivity, the elimination rate of radioactivity from the human lungs was in similar range as in mice (kE, humans: 0.875 ± 0.095 h− 1, mice: 1.52 ± 0.10 h− 1) [9]. Intriguingly, we observed significant sex differences in the lungs with lower kE values in women than in men (Table 1). This agrees well with quantitative proteomics data, which revealed a 30% lower expression of MRP1 in the lungs of women than of men [31], and supports that [11C]BMP-derived radioactivity is eliminated from the lungs by MRP1. We also analysed the myocardium, in which MRP1 is expressed in the sarcolemmal membrane of cardiomyocytes and was shown to protect the heart from doxorubicin-induced cardiotoxicity [32].
Different MRP subtypes (i.e., MRP2 and MRP4) are abundantly expressed in excretory organs (i.e., the kidneys and the liver), where they mediate the urinary and biliary excretion of various drugs and their metabolites [33]. In contrast, MRP1 is only moderately or not at all expressed in the liver and kidneys. As different MRP subtypes show a considerable degree of substrate overlap, it can be expected that [11C]MPG is also transported by other MRP subtypes including those expressed in the kidneys and liver. In fact, experiments in Abcc4(−/−) mice revealed that MRP4 contributed to the urinary excretion of [11C]BMP-derived radioactivity in mice [9]. To assess renal and hepatic MRP function with [11C]BMP PET we included the kidneys, the liver, and the urinary bladder into our analysis. The gall bladder was not always clearly visible on the PET images and could therefore not be analysed in all subjects. Our data revealed that [11C]BMP-derived radioactivity is predominantly excreted into the urine with approximately 50% of the administered activity found in the urinary bladder at the end of the PET scan (Fig. 4a). In contrast, < 5% of the administered activity was in the gall bladder at the end of the PET scan (Fig. 4b), which suggests negligible biliary excretion of [11C]BMP-derived radioactivity. Radio-HPLC analysis of urine samples confirmed excretion of [11C]MPG into the urine but also showed the presence of some other, unidentified radiolabelled species, which were not detected in plasma, suggesting that they were formed in the kidneys (Fig. 2). We found significant sex differences in kE values in the kidneys, pointing to a higher expression of [11C]MPG-excreting transporters in the kidneys of women than men (Table 1). In future studies, the MRP subtype specificity of [11C]MPG needs to be assessed to elucidate which MRP subtypes are involved in its urinary excretion in humans.
To assess the reproducibility of [11C]BMP PET for measurement of tissue MRP function, we performed test-retest scans in 3 male and 3 female subjects. Test and retest scans were not performed on the same study day as this was not feasible within our study set-up. Overall, mean TRTVs were in an acceptable range for all investigated tissues, except for the retina (Table 1), despite the relatively long interval between test and retest scans (20 ± 10 days). The comparatively low reproducibility of kE values in the retina is probably due to the small size of this structure. The between-subject variability of the TRTV values was rather large (Table 1), which may be due to physiological variability in transporter function and different time intervals between the test and retest scans in individual subjects.
We calculated human dosimetry based on the whole-body PET data. The dosimetry calculations benefitted from the rich kinetic information obtained in multiple organs of the body, which would not have been available when performing the dosimetry study on a conventional PET/CT with multiple bed positions. The organ absorbed doses did not show any conspicuous values. For both sexes, the urinary bladder and the kidneys received the highest absorbed doses (Table 2), which was consistent with the predominantly urinary excretion of [11C]BMP-derived radioactivity (Fig. 4). The effective doses (Table 2) were comparable to human effective doses extrapolated from mouse PET data, which had been included in the IMPD of [11C]BMP [18]. The dosimetry of [11C]BMP was in the typical range of other 11C-tracers (average effective dose of 77 different 11C-tracers: 5.2 ± 1.7 µSv/MBq, range: 3.2–14.1 µSv/MBq) [34]. An injected activity of 400 MBq thus corresponds to an effective dose of 1.87 mSv in men and 1.82 mSv in women, which is well below the limit of 10 mSv for studies in healthy volunteers aged less than 50 years [35] and will permit multiple [11C]BMP PET scans in a single subject. Future studies will include a validation of [11C]BMP for measurement of tissue MRP function in humans by performing LAFOV PET/CT scans without and with pre-treatment with an MRP-inhibiting drug, for which the here reported test-retest data will be useful.
In summary, the advent of LAFOV PET/CT offers the unprecedented opportunity to assess molecular targets relevant for drug disposition in humans at a whole-body, multi-tissue level. This innovative research approach holds enormous potential for evaluating the disposition of radiolabelled drugs in humans in health and disease, as well as for investigating factors that may alter drug disposition (e.g., drug-drug interactions). Based on our current data, [11C]BMP is a safe radiotracer that will, in combination with LAFOV PET/CT, potentially allow the comprehensive assessment of MRP function across multiple tissues in the human body.
Data availability
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request (OL).
References
Cole SP. Multidrug resistance protein 1 (MRP1, ABCC1), a multitasking ATP-binding cassette (ABC) transporter. J Biol Chem. 2014;289:30880–8. https://doi.org/10.1074/jbc.R114.609248.
Cole SP. Targeting multidrug resistance protein 1 (MRP1, ABCC1): past, present, and future. Annu Rev Pharmacol Toxicol. 2014;54:95–117. https://doi.org/10.1146/annurev-pharmtox-011613-135959.
Krohn M, Lange C, Hofrichter J, Scheffler K, Stenzel J, Steffen J, et al. Cerebral amyloid-beta proteostasis is regulated by the membrane transport protein ABCC1 in mice. J Clin Invest. 2011;121:3924–31. https://doi.org/10.1172/JCI57867.
van der Deen M, Marks H, Willemse BW, Postma DS, Muller M, Smit EF, et al. Diminished expression of multidrug resistance-associated protein 1 (MRP1) in bronchial epithelium of COPD patients. Virchows Arch. 2006;449:682–8. https://doi.org/10.1007/s00428-006-0240-3.
Hurbain I, Sermet-Gaudelus I, Vallee B, Feuillet MN, Lenoir G, Bernaudin JF, et al. Evaluation of MRP1-5 gene expression in cystic fibrosis patients homozygous for the delta F508 mutation. Pediatr Res. 2003;54:627–34. https://doi.org/10.1203/01.PDR.0000090926.16166.3F.
Bauer M, Tournier N, Langer O. Imaging P-glycoprotein function at the blood-brain barrier as a determinant of the variability in response to central nervous system drugs. Clin Pharmacol Ther. 2019;105:1061–4. https://doi.org/10.1002/cpt.1402.
Okamura T, Kikuchi T, Okada M, Toramatsu C, Fukushi K, Takei M et al. Noninvasive and quantitative assessment of the function of multidrug resistance-associated protein 1 in the living brain. J Cereb Blood Flow Metab. 2009;29:504 – 11. doi:jcbfm2008135 [pii]10.1038/jcbfm.2008.135.
Okamura T, Kikuchi T, Okada M, Wakizaka H, Zhang MR. Imaging of activity of multidrug resistance-associated protein 1 in the lungs. Am J Respir Cell Mol Biol. 2013;49:335–40. https://doi.org/10.1165/rcmb.2012-0275MA.
Zoufal V, Mairinger S, Krohn M, Wanek T, Filip T, Sauberer M, et al. Influence of multidrug resistance-associated proteins on the excretion of the ABCC1 imaging probe 6-bromo-7-[11C]methylpurine in mice. Mol Imaging Biol. 2019;21:306–16. https://doi.org/10.1007/s11307-018-1230-y.
Krohn M, Zoufal V, Mairinger S, Wanek T, Paarmann K, Bruning T, et al. Generation and characterization of an Abcc1 humanized mouse model (hABCC1flx/flx) with knockout capability. Mol Pharmacol. 2019;96:138–47. https://doi.org/10.1124/mol.119.115824.
Zoufal V, Mairinger S, Krohn M, Wanek T, Filip T, Sauberer M, et al. Measurement of cerebral ABCC1 transport activity in wild-type and APP/PS1-21 mice with positron emission tomography. J Cereb Blood Flow Metab. 2020;40:954–65. https://doi.org/10.1177/0271678X19854541.
Mairinger S, Sake JA, Hernández Lozano I, Filip T, Sauberer M, Stanek J, et al. Assessing the activity of multidrug resistance-associated protein 1 at the lung epithelial barrier. J Nucl Med. 2020;61:1650–7. https://doi.org/10.2967/jnumed.120.244038.
Wölfl-Duchek M, Mairinger S, Hernández-Lozano I, Filip T, Zoufal V, Löbsch M, et al. Use of PET imaging to assess the efficacy of thiethylperazine to stimulate cerebral MRP1 transport activity in wild-type and APP/PS1-21 mice. Int J Mol Sci. 2022;23. https://doi.org/10.3390/ijms23126514.
Mairinger S, Hernández-Lozano I, Zachhuber L, Filip T, Löbsch M, Zeitlinger M, et al. Effect of budesonide on pulmonary activity of multidrug resistance-associated protein 1 assessed with PET imaging in rats. Eur J Pharm Sci. 2023;184:106414. https://doi.org/10.1016/j.ejps.2023.106414.
Okamura T, Okada M, Kikuchi T, Wakizaka H, Zhang MR. Mechanisms of glutathione-conjugate efflux from the brain into blood: involvement of multiple transporters in the course. J Cereb Blood Flow Metab. 2020;40:116–25. https://doi.org/10.1177/0271678X18808399.
Okamura T, Kikuchi T, Fukushi K, Arano Y, Irie T. A novel noninvasive method for assessing glutathione-conjugate efflux systems in the brain. Bioorg Med Chem. 2007;15:3127–33. https://doi.org/10.1016/j.bmc.2007.02.045.
Prenosil GA, Sari H, Furstner M, Afshar-Oromieh A, Shi K, Rominger A, et al. Performance characteristics of the Biograph vision quadra PET/CT system with a long axial field of view using the NEMA NU 2-2018 standard. J Nucl Med. 2022;63:476–84. https://doi.org/10.2967/jnumed.121.261972.
Mairinger S, Jackwerth M, Soukup O, Blaickner M, Decristoforo C, Nics L, et al. Advancing 6-bromo-7-[11C]methylpurine to clinical use: improved regioselective radiosynthesis, non-clinical toxicity data and human dosimetry estimates. EJNMMI Radiopharm Chem. 2024;9:34. https://doi.org/10.1186/s41181-024-00265-z.
Shiyam Sundar LK, Yu J, Muzik O, Kulterer O, Fueger BJ, Kifjak D, et al. Fully-automated, semantic segmentation of whole-body (18)F-FDG PET/CT images based on data-centric artificial intelligence. J Nucl Med. 2022. https://doi.org/10.2967/jnumed.122.264063.
Bolch WE, Eckerman KF, Sgouros G, Thomas SR. MIRD pamphlet 21: a generalized schema for radiopharmaceutical dosimetry–standardization of nomenclature. J Nucl Med. 2009;50:477–84. https://doi.org/10.2967/jnumed.108.056036.
Kesner AL, Carter LM, Ramos JCO, Lafontaine D, Olguin EA, Brown JL, et al. MIRD pamphlet 28, part 1: MIRDcalc-a software tool for medical internal radiation dosimetry. J Nucl Med. 2023;64:1117–24. https://doi.org/10.2967/jnumed.122.264225.
Carter LM, Ocampo Ramos JC, Olguin EA, Brown JL, Lafontaine D, Jokisch DW, et al. MIRD pamphlet 28, part 2: comparative evaluation of MIRDcalc dosimetry software across a compendium of diagnostic radiopharmaceuticals. J Nucl Med. 2023;64:1295–303. https://doi.org/10.2967/jnumed.122.264230.
Slart R, Tsoumpas C, Glaudemans A, Noordzij W, Willemsen ATM, Borra RJH, et al. Long axial field of view PET scanners: a road map to implementation and new possibilities. Eur J Nucl Med Mol Imaging. 2021;48:4236–45. https://doi.org/10.1007/s00259-021-05461-6.
Bernstein HG, Holzl G, Dobrowolny H, Hildebrandt J, Trubner K, Krohn M, et al. Vascular and extravascular distribution of the ATP-binding cassette transporters ABCB1 and ABCC1 in aged human brain and pituitary. Mech Ageing Dev. 2014;141–142:12–21. https://doi.org/10.1016/j.mad.2014.08.003.
Kamiie J, Ohtsuki S, Iwase R, Ohmine K, Katsukura Y, Yanai K, et al. Quantitative atlas of membrane transporter proteins: development and application of a highly sensitive simultaneous LC/MS/MS method combined with novel in-silico peptide selection criteria. Pharm Res. 2008;25:1469–83. https://doi.org/10.1007/s11095-008-9532-4.
Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, et al. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J Neurochem. 2011;117:333–45. https://doi.org/10.1111/j.1471-4159.2011.07208.x.
Kikuchi T, Okamura T, Wakizaka H, Okada M, Odaka K, Yui J, et al. OAT3-mediated extrusion of the 99mTc-ECD metabolite in the mouse brain. J Cereb Blood Flow Metab. 2014;34:585–8. https://doi.org/10.1038/jcbfm.2014.20.
Rao VV, Dahlheimer JL, Bardgett ME, Snyder AZ, Finch RA, Sartorelli AC, et al. Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drug-permeability barrier. Proc Natl Acad Sci USA. 1999;96:3900–5. https://doi.org/10.1073/pnas.96.7.3900.
Hellinen L, Sato K, Reinisalo M, Kidron H, Rilla K, Tachikawa M, et al. Quantitative protein expression in the human retinal pigment epithelium: comparison between apical and basolateral plasma membranes with emphasis on transporters. Invest Ophthalmol Vis Sci. 2019;60:5022–34. https://doi.org/10.1167/iovs.19-27328.
Scheffer GL, Pijnenborg AC, Smit EF, Müller M, Postma DS, Timens W, et al. Multidrug resistance related molecules in human and murine lung. J Clin Pathol. 2002;55:332–9. https://doi.org/10.1136/jcp.55.5.332.
Sakamoto A, Matsumaru T, Yamamura N, Uchida Y, Tachikawa M, Ohtsuki S, et al. Quantitative expression of human drug transporter proteins in lung tissues: analysis of regional, gender, and interindividual differences by liquid chromatography-tandem mass spectrometry. J Pharm Sci. 2013;102:3395–406. https://doi.org/10.1002/jps.23606.
Zhang W, Deng J, Sunkara M, Morris AJ, Wang C, St Clair D, et al. Loss of multidrug resistance-associated protein 1 potentiates chronic doxorubicin-induced cardiac dysfunction in mice. J Pharmacol Exp Ther. 2015;355:280–7. https://doi.org/10.1124/jpet.115.225581.
Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–36. https://doi.org/10.1038/nrd3028.
Zanotti-Fregonara P, Lammertsma AA, Innis RB. 11C dosimetry scans should be abandoned. J Nucl Med. 2021;62:158–9. https://doi.org/10.2967/jnumed.120.257402.
ICRP. Radiological Protection in Biomedical Research. Ann ICRP. 1992;22:1–18. https://doi.org/10.1016/j.icrp.2007.10.003. ICRP Publication 62.
Acknowledgements
The authors would like to thank Harald Ibeschitz, Bettina Reiterits, Lea Kum, and Anselm Jorda for providing support during the study days.
Funding
Open access funding provided by Medical University of Vienna. This research was funded by the Austrian Research Promotion Agency (FFG) [882717 PETABC, to Oliver Langer]. PETABC is an EU Joint Programme - Neurodegenerative Disease Research (JPND) project. PETABC is supported through the following funding organisations under the aegis of JPND (www.jpnd.eu): NFR #327571 - Norway, FFG #882717 - Austria, BMBF #01ED2106 - Germany, MSMT #8F21002 - Czech Republic, VIAA #ES RTD/2020/26 - Latvia, ANR #20-JPW2-0002-04 - France and SRC #2020–02905 - Sweden.
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Contributions
S.M., M.J., M.W., M.W.-D., J.P., W.L., M.H, M.Z., and O.L. contributed to the concept and design of the study. S.M., M.J., M.W., L.P., and L.N. acquired the data. S.M., M.J., Z.C., I.R., and O.L. analysed the data. Z.C. performed the dosimetry calculations. O.L. and S.M. wrote the first draft of the manuscript and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was conducted in accordance with the ICH-GCP guidelines and the Declaration of Helsinki. The trial was registered in the EudraCT database (2021-006348-29) and was approved by the Ethics Committee of the Medical University of Vienna and the Austrian Agency for Health and Food Safety.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mairinger, S., Jackwerth, M., Chalampalakis, Z. et al. First-in-human evaluation of 6-bromo-7-[11C]methylpurine, a PET tracer for assessing the function of multidrug resistance-associated proteins in different tissues. Eur J Nucl Med Mol Imaging (2024). https://doi.org/10.1007/s00259-024-06851-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00259-024-06851-2