Abstract
Purpose
To assess if PSMA PET quantitative parameters are associated with pathologic ISUP grade group (GG) and upgrading/downgrading.
Methods
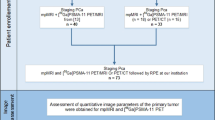
PCa patients undergoing radical prostatectomy with or without pelvic lymph node dissection staged with preoperative PSMA PET at seven referral centres worldwide were evaluated. PSMA PET parameters which included SUVmax, PSMAvolume, and total PSMA accumulation (PSMAtotal) were collected. Multivariable logistic regression evaluated the association between PSMA PET quantified parameters and surgical ISUP GG. Decision-tree analysis was performed to identify discriminative thresholds for all three parameters related to the five ISUP GGs The ROC-derived AUC was used to determine whether the inclusion of PSMA quantified parameters improved the ability of multivariable models to predict ISUP GG ≥ 4.
Results
A total of 605 patients were included. Overall, 2%, 37%, 37%, 10% and 13% patients had pathologic ISUP GG1, 2, 3, 4, and 5, respectively. At multivariable analyses, all three parameters SUVmax, PSMAvolume and PSMAtotal were associated with GG ≥ 4 at surgical pathology after accounting for PSA and clinical T stage based on DRE, hospital and radioligand (all p < 0.05). Addition of all three parameters significantly improved the discrimination of clinical models in predicting GG ≥ 4 from 68% (95%CI 63 – 74) to 74% (95%CI 69 – 79) for SUVmax, 72% (95%CI 67 – 76) for PSMAvolume, 74% (70 – 79) for PSMAtotal and 75% (95%CI 71 – 80) when all parameters were included (all p < 0.05). Decision-tree analysis resulted in thresholds that discriminate between GG (SUVmax 0–6.5, 6.5–15, 15–28, > 28, PSMAvol 0–2, 2–9, 9–20 and > 20 and PSMAtotal 0–12, 12–98 and > 98). PSMAvolume was significantly associated with GG upgrading (OR 1.03 95%CI 1.01 – 1.05). In patients with biopsy GG1-3, PSMAvolume ≥ 2 was significantly associated with higher odds for upgrading to ISUP GG ≥ 4, compared to PSMAvolume < 2 (OR 6.36, 95%CI 1.47 – 27.6).
Conclusion
Quantitative PSMA PET parameters are associated with surgical ISUP GG and upgrading. We propose clinically relevant thresholds of these parameters which can improve in PCa risk stratification in daily clinical practice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of PSMA PET/CT to assist primary staging of prostate cancer (PCa) is characterized by a higher sensitivity for the detection of nodal and distant metastasis compared to conventional imaging [1,2,3,4,5]. More recently, PSMA PET-derived quantified parameters have been proposed to improve risk stratification [6]. One of the most extensively investigated quantitative parameter for analysis of tracer uptake includes the standardized uptake value (SUV). SUVmax is defined as the SUV of the single voxel in a region of interest that presents the highest uptake on the attenuation-corrected PET image [7]. SUVmax has been previously shown to have high reproducibility [8]. Since PSMA expression is observed with the greatest extent and intensity in the highest Gleason primary patterns 4 and 5, SUVmax might improve our ability to risk stratify PCa [9, 10]. Prior studies exploring the association between uptake values have shown that Gleason scores were correlated with the intensity of tracer accumulation in the primary tumor, showing that SUVmax among patients with Gleason scores ≤ 7 were significantly lower compared with patients with Gleason scores > 7 [11]. Similarly, [68 Ga]Ga-PSMA-11 SUVmax was significantly higher among patients with Gleason ≥ 4 + 3 compared with Gleason ≤ 3 + 4 [12]. Besides SUVmax, other quantitative PSMA PET parameters such as intraprostatic PSMAvolume and PSMAtotal have been reported to be significantly associated with surgical outcomes. PSMAvolume is the total quantified PSMA positive volume of the prostate tumor, whereas PSMAtotal represents the total PSMA accumulation (PSMAvolume x SUVmean) of the tumor. These parameters could add value to SUVmax, in terms of PCa prognostication, as they also provide information regarding the size and total uptake of the region of interest. For example, both PSMAvolume and PSMAtotal were associated with lymph node involvement (LNI) at pelvic lymph node dissection [13]. However, it is unclear how these parameters relate to histopathological features such as the International Society of Urological Pathology (ISUP) Grade Group (GG) of the primary tumor, and if these parameters provide additional predictive value to SUVmax alone.
Although previous studies confirm the association of quantitative PSMA PET parameters with PCa histopathological findings, reliable and reproducible thresholds to further guide clinical decision-making are lacking. As most prior studies on this subject include single-center, relatively small cohorts, there is an urgent need for studies with larger sample sizes. In addition, the majority of studies describe the use of [68 Ga]Ga-PSMA-11, and it is unclear how quantitative parameters and their association with histopathology relate among other radioligands. In the face of such a paucity of data, we sought to evaluate the association between PSMA PET quantitative parameters with disease aggressiveness (namely, pathologic ISUP grade group) in a large international multi-center cohort of PCa patients undergoing radical prostatectomy.
Methods
Patient population
Men with histopathologically proven PCa undergoing radical prostatectomy with or without pelvic lymph node dissection and preoperatively staged with PSMA PET/CT in the period 2016 to 2023 at seven tertiary referral centers were included. Patients were excluded if they underwent prior other (systemic) therapy for PCa. Histopathological reporting of the surgical specimen was done by local dedicated uropathologists according to the ISUP guidelines [14].
PSMA PET/CT procedures
All PSMA PET scans were performed at the tertiary referral centers according to the local protocol. A description of the PET protocols used per hospital is presented in Supplementary Table 1. The inclusion of PET scans performed externally for referred patients was allowed. These PET scans were re-read by the local team. PET images were made from mid-thigh to skull base and combined with a low-dose CT scan or a diagnostic CT scan for anatomical correlation. All PSMA PET scans were evaluated by an experienced nuclear medicine physician (> 5 yr experience and/or > 500 studies) at each referral center. The radioligands used included [68 Ga]Ga-PSMA-11, [18F]PSMA-1007, [18F]DCF-PyL, and [18F]-JK-PSMA-7, according to specific center preference. Images were acquired according to European Association of Nuclear Medicine/Society of Nuclear Medicine and Molecular Imaging criteria [15].
PSMA PET/CT parameters
To collect additional PSMA parameters not standardly reported during routine clinical care, all PSMA PET scans were prospectively reassessed and read by the local nuclear medicine physician or research fellow under the direct supervision of the nuclear medicine staff physician. PSMA parameters were assessed by delineating the PSMA-expressing tumors, which represent the volume of interest, manually within the prostate with the threshold set to SUVmax ≥ 4. Neighboring anatomical tissues with high PSMA accumulation (e.g. urinary bladder) were excluded. PSMA parameters calculated one whole-gland level included SUVmax, PSMA positive volume (PSMAvolume), and total PSMA accumulation (PSMAvolume × SUVmean [of the selected volume of interest] = PSMAtotal).
Statistical analysis
Pairwise comparison of the distribution of PSMA PET/CT parameters per ISUP GG
Since data of PSMA PET parameters were not normally distributed, non-parametric tests were employed. Median values of all three PSMA PET parameters were assessed per surgical ISUP GG, and pairwise comparisons of median values per pathologic ISUP GG were performed. The Kruskall-Wallis test was used to compare > 2 independent groups, including post-hoc pairwise comparisons of all separate ISUP GG (1 to 5) using Dunn’s test and applying the Bonferroni correction.
Multivariable logistic regression analyses predicting pathologic ISUP GG ≥ 4
Uni- and multivariable logistic regression analysis assessed the association of SUVmax, PSMAvolume, and PSMAtotal with pathologic ISUP GG ≥ 4 after adjusting for potential confounders. To establish whether the potential association varied among radioligands, multivariable logistic regression analysis was done including patients undergoing PSMA PET with use of either [68 Ga]Ga-PSMA-11 or [18F]PSMA-1007, adjusting for clinical stage based on DRE, preoperative PSA and hospital. The ROC-derived AUC of models predicting ISUP GG ≥ 4 was calculated before (clinical variables only) and after including PSMA PET parameters.
Decision-tree analysis for discerning thresholds related to ISUP GG 1 to 5
We then employed decision tree analysis, a machine learning technique, to identify discriminative thresholds for SUVmax, PSMAvolume, and PSMAtotal related to the five ISUP GGs. The aim of this analysis was to explore presence of cut-offs who are directly proportional to ISUP GG histology. The decision tree model was trained on the dataset, iteratively splitting subclasses based on the values of the continuous variables to create a tree structure, using the CHAID (Chi-square Automatic Interaction Detection) method. To reduce overfitting, tenfold cross validation was employed [16, 17].
Association between PSMA PET parameters and GG upgrading and downgrading
The association between SUVmax, PSMAvolume, and PSMAtotal and GG upgrading among patients with biopsy ISUP GG < 5, and downgrading among patients with biopsy ISUP GG > 1, were assessed using univariable and multivariable logistic regression analysis. The thresholds resulting from the decision-tree analysis were explored to assess most optimum cut-offs for the prediction of both upgrading and downgrading.
All statistical analyses were done using SPSS (IBM Corp. Version 25.0. Armonk, NY) and R v4.2.1. (R Project for Statistical Computing, www.r-project.org).
Results
Patient baseline characteristics
A total of 605 patients were included per analysis. The median age at surgery was 66 years (IQR 62 – 71) and the median preoperative serum PSA level was 9.5 ng/ml (IQR 6.4 – 16.1). Overall, 2%, 43%, and 56% of patients had EAU low-, intermediate- and high-risk PCa. MRI information (PI-RADS score and staging info) was available in 534 (88%) of patients. Among patients with PI-RADS 3 or higher on MRI, target biopsy was performed in 77% of cases. In the vast majority of cases (95%), radioligands [68 Ga]Ga-PSMA-11 (62%) and [18F]PSMA-1007 (33%) were used. The median SUVmax, PSMAvolume, and PSMAtotal were 9.8 (IQR 6.1 – 16.4), 4.6 (IQR 1.4 – 10.7), and 29.8 (IQR 8.0 – 77.5), respectively (Table 1). Boxplots of all three parameters per ISUP GG are shown in Supplementary Fig. 1. At final surgical pathology, 136 patients (23%) had ISUP grade ≥ 4, while 29% of men had localized disease (pT2), and extraprostatic extension (pT3a) and seminal vesicle invasion (pT3b) were observed in respectively 49% and 22% (Table 1).
Pairwise comparison of the distribution of PSMA PET/CT parameters per ISUP GG
The median values of all three PSMA parameters differed significantly per ISUP GG and were directly proportional in value (Table 2). In the pairwise comparative analysis of each GG pair, SUVmax showed highest heterogeneity in the pairwise comparison of median values per ISUP GG, showing significant differences for all GG pairs except GG1 vs GG2, GG3 vs GG4 and GG4 vs GG5 (Table 3). Comparing median values per GG, [18F]PSMA-1007 vs. [68 Ga]Ga-PSMA-11, PSMAtotal and SUVmax median values per GG showed no significant differences in median values comparing both radioligands. For PSMAvolume significant differences in median values were observed for GG2 and GG3 comparing both radioligands (Supplementary Table 2).
Uni- and multivariable regression analysis identifying predictors of ISUP GG ≥ 4
At uni- and multivariable logistic regression analyses of all three PSMA parameters separately, SUVmax, PSMAvolume and PSMAtotal were significantly associated with a pathologic GG ≥ 4 (Supplementary Table 3, all p < 0.05). Combining all three parameters in multivariable analysis showed that PSMAtotal was significantly associated with ISUP GG ≥ 4 (OR 1.005 95%CI 1.002 – 1.007), whereas SUVmax and PSMAvolume were not (Table 4). PSMA quantified parameters significantly improved the discrimination in terms of AUC of the model with clinical parameters in predicting GG ≥ 4 from 68% (95%CI 63 –74) to respectively 74% (95%CI 69 – 79), p < 0.001, for SUVmax, 72% (95%CI 67 –76), p = 0.006, for PSMAvolume, 74% (95%CI 70 – 79), p = 0.003, for PSMAtotal and 75% (95%CI 71 – 80), p = 0.001, with all three parameters included (Table 4 and Fig. 1). Results of additional analyses evaluating the impact of hospital and radioligand on the multivariable models are shown in Supplementary Table 4. When excluding hospital as a covariate (model B), SUVmax remained significantly associated with ISUP GG ≥ 4 (OR 1.031, 95%CI 1.009 – 1.054). While addition of hospital as a covariate resulted in a significant increase in AUC from 71% (Model B) to 75% (Model D), p = 0.01), accounting for radioligand as a covariate did not significantly change AUC (71% vs 71%, p = 0.6) (Supplementary Table 4 and Fig. 2).
ROC curves of multivariable logistic regression analysis of high-risk surgical ISUP grade group (model 1 to 5) (Table 4)
ROC curves of multivariable logistic regression models assessing the impact of hospital and radioligand type on model discrimination (model A to D) (Supplementary Table 4)
Decision-tree analysis for discerning thresholds for all parameters related to ISUP GG 1 to 5
Decision-tree analysis resulted in thresholds that discriminate between GG (SUVmax 0–6.5, 6.5–15, 15–28, > 28, PSMAvolume 0–2, 2–9, 9–20 and > 20 and PSMAtotal 0–12, 12–98 and > 98). For all three parameters, an absolute increase in proportion of patients with ISUP grade 4 and 5 was observed, directly proportional with PSMA parameters values for each node. An inversely proportional association was observed for proportions of patients with ISUP grade 1 and 2, whereas proportions of patients with ISUP grade 3 remained stable among nodes (Fig. 3a, b, and c.).
Association between PSMA PET parameters and GG upgrading and downgrading
Upgrading and downgrading were observed in respectively 97 (16%) and 207 (35%) patients. As shown in Table 5, upgrading occurred most frequently in ISUP grade group 1 (n = 22, 73%), which included upgrading to GG2 in 53%, GG3 in 17% and GG5 in 5% of cases, respectively. On multivariable analysis including all three PSMA parameters, PSMAvolume was significantly associated with GG upgrading (OR 1.027 95%CI 1.007 – 1.049), whereas SUVmax and PSMAtotal were not (Supplementary Table 6). Among patients with biopsy GG1-3 (n = 352), upgrading to GG ≥ 4 was observed in 23 (7%) of patients. In patients with biopsy GG1-3, PSMAvolume ≥ 2 was significantly associated with higher odds for upgrading to ISUP GG ≥ 4, compared to PSMAvolume < 2 (OR 6.36, 95%CI 1.47 – 27.6). PSMAvolume was also the only PSMA parameter significantly (inversely) associated with GG downgrading on multivariable analysis (Supplementary Table 8). Among patients with biopsy GG ≥ 4 (n = 248), 44 patients (18%) experienced downgrading to GG ≤ 2. Patients with biopsy GG ≥ 4 and PSMAvolume ≥ 2, had significantly lower odds (OR 0.42 95%CI 0.21 – 0.87) for downgrading to GG ≤ 2, compared with those with PSMAvolume < 2.
Discussion
Although previous studies proposed an association between PSMA PET quantitative parameters and disease aggressiveness, their results are poorly generalizable due to the inclusion of small sample sizes and the lack of a comprehensive assessment of all available tracers. As such, we aimed to evaluate the association between PSMA PET quantitative parameters with surgical ISUP GG in a large multi-center cohort of PCa patients undergoing RP treated worldwide. Our multicenter analyses allowed us to propose a clinically relevant subclassification of SUVmax, PSMAvolume and PSMAtotal associated with ISUP GG ≥ 4 at histopathological evaluation after RP. Analyses of their median values per ISUP GG revealed that these are directly proportionally associated with ISUP grading. SUVmax had the best discriminative ability at pairwise ISUP GG comparative analysis. Multivariable analyses including all three PSMA PET parameters, showed that PSMAtotal was significantly associatied with GG ≥ 4, whereas PSMAvolume was associated with upgrading and downgrading. Our findings have clinical implications, as our proposed subclassification per parameter can assist PCa risk stratification and guide clinical decision-making.
Previous studies proposed that PSMA parameters can be used to discriminate PCa from benign tissue. For example, Jiao and colleagues evaluated 135 patients who underwent [68 Ga]Ga-PSMA-11 PET/CT and showed that using SUVmax with a cutoff value of 5.30 can assist with discriminating clinically significant PCa from benign prostatic diseases [18]. Fendler and colleagues proposed an optimal SUVmax cutoff of 6.5 for discrimination between histopathology-positive segments from histopathology-negative segments (AUC 0.84, p < 0.001) [19]. In the PRIMARY study, Emmet and colleagues found PSMA intensity to be associated with both PI-RADS and biopsy grade (p < 0.001). They also reported a median PSMA SUVmax for men without cancer on biopsy of 4.0 (interquartile range [IQR]: 3.4–5.1) versus 12.3 (IQR: 6.3–15.6) for ISUP grade group 5 malignancy [20]. Our analyses suggest that SUVmax can assist with discrimination of lower ISUP grades 1, 2 and 3 from the most aggressive ISUP GG 4 and 5. Our findings regarding SUVmax are also concordant with those reported by Xue and colleagues, who found that median SUVmax was directly proportionally related to percentage of Gleason 4 pattern present in prostate segments after prostatectomy. SUVmax was a fair discriminator of > 50%, > 20% and > 10% Gleason pattern 4 per segment, with AUCs of 78%, 74% and 74%, respectively [21]. In addition, our study showed the added value of PSMAtotal, which remained associated with ISUP GG ≥ 4 when adjusted for SUVmax and PSMAvolume and other confounders including hospital.
The clinical relevance of relating PSMA PET parameters to ISUP GG at surgical pathology perhaps mostly entails prediction of upgrading. The underlying hypothesis is that in the presence of discrepancy between relatively high uptake values of the tumour at PSMA PET/CT and low ISUP GG on biopsy (e.g. ISUP GG1), there might be an underestimation of histopathological grading. In previous relatively smaller series, SUVmax ≥ 5.6 was proposed as the only independent predictor of pathological upgrading from ISUP GG1 to GG ≥ 4, adjusting for maximal tumor core involvement and PI-RADS score of the mpMRI index lesion [22]. Although this analysis should be interpreted cautiously due to the risk of overfitting given the low event-per-variable rate, it suggests the added value of SUVmax for risk stratification. Demirci and colleagues studied 141 patients undergoing RP and proposed that SUVmax values significantly correlate to ISUP GG of the primary tumor. In particular, SUVmax of high-risk patients were significantly higher than those of low-risk patients. Using a SUVmax cut-off of 9.1 would have predicted upgrading from GG1-2 to GG3-4–5 in 63% of the patients [23]. Raveenthiran and colleagues reported that in patients with ISUP GG ≤ 2 and a SUVmax < 5, only 10% were upgraded to ISUP GG ≥ 3 at surgical pathology, compared to 90% if the SUVmax was > 11 [24]. In this study, although SUVmax was a significant discriminator in terms of median values comparing ISUP GG pairs, PSMAvolume was significantly associated with both upgrading and downgrading on multivariable analysis. Our subgroup analysis revealed that a threshold of 2 could assist in predicting upgrading to GG ≥ 4 for patients with biopsy GG ≤ 3, as well as the likelihood of downgrading to GG ≤ 2 in patients with GG ≥ 4. However, it should be emphasized that this is a retrospective cohort of patients treated in routine clinical care who had the indication for undergoing a staging PSMA PET/CT, including mostly unfavorable intermediate-risk and high-risk patients (71% had GG ≥ 3 at biopsy). Given the relatively low number of cases with GG1 and GG2, evaluation of the relevance of all three PET parameters in the prediction of biopsy upgrading in larger populations of patients with GG ≤ 2, classified as low- or intermediate-risk at diagnosis, is required.
The inclusion of substantial numbers of patients undergoing either [68 Ga]Ga-PSMA-11 and [18F]PSMA-1007 in this study enabled in-between assessment of these radioligands regarding of the predictive value of PSMA PET parameters. No significant differences in median values of SUVmax and PSMAtotal were observed per ISUP GG. These findings contradict the results previously reported by Kuten and colleagues, who reported significantly higher median SUVmax in the primary dominant intraprostatic tumors for [18F]PSMA-1007 compared with [68 Ga]Ga-PSMA-11 (8.7 vs 6.9, p = 0.002) [25]. Huang and colleagues also reported contradicting results in their meta-analysis, describing lesion SUVmax of [18F]PSMA-1007 was significantly higher than [68 Ga]Ga-PSMA-11 [26]. When adjusting for these radioligands on logistic regression, the odds of ISUP GG ≥ 4, were not statistically significant for all three parameters. However, for ISUP GG2 and GG3, significant differences in median PSMAvolume were observed. This may suggest estimation of this parameter is susceptible to in-between radioligand differences. An important limitation of this sub-analysis is that the majority of [18F]PSMA-1007 PET/CT were performed at 1 hospital (176/200, [88%]), and therefore interobserver variability as a confounder cannot be excluded. Nevertheless, at multivariable analysis, PSMAvolume remained significantly associated with ISUP GG ≥ 4, adjusting for confounders including radioligand and hospital, which shows its clinical relevance.
Our findings emphasize the complexity of in-between radioligand quantitative parameters; reflected by the wide IQR observed of all three parameters per ISUP GG. However, it is known that SUV values can be influenced by several factors such as time of SUV evaluation (injection-to-midacquisition time), scanner type, body size as well as techniques used in reconstruction [27]. Partly due to these limitations, the PRIMARY score (1 to 5) was developed using parameters beyond solely quantitative parameters, including a combination of pattern, zonal location and SUVmax (using a threshold of ≥ 12). High SUVmax ≥ 12 represents the top score (PRIMARY score of 5), because of its observed 100% specificity of significant malignancy [28]. However, the PRIMARY score has been developed as a risk score to assist diagnosis of clinically significant PCa. The proposed subclassification of SUVmax in this study can be complimentary to the PRIMARY score, as it provides additional information regarding the aggressiveness of the cancer. For instance, among patients with SUVmax > 28, 50% had ISUP GG ≥ 4 at surgical pathology, whereas this accounted for 11.6% of patients with SUVmax ≤ 6.5, respectively. In conclusion, if quantitative PET parameters are used for PCa risk prediction, the adoption of clinically relevant thresholds instead of a single numeric values are recommended, as this may lead to more accurate and reproducible predictions. The proposed clinically meaningful thresholds in this study showing to be related to ISUP GG at histopathology, providing additive information to other classification systems such as the PRIMARY score.
Besides their association with surgical ISUP GG, PSMA PET parameters have shown their potential to assist in prediction of presence of pelvic LNI. Muehlematter and colleagues showed significant higher median values in PSMAvolume and PSMAtotal comparing patients with and without LNI at histopathological evaluation and this was confirmed at external validation [29]. In addition, Laudicella and colleagues showed PSMAtotal and PSMAvolume to be significantly associated with pathological T stage after RP. They reported that using PSMAtotal and PSMAvolume for the prediction of extraprostatic extension resulted in AUCs of 71% and 72%, respectively. By using their proposed cutoff of 24.6 g/ml x cm3 for PSMAtotal and 4.41 cm3 for PSMAvolume, sensitivity for the detection of EPE of 71% was reached [6]. However, this study is limited by its single-center nature and small sample size. Lastly, PSMA whole body uptake (total volume of PSMA-avid tumor) has been shown to have a direct and positive correlation with serum PSA values in prostate cancer patients with biochemical recurrence [30]. Although outside of the scope of current study, these preliminary findings regarding the predictive value of PSMAtotal and PSMAvolume for local tumor stage and presence of LNI should be validated using large multi-center and multi-tracer patient populations. In this future study, focus should also be on identification of clinically relevant and reproducible thresholds for accurate predictive modelling among different patient populations. A pragmatic subclassification, as proposed in this study, could account for the variability regarding uptake parameters, and validation of our classification system in external cohorts is crucial to answer this question. In addition, future studies should also focus on the association between PSMA PET parameters and oncological outcomes including biochemical recurrence and development of metastastatic disease, which have been described previously [31, 32]. Unfortunately, this study is limited by the lack of data on follow-up and recurrence and these outcome parameters where therefore not evaluated.
Although our study has several strengths, such as a multicenter international study with one of the largest series of patients available describing the predictive value of PSMA PET quantitative parameters using different radioligands, it is not devoid of limitations. First, our study did not include central review or second reading of PSMA PET/CT. This could potentially have introduced interobserver variability. Second, we did not include intra-individual comparisons between different tracers, and although we adjusted for potential confounders on multivariate analysis, this could introduce selection bias. Third, no restrictions were used regarding type of radioligands, scanners as well as used software, which could also have led to information bias. However, the incorporation of different protocols and scanners may also be seen as a strength, as the incorporation of this heterogeneity potentially enables more robust estimations, and the variability reflects the real-world clinical situation. In addition, data regarding the location of PSMA uptake in the prostate, physiological PSMA uptake in non-malignant tissue and scoring systems integrating this information (e.g. PSMA expression V score and the PRIMARY score), were unfortunately not available in this study [28, 33]. Lastly, in-between hospital differences in selection of patients for PSMA PET/CT as well as the lack of central histopathological review. This could explain hospital to be significantly associated with ISUP GG ≥ 4 on multivariable analysis, potentially introducing selection and information bias, which could limit the generalizability of the results.
Conclusions
We demonstrated that PSMA PET parameters SUVmax, PSMAvolume and PSMAtotal are associated with ISUP GG found at final histopathological evaluation. Our results suggest a robust classification system with clinically relevant thresholds, which has the potential to assist in prostate cancer risk stratification in daily clinical practice.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
van Kalmthout LWM, van Melick HHE, Lavalaye J, Meijer RP, Kooistra A, de Klerk JMH, et al. Prospective Validation of Gallium-68 Prostate Specific Membrane Antigen-Positron Emission Tomography/Computerized Tomography for Primary Staging of Prostate Cancer. J Urol. 2020;203:537–45. https://doi.org/10.1097/JU.0000000000000531.
Jansen BHE, Bodar YJL, Zwezerijnen GJC, Meijer D, van der Voorn JP, Nieuwenhuijzen JA, et al. Pelvic lymph-node staging with (18)F-DCFPyL PET/CT prior to extended pelvic lymph-node dissection in primary prostate cancer - the SALT trial. Eur J Nucl Med Mol Imaging. 2021;48:509–20. https://doi.org/10.1007/s00259-020-04974-w.
Hope TA, Eiber M, Armstrong WR, Juarez R, Murthy V, Lawhn-Heath C, et al. Diagnostic Accuracy of 68Ga-PSMA-11 PET for Pelvic Nodal Metastasis Detection Prior to Radical Prostatectomy and Pelvic Lymph Node Dissection: A Multicenter Prospective Phase 3 Imaging Trial. JAMA Oncol. 2021;7:1635–42. https://doi.org/10.1001/jamaoncol.2021.3771.
Hermsen R, Wedick EBC, Vinken MJM, van Kalmthout LWM, Kusters-Vandevelde HVN, Wijers CHW, et al. Lymph node staging with fluorine-18 prostate specific membrane antigen 1007-positron emission tomography/computed tomography in newly diagnosed intermediate- to high-risk prostate cancer using histopathological evaluation of extended pelvic node dissection as reference. Eur J Nucl Med Mol Imaging. 2022;49:3929–37. https://doi.org/10.1007/s00259-022-05827-4.
Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395:1208–16. https://doi.org/10.1016/S0140-6736(20)30314-7.
Laudicella R, Skawran S, Ferraro DA, Muhlematter UJ, Maurer A, Grunig H, et al. Quantitative imaging parameters to predict the local staging of prostate cancer in intermediate- to high-risk patients. Insights Imaging. 2022;13:75. https://doi.org/10.1186/s13244-022-01217-4.
Fendler WP, Eiber M, Beheshti M, Bomanji J, Calais J, Ceci F, et al. PSMA PET/CT: joint EANM procedure guideline/SNMMI procedure standard for prostate cancer imaging 2.0. Eur J Nucl Med Mol Imaging. 2023;50:1466–86. https://doi.org/10.1007/s00259-022-06089-w.
Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart HG, Hadaschik BA, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40:486–95. https://doi.org/10.1007/s00259-012-2298-2.
Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma. Cancer. 1998;82:2256–61. https://doi.org/10.1002/(sici)1097-0142(19980601)82:11%3c2256::aid-cncr22%3e3.0.co;2-s.
Woythal N, Arsenic R, Kempkensteffen C, Miller K, Janssen JC, Huang K, et al. Immunohistochemical Validation of PSMA Expression Measured by (68)Ga-PSMA PET/CT in Primary Prostate Cancer. J Nucl Med. 2018;59:238–43. https://doi.org/10.2967/jnumed.117.195172.
Uprimny C, Kroiss AS, Decristoforo C, Fritz J, von Guggenberg E, Kendler D, et al. (68)Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur J Nucl Med Mol Imaging. 2017;44:941–9. https://doi.org/10.1007/s00259-017-3631-6.
Roberts MJ, Morton A, Donato P, Kyle S, Pattison DA, Thomas P, et al. (68)Ga-PSMA PET/CT tumour intensity pre-operatively predicts adverse pathological outcomes and progression-free survival in localised prostate cancer. Eur J Nucl Med Mol Imaging. 2021;48:477–82. https://doi.org/10.1007/s00259-020-04944-2.
Ferraro DA, Muehlematter UJ, Garcia Schuler HI, Rupp NJ, Huellner M, Messerli M, et al. (68)Ga-PSMA-11 PET has the potential to improve patient selection for extended pelvic lymph node dissection in intermediate to high-risk prostate cancer. Eur J Nucl Med Mol Imaging. 2020;47:147–59. https://doi.org/10.1007/s00259-019-04511-4.
Epstein J, Egevad L, Mahul A, Delahunt B, Srigley J, Humphrey P. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol. 2019;40:244–52.
Fendler WP, Eiber M, Beheshti M, Bomanji J, Ceci F, Cho S, et al. (68)Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 10. Eur J Nucl Med Mol Imaging. 2017;44:1014–24. https://doi.org/10.1007/s00259-017-3670-z.
Kass G. An Exploratory Technique for Investigating Large Quantities of Categorical Data. J Roy Stat Soc: Ser C (Appl Stat). 1980;29:119–217.
Blockeel H, Struyf J. Efficient Algorithms for Decision Tree Cross-validation. J Mach Learn Res. 2002;3:621–50.
Jiao J, Kang F, Zhang J, Quan Z, Wen W, Zhao X, et al. Establishment and prospective validation of an SUV(max) cutoff value to discriminate clinically significant prostate cancer from benign prostate diseases in patients with suspected prostate cancer by (68)Ga-PSMA PET/CT: a real-world study. Theranostics. 2021;11:8396–411. https://doi.org/10.7150/thno.58140.
Fendler WP, Schmidt DF, Wenter V, Thierfelder KM, Zach C, Stief C, et al. 68Ga-PSMA PET/CT Detects the Location and Extent of Primary Prostate Cancer. J Nucl Med. 2016;57:1720–5. https://doi.org/10.2967/jnumed.116.172627.
Emmett L, Buteau J, Papa N, Moon D, Thompson J, Roberts MJ, et al. The Additive Diagnostic Value of Prostate-specific Membrane Antigen Positron Emission Tomography Computed Tomography to Multiparametric Magnetic Resonance Imaging Triage in the Diagnosis of Prostate Cancer (PRIMARY): A Prospective Multicentre Study. Eur Urol. 2021;80:682–9. https://doi.org/10.1016/j.eururo.2021.08.002.
Xue AL, Kalapara AA, Ballok ZE, Levy SM, Sivaratnam D, Ryan A, et al. (68)Ga-Prostate-Specific Membrane Antigen Positron Emission Tomography Maximum Standardized Uptake Value as a Predictor of Gleason Pattern 4 and Pathological Upgrading in Intermediate-Risk Prostate Cancer. J Urol. 2022;207:341–9. https://doi.org/10.1097/JU.0000000000002254.
Esen B, Seymen H, Gurses B, Armutlu A, Koseoglu E, Tarim K, et al. The role of PSMA PET/CT to predict upgrading in patients undergoing radical prostatectomy for ISUP grade group 1 prostate cancer. Prostate. 2024;84:32–8. https://doi.org/10.1002/pros.24621.
Demirci E, Kabasakal L, Sahin OE, Akgun E, Gultekin MH, Doganca T, et al. Can SUVmax values of Ga-68-PSMA PET/CT scan predict the clinically significant prostate cancer? Nucl Med Commun. 2019;40:86–91. https://doi.org/10.1097/MNM.0000000000000942.
Raveenthiran S, Yaxley WJ, Franklin T, Coughlin G, Roberts M, Gianduzzo T, et al. Findings in 1,123 Men with Preoperative (68)Ga-Prostate-Specific Membrane Antigen Positron Emission Tomography/Computerized Tomography and Multiparametric Magnetic Resonance Imaging Compared to Totally Embedded Radical Prostatectomy Histopathology: Implications for the Diagnosis and Management of Prostate Cancer. J Urol. 2022;207:573–80. https://doi.org/10.1097/JU.0000000000002293.
Kuten J, Fahoum I, Savin Z, Shamni O, Gitstein G, Hershkovitz D, et al. Head-to-Head Comparison of (68)Ga-PSMA-11 with (18)F-PSMA-1007 PET/CT in Staging Prostate Cancer Using Histopathology and Immunohistochemical Analysis as a Reference Standard. J Nucl Med. 2020;61:527–32. https://doi.org/10.2967/jnumed.119.234187.
Huang S, Ong S, McKenzie D, Mirabelli A, Chen DC, Chengodu T, et al. Comparison of (18)F-based PSMA radiotracers with [(68)Ga]Ga-PSMA-11 in PET/CT imaging of prostate cancer-a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2023. https://doi.org/10.1038/s41391-023-00755-2.
Thie J. Understanding the Standardized Uptake Value, Its Methods, and Implications for Usage. J Nucl Med. 2004;45(9):1431.
Emmett L, Papa N, Buteau J, Ho B, Liu V, Roberts M, et al. The PRIMARY Score: Using Intraprostatic (68)Ga-PSMA PET/CT Patterns to Optimize Prostate Cancer Diagnosis. J Nucl Med. 2022;63:1644–50. https://doi.org/10.2967/jnumed.121.263448.
Muehlematter UJ, Schweiger L, Ferraro DA, Hermanns T, Maurer T, Heck MM, et al. Development and external validation of a multivariable [(68)Ga]Ga-PSMA-11 PET-based prediction model for lymph node involvement in men with intermediate or high-risk prostate cancer. Eur J Nucl Med Mol Imaging. 2023;50:3137–46. https://doi.org/10.1007/s00259-023-06278-1.
Santos A, Mattiolli A, Carvalheira JB, Ferreira U, Camacho M, Silva C, et al. PSMA whole-body tumor burden in primary staging and biochemical recurrence of prostate cancer. Eur J Nucl Med Mol Imaging. 2021;48:493–500. https://doi.org/10.1007/s00259-020-04981-x.
Wang Z, Zheng A, Li Y, Dong W, Liu X, Yuan W, et al. (18)F-PSMA-1007 PET/CT Performance on Risk Stratification Discrimination and Distant Metastases Prediction in Newly Diagnosed Prostate Cancer. Front Oncol. 2021;11: 759053. https://doi.org/10.3389/fonc.2021.759053.
Moradi F, Duan H, Song H, Davidzon GA, Chung BI, Thong AEC, et al. (68)Ga-PSMA-11 PET/MRI in Patients with Newly Diagnosed Intermediate- or High-Risk Prostate Adenocarcinoma: PET Findings Correlate with Outcomes After Definitive Treatment. J Nucl Med. 2022;63:1822–8. https://doi.org/10.2967/jnumed.122.263897.
Ceci F, Oprea-Lager DE, Emmett L, Adam JA, Bomanji J, Czernin J, et al. E-PSMA: the EANM standardized reporting guidelines v1.0 for PSMA-PET. Eur J Nucl Med Mol Imaging. 2021;48:1626–38. https://doi.org/10.1007/s00259-021-05245-y.
Funding
The authors declare that no funds, grants or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the study conception and design. Material preparation and analysis were performed by Timo F.W. Soeterik. Data collection was done by Timo F.W Soeterik, Joris G. Heetman, Rick Hermsen, Lieke Wever, Maarten Vinken, Clint D. Bahler, Courtney Yong, Mark Tann, Claudia Kesch, Robert Seifert, Tugce Telli, Peter Ka-Fung Chiu, Kwan Kit Wu, Fabio Zattoni, Laura Evangelista, Emma Segalla, and Antonio Barone. The first manuscript draft was written by Timo F.W. Soeterik. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This is an observational study. The Medical research Ethics Committees United has confirmed that no ethical approval is required (AW22.067/W18.055). Written informed consent was waived due to the retrospective nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 106 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soeterik, T.F.W., Heetman, J.G., Hermsen, R. et al. The association of quantitative PSMA PET parameters with pathologic ISUP grade: an international multicenter analysis. Eur J Nucl Med Mol Imaging (2024). https://doi.org/10.1007/s00259-024-06847-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00259-024-06847-y