Abstract
Purpose
The angiotensin converting enzyme 2 (ACE2) plays a regulatory role in the cardiovascular system and serves SARS-CoV-2 as an entry receptor. The aim of this study was to synthesize and evaluate radiofluorinated derivatives of the ACE2 inhibitor MLN-4760. [18F]F-MLN-4760 and [18F]F-Aza-MLN-4760 were demonstrated to be suitable for non-invasive imaging of ACE2, potentially enabling a better understanding of its expression dynamics.
Methods
Computational molecular modeling, based on the structures of human ACE2 (hACE2) and mouse ACE2 (mACE2), revealed that the ACE2-binding modes of F-MLN-4760 and F-Aza-MLN-4760 were similar to that of MLN-4760. Co-crystallization of the hACE2/F-MLN-4760 protein complex was performed for confirmation. Displacement experiments using [3H]MLN-4760 enabled the determination of the binding affinities of the synthesized F-MLN-4760 and F-Aza-MLN-4760 to hACE2 expressed in HEK-ACE2 cells. Aryl trimethylstannane-based and pyridine-based radiofluorination precursors were synthesized and used for the preparation of the respective radiotracers. [18F]F-MLN-4760 and [18F]F-Aza-MLN-4760 were evaluated with regard to the uptake in HEK-ACE2 and HEK-ACE cells and in vitro binding to tissue sections of HEK-ACE2 xenografts and normal organs of mice. Biodistribution and PET/CT imaging studies of [18F]F-MLN-4760 and [18F]F-Aza-MLN-4760 were performed using HEK-ACE2 and HEK-ACE xenografted nude mice.
Results
Crystallography data revealed an equal hACE2-binding mode for F-MLN-4760 as previously found for MLN-4760. Moreover, computer-based modeling indicated that similar binding to hACE2 and mACE2 holds true for both, F-MLN-4760 and F-Aza-MLN-4760, as is the case for MLN-4760. The IC50 values were three-fold and seven-fold higher for F-MLN-4760 and F-Aza-MLN-4760, respectively, than for MLN-4760. [18F]F-MLN-4760 and [18F]F-Aza-MLN-4760 were obtained in 1.4 ± 0.3 GBq and 0.5 ± 0.1 GBq activity with > 99% radiochemical purity in a 5.3% and 1.2% radiochemical yield, respectively. Uptake in HEK-ACE2 cells was higher for [18F]F-MLN-4760 (67 ± 9%) than for [18F]F-Aza-MLN-4760 (37 ± 8%) after 3-h incubation while negligible uptake was seen in HEK-ACE cells (< 0.3%). [18F]F-MLN-4760 and [18F]F-Aza-MLN-4760 accumulated specifically in HEK-ACE2 xenografts of mice (13 ± 2% IA/g and 15 ± 2% IA/g at 1 h p.i.) with almost no uptake observed in HEK-ACE xenografts (< 0.3% IA/g). This was confirmed by PET/CT imaging, which also visualized unspecific accumulation in the gall bladder and intestinal tract.
Conclusion
Both radiotracers showed specific and selective binding to ACE2 in vitro and in vivo. [18F]F-MLN-4760 was, however, obtained in higher yields and the ACE2-binding affinity was superior over that of [18F]F-Aza-MLN-4760. [18F]F-MLN-4760 would, thus, be the candidate of choice for further development in view of its use for PET imaging of ACE2.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In March 2020, the World Health Organization announced the coronavirus disease 2019 (Covid-19) pandemic due to the worldwide increasing number of infections by the acute severe respiratory syndrome Coronavirus-2 (SARS-CoV-2) and the poorly understood risk factors for severe disease progression [1, 2]. It is known that SARS-CoV-2 interacts with the human angiotensin converting enzyme 2 (hACE2) and uses it as a cell entry receptor [3]. ACE2 is an enzyme involved in the renin-angiotensin-aldosterone-system (RAAS), as is the case also for the better-known angiotensin converting enzyme (ACE) [4]. The primary functions of ACE are the conversion of angiotensin I to angiotensin II and the hydrolysis of bradykinin, which leads to vasoconstriction and related effects. ACE2 plays a counterregulatory role by cleaving angiotensin II to angiotensin 1–7 which interacts with the MAS receptor to induce vasodilatation and, hence, cardioprotective, anti-inflammatory effects. The interplay of ACE and ACE2 is essential for the regulation of the cardiovascular system (Fig. 1).
It remains unclear to which extent the expression level and dynamics of ACE2 contribute to variations in infection susceptibility among individuals and whether the function of ACE2 is affected upon infection by SARS-CoV-2 [5, 6]. A hypothetical downregulation of ACE2 upon SARS-CoV-2 infection may lead to increased angiotensin II levels and related symptoms, which are consistent with clinical symptoms observed in severe Covid-19 cases [7,8,9]. This is in line with the fact that alterations in ACE2 expression levels were associated with cardiac and vascular pathophysiologies such as hypertension, ischemia and atherosclerosis [10,11,12].
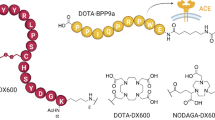
Non-invasive imaging of ACE2 may contribute to a better understanding of the regulation of this important enzyme and potentially identify patients at risk for severe Covid-19 disease outcomes. Among the various imaging technologies, positron emission tomography (PET) is particularly useful to investigate (patho)physiological processes in cardiovascular diseases [13]. Several PET imaging studies have been performed at many nuclear medicine centers worldwide using [18F]fluorodeoxyglucose ([18F]FDG) to detect inflammation and pulmonary lesions in patients with Covid-19 [14,15,16]. Target-specific radiotracers for imaging ACE2 were also proposed [17]. Several preclinical studies reported on the derivatization of DX600, an ACE2-targeting cyclic peptide, using DOTA, NODAGA or NOTA chelators to enable radiolabeling with gallium-68, copper-64 or aluminium-fluoride-18 [18,19,20]. The Al[18F]F-NOTA-DX600 radiopeptide is currently being investigated in a clinical trial for non-invasive mapping of ACE2 (NCT04542863) [21]. Using this same concept, we derivatized DX600 not only with DOTA and NODAGA but also with an HBED-CC chelator for labeling with gallium-67/68 [22]. [67Ga]Ga-HBED-CC-DX600 showed distinct uptake in ACE2-positive human embryonic kidney (HEK) cell xenografts and rapid clearance from the blood and kidneys of mice. Preclinical data obtained with our own DX600-based radiopeptides and those of others were promising [18,19,20, 22], however, clinical data of patients showed only low accumulation of [68Ga]Ga-HZ20 in the heart and lungs that are known to express ACE2 [20]. It remains unexplored whether this observation can be ascribed to the relatively low ACE2-binding affinity of DX600-based radiopeptides (KD ~100 nM) or whether it was due to the low ACE2 expression level in these organs.
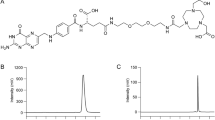
The aim of this study was to develop a 18F-based radiotracer for PET imaging of ACE2 [23]. The small-molecular-weight ACE2 inhibitor MLN-4760 was identified as a suitable lead structure as it was reported to bind to hACE2 with high affinity (IC50 = 0.44 nM [24]) and to show cross reactivity to the mouse ACE2 (mACE2) but no binding to ACE (Fig. 2a) [25, 26]. The radiosynthesis of [18F]F-MLN-4760 and [18F]F-Aza-MLN-4760 was established based on specifically designed precursor molecules (Fig. 2b/c). The non-radioactive reference compounds, F-MLN-4760 and F-Aza-MLN-4760, were synthesized to determine ACE2-binding affinity. The radiotracers were evaluated using HEK cells expressing hACE2 or hACE as well as the respective xenograft mouse model which was previously established in the group [22].
Methods
Computational modeling and co-crystallization of ACE2-bound compounds
Computational molecular modeling techniques were applied to predict the binding mode of F-MLN-4760 and F-Aza-MLN-4760 in hACE2 and mACE2, based on a previously reported crystal structure of the hACE2/MLN-4760 complex [27] using SwissDock program [28] (Supplementary Material). The sequence alignment of hACE2 and mACE2 was performed using the program ClustalW [29].
The extracellular part of hACE2 with a C-terminal His-tag was expressed in insect cells and purified to achieve a quality corresponding to crystallization standard (Supplementary Material). Crystals of the concentrated protein, complexed with F-MLN-4760, were grown. A dataset to 2.5 Å was collected from a single crystal at 100 K at the X06SA beamline of the Swiss Light Source synchrotron at the Paul Scherrer Institute, using a wavelength of 1.0 Å. The data was processed using XDS X-ray detector software [30]. The structure was solved by molecular replacement with Phaser [31] using an AlphaFold model [32] of hACE2 as the search model. There were two hACE2 molecules in the asymmetric unit. The model was built and refined iteratively [33, 34] and the geometry and stereochemistry were validated [35].
Synthesis of MLN-4760, F-MLN-4760 and F-Aza-MLN-4760
MLN-4760 was synthesized according to previously published procedures with slight modifications (Supplementary Material; Scheme S1) [24, 36]. F-MLN-4760 was prepared according to the same synthesis approach as applied for MLN-4760, however, instead of using (3,5-dichlorophenyl)methanol the (3-chloro-5-fluorophenyl)methanol was employed in the respective synthesis step (Supplementary Material; Scheme S1). F-Aza-MLN-4760 was produced based on a novel synthesis approach that involved tert-butyl protection of the carboxylic groups (Supplementary Material; Scheme S2). HPLC purification of the (S, S)-MLN-4760 was based on the comparison with the commercially available compound (MLN-4760; MW: 428.31; Merck, CAS N° 305335-31-3). The isolation of the desired (S,S)-diastereoisomers of the respective fluorinated MLN-4760 derivatives was based on the assumption that the HPLC elution sequence of the diastereoisomers would be identical to that of the MLN-4760 lead compound.
Synthesis of precursor molecules for the preparation of the radiotracers
The synthetic approach adopted for the preparation of the aryl-trimethylstannane precursor 1 was based on the same reaction sequence described for F-Aza-MLN-4760 (Supplementary Material; Scheme S2). This allowed us to obtain a tert-butyl-protected compound suitable for the subsequent radiolabeling step. One of the chlorine atoms on the aromatic ring of MLN-4760 was substituted with iodine to enable a palladium-catalyzed stannylation as the last synthetic step (Supplementary Material, Scheme S3). The production of the pyridine-based precursor 2 was based on the same reaction sequence, however, a chlorinated pyridine was employed as an alkylating agent in the respective synthesis step (Supplementary Material, Scheme S4). The desired (S,S)-diastereomers of both precursor 1 and 2 were isolated by preparative HPLC based on the assumption that the elution sequence of the diastereoisomers would be identical to that of the MLN-4760 lead compound.
Radiosynthesis of [18F]F-MLN-4760 and [18F]F-Aza-MLN-4760
Precursors 1 and 2 were used for 18Ffluorination to prepare [18F]F-MLN-4760 and [18F]F-Aza-MLN-4760, respectively (Scheme 1). The synthesis of [18F]F-MLN-4760 was performed by mixing the precursor 1, Cu(OTf)2(Py)4 and azeotropically dried K[18F]F in N,N-dimethylacetamide followed by heating at 110 °C for 10 min. The subsequent deprotection reaction was performed by adding 85% orthophosphoric acid and heating at 110 °C for 15 min (Supplementary Material). [18F]F-Aza-MLN-4760 was prepared by heating a solution of precursor 2, Kryptofix® 222 and azeotropically dried Cs[18F]F in dimethylsulfoxide at 195 °C for 20 min. The tBu groups were cleaved by the addition of HCl 4 M and further stirring at 80 °C for 20 min (Supplementary Material). After partial neutralization of the acidic reaction mixtures, both [18F]F-MLN-4760 and [18F]F-Aza-MLN-4760 were purified by semipreparative HPLC and subsequently trapped using a C18 or cation-exchange cartridge, respectively, which allowed the formulation of these radiotracers in phosphate buffered saline pH 7.4 (PBS) using a maximum ethanol content of 10% (v/v).
In vitro stability and distribution coefficient of [18F]F-MLN-4760 and [18F]F-Aza-MLN-4760
The radiolytic stability of [18F]F-MLN-4760 and [18F]F-Aza-MLN-4760 formulated in PBS containing 10% EtOH was investigated over 3-h incubation at room temperature (Supplementary Material). The metabolic stability was determined after incubation of the radiotracers in mouse and human blood plasma at 37 °C (Supplementary Material). A shake flask method was employed to determine the n-octanol/PBS pH 7.4 distribution coefficient (logD value) of the 18F-based radiotracers as previously reported (Supplementary Material) [22]. The result were presented as the average value (± standard deviation, SD) of three independent logD experiments each performed with five replicates.
Cell culture
HEK cells transfected with hACE2 (HEK-ACE2) or hACE (HEK-ACE), respectively, were obtained from Innoprot (Innovative Technologies in Biological Systems S.L. Bizkaia, Spain) and cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with non-essential amino acids, fetal calf serum, antibiotics and hygromycin B using standard culture conditions (Supplementary Material) [22].
ACE2-binding affinity of F-MLN-4760 and F-Aza-MLN-4760
The IC50 values of MLN-4760, F-MLN-4760 and F-Aza-MLN-4760 were determined using HEK-ACE2 cells (Supplementary Material). In brief, the cells were seeded in 48-well plates, allowing adhesion and growth overnight. [3H]MLN-4760 (RC Tritec AG, Teufen, Switzerland) was added to each well (10 µL, 3.2 pmol, 6.2 nM) together with increasing concentrations (100 µM‒0.01 nM) of the test agents, MLN-4760, F-MLN-4760 or F-Aza-MLN-4760. After a 1-h incubation time at 37 °C, the cells were rinsed with PBS followed by lysis using NaOH (1 M, 600 µL) and transferred to scintillation vials. After the addition of 5 mL scintillation cocktail (Ultima Gold, Perkin Elmer), the samples were counted in a liquid scintillation counter (TRI-CARB 2250CA, Packard) and the counts were plotted against the logarithmic concentration of the test agents to obtain the IC50 values using GraphPad Prism software (version 8.3.1). The relative binding affinities were defined as the inverse molar ratio of compound required to displace 50% of [3H]MLN-4760 bound to ACE2 on HEK-ACE2 cells, and the relative affinity of MLN-4760 was set as 1.0.
Uptake of 18F-radiotracers in HEK-ACE2 and HEK-ACE cells
The uptake and internalization of [18F]F-MLN-4760 and [18F]F-Aza-MLN-4760 in HEK-ACE2 and HEK-ACE cells were investigated according to a previously published procedure (Supplementary Material) [22]. In brief, cells were seeded in 12-well plates and let to grow and adhere overnight. After the addition of the radiotracer, the cells were incubated for 1–3 h in fresh medium. The supernatant was removed and the cells were rinsed with PBS or additionally with acidic stripping buffer to determine the total uptake and the internalized fraction, respectively. The cells were lysed with NaOH (1 M, 1 mL) and transferred to radioimmunoassay tubes for counting in a γ-counter (Perkin Elmer, Wallac Wizard 1480). The uptake and internalized fraction of the radiotracers were expressed as the percentage of total added activity to each well.
Immunohistochemistry and in vitro autoradiography studies
Immunohistochemical staining of ACE2 was performed with an anti-ACE2 antibody (EPR4435(2) Abcam, Cambridge, UK) on 2-µm thick paraffin sections of HEK-ACE and HEK-ACE2 xenografts as well as on sections of the heart, lungs, kidneys and brain (Supplementary Material). Autoradiography studies were performed on 10-µm thick frozen tissue sections of HEK-ACE and HEK-ACE2 xenografts as well as on sections of the kidneys, heart, lung and brain. The tissue sections were thawed and hydrated in Tris-HCl buffer containing 0.25% bovine serum albumin (BSA) followed by incubation with [18F]F-MLN-4760 or [18F]F-Aza-MLN-4760 (225 kBq/150 µL) in Tris-HCl buffer containing 1% BSA with or without excess of MLN-4760 (10 µM) for 1 h at room temperature. After several rinsing steps and drying of the tissue sections, autoradiographic images were obtained after exposure of the tissue sections to a phosphor screen (Super resolution) using a storage phosphor system (Cyclone Plus; Perkin Elmer). Quantification of the signals was performed using OptiQuant software (version 5.0) based on standards exposed at the same time. The unspecific binding of the radiotracers was determined in the presence of excess MLN-4760, which blocked the binding sites of ACE2. This value was subtracted from the total binding to obtain the specific binding of the radiotracers on each tissue section. The presented data were obtained from two independent experiments performed in duplicates of normal organs and xenografts from n = 2‒3 different mice.
In vivo studies
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. In particular, all animal experiments were carried out according to the guidelines of the Swiss Regulations for Animal Welfare. The preclinical studies have been ethically approved by the Cantonal Committee of Animal Experimentation and permitted by the responsible cantonal authorities (License N° 75743).
Biodistribution and PET/CT imaging studies
Five-week-old female Crl: CD1-Foxnnu (CD1 nude) mice were obtained from Charles River Laboratories (Sulzfeld, Germany). HEK-ACE2 cells (8 × 106 cells in 100 µL PBS) and HEK-ACE cells (4 × 106 cells in 100 µL PBS) were subcutaneously inoculated on the right and left shoulder, respectively, as previously reported [22]. Biodistribution studies were performed 2–4 weeks later by intravenous injection of [18F]F-MLN-4760 or [18F]F-Aza-MLN-4760 (5 MBq, 100 µL) diluted in NaCl 0.9% containing 0.05% BSA. Mice were sacrificed and dissected at 15 min, 1–3 h after injection. Selected organs and tissues were collected, weighed and counted in a γ-counter (Perkin Elmer, Wallac Wizard 1480) for activity at the same time as standards of the original injection solution. The decay-corrected data were expressed as percent of the injected activity per gram of tissue mass (% IA/g). PET/CT imaging was performed at 15 min, 1 h and 3 h after injection of the radiotracers using a small-animal PET/CT scanner (G8 PET/CT; SOFIE, Dulles, U.S.A. [37]) as previously reported (Supplementary Material) [38].
Results
Computational model and crystal structure of ACE2 complexes of MLN-4760 derivatives
The sequence alignment of hACE2 and mACE2 revealed 82.1% sequence identity between the two proteins (Supplemenatary Material, Fig. S1). Computational models of F-MLN-4760 and F-Aza-MLN-4760 bound to hACE2 and mACE2 confirmed a similar binding mode of these derivatives to the previously published binding mode of MLN-4760 (Supplementary Material, Fig. S2) [27]. The X-ray diffraction analysis of the co-crystallized hACE2/F-MLN-4760 further corroborated the analogous binding mode of F-MLN-4760 and MLN-4760 characterized by direct interactions between a carboxylate group of these compounds and the zinc ion site in a closed conformation of the hACE2 peptidase domain (Fig. 3, Supplementary Material, Table S1). The X-ray diffraction analysis confirmed an equal configuration of the F-MLN-4760 as for the known (S,S)-MLN-4760, indicating that the (S,S)-diastereoisomer of the synthesized F-MLN-4760 was correctly identified.
Superimposition of the hACE2/MLN-4760 complex (PDB:1R4L [27]) on the chain A of the hACE2/F-MLN-4760 (this work). A selection of key hACE2 residues at the binding site is shown with yellow carbon atoms and only for the hACE2/F-MLN-4760 complex. MLN-4760 is shown in grey and the fluorinated compound (F-MLN-4760) in red (atom colors: N = blue, O = red, Cl = light green, F = light blue). The figure was created using PyMOL (The PyMOL Molecular Graphics System, Version 2.5.3, Schrödinger LLC)
Synthesis of MLN-4760, F-MLN-4760 and F-Aza-MLN-4760
MLN-4760, F-MLN-4760 and F-Aza-MLN-4760 were obtained in overall yields of 7%, 5% and 7%, respectively, and a purity of > 99%. HRMS and NMR data correlated well with the theoretical values, confirming the structural identity of the obtained compounds (Supplementary Material).
Synthesis of the MLN-4760-based precursors for radiofluorination
Precursors 1 and 2 were obtained with a purity of > 99% and an overall yield of 8% and 28%, respectively (Supplementary Material). The carboxylic functions of both precursors were protected by tert-butyl esters. In precursor 1, one of the chloride atoms of the MLN-4760 structure was replaced with a SnMe3-leaving group to enable radiofluorination via a copper-catalyzed reaction to obtain [18F]F-MLN-4760. The benzene-to-pyridine substitution in precursor 2 was suitable for radiofluorination by a halogen exchange reaction to obtain [18F]F-Aza-MLN-4760.
Radiochemical synthesis, stability and n-octanol/PBS distribution coefficients
[18F]F-MLN-4760 and [18F]F-Aza-MLN-4760 were obtained with radiochemical purities of > 99% and an average decay-corrected radiochemical yield of 5.3% and 1.2%, respectively. The molar activities of [18F]F-MLN-4760 and [18F]F-Aza-MLN-4760 at the end of synthesis ranged from 21 to 38 GBq/µmol (n = 5) and from 78 to 81 GBq/µmol (n = 3), respectively. The chemical identity of the final products was confirmed by congruent UV-HPLC and radio-HPLC chromatogram peaks obtained during the co-injection of individual radiotracers with the respective (S, S)-diastereomers of the non-radioactive reference compound (Supplementary Material, Fig. S3 and S4). In the final formulation, both [18F]F-MLN-4760 and [18F]F-Aza-MLN-4760 were entirely stable (> 97% intact radiotracer) for at least 3 h at room temperature. Both radiotracers were also metabolically stable in vitro, demonstrated by > 97% intact radiotracer after 3-h incubation period in murine and human blood plasma at 37 °C (Supplementary Material, Fig. S5). The logD values of [18F]F-MLN-4760 (logD: -1.32 ± 0.04, n = 3) was slightly higher than that of [18F]F-Aza-MLN-4760 (logD: -2.02 ± 0.21, n = 3).
ACE2-affinity of the fluorinated MLN-4760 derivatives relative to MLN-4760
The IC50 values of F-MLN-4760 and F-Aza-MLN-4760, obtained under the given experimental conditions, were in the high nM range similar to the value determined for MLN-4760 (Supplementary material, Fig. S6). As IC50 values depend on the experimental setting, the relative ACE2-binding affinities were calculated. A 3-fold and 7-fold lower binding affinity was revealed for F-MLN-4760 (relative binding affinity: 0.35) and F-Aza-MLN-4760 (relative binding affinity: 0.13), respectively, as compared to that of MLN-4760 (set as 1.0).
Uptake and internalization of the radiotracers in HEK-ACE2 and HEK-ACE cells
The HEK-ACE2 cell uptake of [18F]F-MLN-4760 was 49 ± 10% and 67 ± 9% after 1 h and 3 h, respectively, while that of [18F]F-Aza-MLN-4760 was 28 ± 10% and 37 ± 8%, respectively, after the same incubation times (Fig. 4a). Both radiotracers showed only moderate internalization (< 11%) even after a 3-h incubation period, while co-incubation of the cells with an excess of MLN-4760 blocked ACE2 and prevented the uptake of the radiotracers almost completely (< 1.5%, Supplementary Material, Fig. S7). Both [18F]F-MLN-4760 and [18F]F-Aza-MLN-4760, showed only negligible uptake (< 0.3%) in HEK-ACE cells even after incubation for 3 h (Fig. 4b).
ACE2 expression and radiotracer binding to murine tissue and HEK-xenografts
The staining of the HEK-ACE2 xenograft tissue sections revealed pronounced expression of hACE2, while only faint staining was seen in ACE2-negative HEK-ACE xenograft sections. Physiological expression of mACE2 was clearly detectable in the heart, lungs, kidney and brain, but at considerably lowers than in the xenograft (Supplementary Material Fig. S8).
Investigation of frozen HEK-ACE2 and HEK-ACE xenograft sections by means of in vitro autoradiography confirmed the selectivity of [18F]F-MLN-4760 and [18F]F-Aza-MLN-4760 as negligible binding of the radiotracer to ACE-expressing tissue was observed. [18F]F-MLN-4760 revealed significantly higher binding to HEK-ACE2 xenografts compared to [18F]F-Aza-MLN-4760 (Fig. 5a). Specific binding of the [18F]F-MLN-4760 was observed in kidney, heart and lung tissue as well as in some parts of the brain of mice. Quantification of the activity per area of the tissues revealed that among the physiological tissue of CD1 nude mice, the heart showed the highest radiotracer binding. Specific binding of [18F]F-Aza-MLN-4760 was, however, considerably lower than [18F]F-MLN-4760 in all represented organs (Fig. 5b). Representative autoradiograms of heart, brain, lung and kidney tissue incubated in the presence and absence of an excess of MLN-4760 can be found in the Supplementary Material (Fig. S9).
Biodistribution of [18F]F-MLN-4760 and [18F]F-Aza-MLN-4760 in xenograft-bearing mice
The uptake of [18F]F-MLN-4760 in HEK-ACE2 xenografts reached 11 ± 1% IA/g already at 15 min p.i. and was well retained over the first hour (13 ± 2% IA/g at 1 h p.i.). At 3 h after injection of [18F]F-MLN-4760, still 5.8 ± 0.9% IA/g were measured in the HEK-ACE2 xenograft. A similar uptake of 15 ± 2% IA/g was obtained at 1 h after injection of [18F]F-Aza-MLN-4760 in the HEK-ACE2 xenografts. Activity accumulation in the HEK-ACE xenografts was negligible for both radiotracers (< 0.3% IA/g) (Fig. 6). Initial uptake of [18F]F-MLN-4760 in the kidneys reached 44 ± 8% IA/g (15 min p.i.), however, over the following hours, renal activity was rapidly cleared (5.1 ± 1.5% IA/g at 1 h p.i. and < 0.5% IA/g at 3 h p.i.). In the case of [18F]F-Aza-MLN-4760, the renal uptake was 2.2 ± 0.6% IA/g at 1 h p.i. Both radiotracers showed substantial retention in the intestines, reaching a maximum of 28 ± 5% IA/g and 10 ± 5% IA/g at 1 h after injection of [18F]F-MLN-4760 and [18F]F-Aza-MLN-4760, respectively (Fig. 6; Supplementary Material, Table S2). No appreciable pharmacologic effects were noticed following the administration of the 18F-based radiotracers and for the entire duration of the study.
PET/CT imaging studies
PET/CT of mice bearing HEK-ACE2 and HEK-ACE xenografts visualized the distribution profile of [18F]F-MLN-4760 and [18F]F-Aza-MLN-4760 (Fig. 7a/b). While an intense signal was obtained from the HEK-ACE2 xenografts already at early timepoints after injection of [18F]F-MLN-4760 and [18F]F-Aza-MLN-4760, no activity accumulation was seen in the HEK-ACE xenografts. The PET images of mice injected with either radiotracer revealed substantial activity in the kidneys at early timepoints, which was entirely cleared over the first hour after injection. The highest activity retention was found in the gallbladder and the intestines throughout the entire time of investigation of both radiotracers.
(a/b) PET/CT images of mice injected with the radiotracers. (a) PET/CT images acquired 15 min, 1 h and 3 h after injection of [18F]F-MLN-4760 in nude mice bearing HEK-ACE2 xenografts on the right shoulder and HEK-ACE xenografts on the left shoulder. (b) PET/CT images acquired at the same timepoints after injection of [18F]F-Aza-MLN-4760 into the same xenograft mouse model. (HEK-ACE2 xenograft, indicated as ACE2 + with green arrow, HEK-ACE xenograft, indicated as ACE + with grey arrow; GB, gall bladder; Ki, kidney, Int, intestines; UB, urinary bladder)
Discussion
Two 18F-based radiotracers were developed based on the structure of MLN-4760. Computer generated models suggested that the replacement of the chlorinated benzene ring of MLN-4760 with a fluorophenyl (F-MLN-4760) or fluoropyridine (F-Aza-MLN-4760) entity would not substantially affect the binding mode to ACE2 of human and mouse origin. In order to confirm this hypothesis, the reference compounds were synthesized. F-MLN-4760 was prepared according to previously reported procedures [24, 36], while a new synthetic pathway involving tert-butyl protection of the carboxylic functions was applied for the preparation of F-Aza-MLN-4760. In both cases, the biologically active (S, S)-diastereoisomers were identified based on the analogous HPLC elution sequence of the diastereoisomers of MLN-4760 of which the retention time of the (S, S)-diastereoisomer was verified using the commercially available (S, S)-MLN-4760. The co-crystallization of F-MLN-4760 with hACE2 confirmed the correct (S,S)-configuration of the stereocenters of F-MLN-4760 and its conserved binding mode to the enzyme. Moreover, the obtained data highlighted that the para-position of the benzene ring does not have direct interaction with hACE2 and, hence, the insertion of a nitrogen atom in this position would not affect the binding mode of the resulting pyridine-based compound, F-Aza-MLN-4760. This was further corroborated in preclinical in vitro displacement studies using [3H]MLN-4760, which showed that the affinity of F-MLN-4760 and F-Aza-MLN-4760 to HEK-ACE2 cells was in a similar range, albeit 3-fold and 7-fold lower than that of MLN-4760.
The preparation of the precursors of [18F]F-MLN-4760 and [18F]F-Aza-MLN-4760, respectively, was based on the synthesis pathway of F-Aza-MLN-4760 with minor modifications. The introduction of tert-butyl protecting groups for the carboxylic acid functionalities of the MLN-4760 scaffold allowed deprotection after radiofluorination under relatively mild conditions. As a result, the radiotracers were obtained in high yields within a short time. The design of [18F]F-Aza-MLN-4760 was based on the assumption that the radiofluorination of a pyridine-based precursor would enable a straightforward SNAr-reaction while avoiding the handling of toxic reagents required for the production of the stannylated precursor of [18F]F-MLN-4760. Nevertheless, [18F]F-MLN-4760 was obtained in a 4-fold higher radiochemical yield than [18F]F-Aza-MLN-4760 and within a shorter time period.
In comparison to DX600-based radiopeptides, [18F]fluorinated small molecule-based radiotracers would be more favorable in view of a clinical translation. The longer half-life of fluorine-18 as compared to that of gallium-68 (T1/2 = 110 min vs. 68 min) and the better image resolution that can be obtained, make fluorine-18 clearly the preferred PET nuclide at current stage [39, 40].
Preclinical investigations confirmed the specific binding of both radiotracers to HEK-ACE2 cells in vitro and selective binding to ACE2-expressing xenografts in vivo, while almost no uptake was seen in ACE-expressing cells and xenografts. These findings are relevant as the developed PET agent should finally serve for specific and selective imaging of ACE2 without cross reacting with ACE.
In vitro autoradiography studies demonstrated the feasibility of imaging ACE2 expression at physiological expression levels in mouse tissue. This is in line with molecular modeling studies indicating the conserved binding mode of F-MLN-4760 and F-Aza-MLN-4760 to hACE2 and mACE2. The signals in autoradiograms were indeed congruent with the ACE2 tissue expression detected by immunohistochemical staining and known from the literature [41]. [18F]F-Aza-MLN-4760 showed, however, a lower signal on autoradiograms as well as lower uptake in HEK-ACE2 cells in vitro than [18F]F-MLN-4760. This could be ascribed to the approximately 3-fold weaker ACE2-binding affinity of [18F]F-Aza-MLN-4760 as compared to that of [18F]F-MLN-4760. Even though the ACE2-binding affinity did not seem to affect the accumulation in the xenografts in vivo, which was similar for both radiotracers, the higher binding affinity of [18F]F-MLN-4760 would most probably be essential in view of the imaging of considerably lower levels of ACE2 under (patho)physiological conditions.
Limitations of the newly developed radiotracers refer to their relatively high retention of activity in the gall bladder and intestinal tract as compared to the previously-developed ACE2-targeting DX600-based radiopeptides [22]. This can be ascribed to the rather lipophilic properties of these 18F-based radiotracers, consistent with their relatively high logD values. None of the two radiotracers accumulated in the heart, lungs or brain, although these tissues are known to express ACE2. Most probably this was due to the predominant accumulation of the radiotracers in the HEK-ACE2 xenografts that express ACE2 at much higher levels. Although the initial signal in the kidneys was substantial after injection of either of the two radiotracers, only background levels of retained renal activity were seen at later timepoints. It remained unexplored whether the renal activity was due to ACE2-binding of the radiotracers or simply a result or the radiotracers’ excretion route.
As expected, no pharmacological effects were observed following radiotracer administration as only minute amounts of compound (< 4 µg/kg body mass) were injected. In order to reach pharmacological activity as a result of ACE2 inhibition, at least two orders of magnitude higher quantities of MLN-4760 (1 mg/kg body mass) would be necessary [25].
Further investigations involving transgenic mouse models that express hACE2, in either healthy condition or after SARS-CoV-2 infection, would be necessary to comprehensively define the potential of the developed radiotracers to detect (patho)physiological levels of ACE2 in vivo. This in turn would be challenging, however, due to restrictions of working with virus-infected mice in a standard imaging laboratory.
Conclusion
Based on the more efficient production and the anticipated better performance in detecting low levels of ACE2 as demonstrated by in vitro autoradiograhy, [18F]F-MLN-4760 evolved as the preferred candidate for further preclinical investigations and potential translation to the clinics. It is relevant to note that such a radiotracer may not only serve to image ACE2 expression dynamics during and after Covid-19, but also be a useful tool to investigate the role of ACE2 in cardiovascular diseases. A better understanding of the ACE2 regulatory function as part of the RAAS may serve future drug development processes for the treatment of related diseases.
Data availability
The article is available as a preprint on bioRχiv [23]. The raw data of the results presented in this study are available on request from the corresponding author. Atomic coordinates of the crystal structure have been deposited in the Protein Data Bank database under accession number 9FMM.
References
Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–23. https://doi.org/10.1016/S0140-6736(20)30154-9.
Petersen E, Koopmans M, Go U, Hamer DH, Petrosillo N, Castelli F, et al. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis. 2020;20:e238–44. https://doi.org/10.1016/S1473-3099(20)30484-9.
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–e808. https://doi.org/10.1016/j.cell.2020.02.052.
Singh KD, Karnik SS. Angiotensin receptors: structure, function, signaling and clinical applications. J Cell Signal. 2016;1. https://doi.org/10.4172/jcs.1000111.
Mehrabadi ME, Hemmati R, Tashakor A, Homaei A, Yousefzadeh M, Hemati K, et al. Induced dysregulation of ACE2 by SARS-CoV-2 plays a key role in COVID-19 severity. Biomed Pharmacother. 2021;137:111363. https://doi.org/10.1016/j.biopha.2021.111363.
Shahbaz S, Oyegbami O, Saito S, Osman M, Sligl W, Elahi S. Differential effects of age, sex and dexamethasone therapy on ACE2/TMPRSS2 expression and susceptibility to SARS-CoV-2 infection. Front Immunol. 2022;13:1021928. https://doi.org/10.3389/fimmu.2022.1021928.
Tucker NR, Chaffin M, Bedi KC Jr., Papangeli I, Akkad AD, Arduini A, et al. Myocyte-specific upregulation of ACE2 in cardiovascular disease: implications for SARS-CoV-2-mediated myocarditis. Circulation. 2020;142:708–10. https://doi.org/10.1161/CIRCULATIONAHA.120.047911.
Hirano T, Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52:731–3. https://doi.org/10.1016/j.immuni.2020.04.003.
Jiang D, Hong H. Mapping COVID-19 with nuclear imaging: from infection to functional sequelae. Am J Nucl Med Mol Imaging. 2021;11:59–63.
Oudit GY, Crackower MA, Backx PH, Penninger JM. The role of ACE2 in cardiovascular physiology. Trends Cardiovasc Med. 2003;13:93–101. https://doi.org/10.1016/s1050-1738(02)00233-5.
Raizada MK, Ferreira AJ. ACE2: a new target for cardiovascular disease therapeutics. J Cardiovasc Pharmacol. 2007;50:112–9. https://doi.org/10.1097/FJC.0b013e3180986219.
Rabelo LA, Alenina N, Bader M. ACE2-angiotensin-(1–7)-Mas axis and oxidative stress in cardiovascular disease. Hypertens Res. 2011;34:154–60. https://doi.org/10.1038/hr.2010.235.
Dobrucki LW, Sinusas AJ. PET and SPECT in cardiovascular molecular imaging. Nat Rev Cardiol. 2010;7:38–47. https://doi.org/10.1038/nrcardio.2009.201.
Minamimoto R, Hotta M, Ishikane M, Inagaki T. FDG-PET/CT images of COVID-19: a comprehensive review. Glob Health Med. 2020;2:221–6. https://doi.org/10.35772/ghm.2020.01056.
Sollini M, Ciccarelli M, Cecconi M, Aghemo A, Morelli P, Gelardi F, et al. Vasculitis changes in COVID-19 survivors with persistent symptoms: an [18F]FDG-PET/CT study. Eur J Nucl Med Mol Imaging. 2021;48:1460–6. https://doi.org/10.1007/s00259-020-05084-3.
Fields BKK, Demirjian NL, Dadgar H, Gholamrezanezhad A. Imaging of COVID-19: CT, MRI, and PET. Semin Nucl Med. 2021;51:312–20. https://doi.org/10.1053/j.semnuclmed.2020.11.003.
Neumaier F, Zlatopolskiy BD, Neumaier B. Nuclear medicine in times of COVID-19: how radiopharmaceuticals could help to fight the current and future pandemics. Pharmaceutics. 2020;12. https://doi.org/10.3390/pharmaceutics12121247.
Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–4. https://doi.org/10.1038/nature02145.
Parker MFL, Blecha J, Rosenberg O, Ohliger M, Flavell RR, Wilson DM. Cyclic 68Ga-labeled peptides for specific detection of human angiotensin-converting enzyme 2. J Nucl Med. 2021;62:1631–7. https://doi.org/10.2967/jnumed.120.261768.
Zhu H, Zhang H, Zhou N, Ding J, Jiang J, Liu T, et al. Molecular PET/CT profiling of ACE2 expression in vivo: implications for infection and outcome from SARS-CoV-2. Adv Sci (Weinh). 2021;8:e2100965. https://doi.org/10.1002/advs.202100965.
Ding J, Zhang Q, Jiang J, Zhou N, Yu Z, Wang Z et al. Preclinical evaluation and pilot clinical study of Al18F-DX600-BCH for non-invasive PET mapping of Angiotensin converting enzyme 2 in Mammal. J Nucl Med. 2023;64.
Beyer D, Vaccarin C, Deupi X, Mapanao AK, Cohrs S, Sozzi-Guo F, et al. A tool for nuclear imaging of the SARS-CoV-2 entry receptor: molecular model and preclinical development of ACE2-selective radiopeptides. EJNMMI Res. 2023;13:32. https://doi.org/10.1186/s13550-023-00979-2.
Wang J, Beyer D, Vaccarin C, He Y, Tanriver M, Benoit R et al. Development of radiofluorinated MLN-4760 derivatives for PET imaging of the SARS-CoV-2 entry receptor ACE2. 2024. https://doi.org/10.1101/2024.03.20.585792.
Dales NA, Gould AE, Brown JA, Calderwood EF, Guan B, Minor CA, et al. Substrate-based design of the first class of angiotensin-converting enzyme-related carboxypeptidase (ACE2) inhibitors. J Am Chem Soc. 2002;124:11852–3. https://doi.org/10.1021/ja0277226.
Ye M, Wysocki J, Gonzalez-Pacheco FR, Salem M, Evora K, Garcia-Halpin L, et al. Murine recombinant angiotensin-converting enzyme 2: effect on angiotensin II-dependent hypertension and distinctive angiotensin-converting enzyme 2 inhibitor characteristics on rodent and human angiotensin-converting enzyme 2. Hypertension. 2012;60:730–40. https://doi.org/10.1161/HYPERTENSIONAHA.112.198622.
Joshi S, Balasubramanian N, Vasam G, Jarajapu YP. Angiotensin converting enzyme versus angiotensin converting enzyme-2 selectivity of MLN-4760 and DX600 in human and murine bone marrow-derived cells. Eur J Pharmacol. 2016;774:25–33. https://doi.org/10.1016/j.ejphar.2016.01.007.
Towler P, Staker B, Prasad SG, Menon S, Tang J, Parsons T, et al. ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J Biol Chem. 2004;279:17996–8007. https://doi.org/10.1074/jbc.M311191200.
Grosdidier A, Zoete V, Michielin O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011;39:W270–7. https://doi.org/10.1093/nar/gkr366.
Sievers F, Barton GJ, Higgins DG. Multiple sequence alignments. Bioinformatics. 2020:227–50.
Kabsch W XDS, Acta Crystallogr D. Biol Crystallogr. 2010;66:125–32. https://doi.org/10.1107/S0907444909047337.
McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–74. https://doi.org/10.1107/S0021889807021206.
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–9. https://doi.org/10.1038/s41586-021-03819-2.
Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. https://doi.org/10.1107/S0907444910007493.
Afonine PV, Grosse-Kunstleve RW, Echols N, Headd JJ, Moriarty NW, Mustyakimov M, et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. Biol Crystallogr. 2012;68:352–67. https://doi.org/10.1107/S0907444912001308.
Chen VB, Arendall WB 3rd, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr. 2010;66:12–21. https://doi.org/10.1107/S0907444909042073.
Raimondi W, Baslé O, Constantieux T, Bonne D, Rodriguez J. Activation of 1,2-Keto Esters with Takemoto’s Catalyst toward Michael Addition to Nitroalkenes. Adv Synth Catal. 2012;354:563–8. https://doi.org/10.1002/adsc.201100739.
Gu Z, Taschereau R, Vu NT, Prout DL, Silverman RW, Lee JT, et al. Performance evaluation of G8, a high-sensitivity benchtop preclinical PET/CT tomograph. J Nucl Med. 2019;60:142–9. https://doi.org/10.2967/jnumed.118.208827.
Guzik P, Fang HY, Deberle LM, Benesova M, Cohrs S, Boss SD, et al. Identification of a PET radiotracer for imaging of the folate receptor-alpha: a potential tool to select patients for targeted tumor therapy. J Nucl Med. 2021;62:1475–81. https://doi.org/10.2967/jnumed.120.255760.
Kesch C, Kratochwil C, Mier W, Kopka K, Giesel FL. 68Ga or 18F for prostate cancer imaging? J Nucl Med. 2017;58:687–8. https://doi.org/10.2967/jnumed.117.190157.
Sahnoun S, Conen P, Mottaghy FM. The battle on time, money and precision: Da[18F] id vs. [68Ga]liath. Eur J Nucl Med Mol Imaging. 2020;47:2944–6. https://doi.org/10.1007/s00259-020-04961-1.
Hikmet F, Mear L, Edvinsson A, Micke P, Uhlen M, Lindskog C. The protein expression profile of ACE2 in human tissues. Mol Syst Biol. 2020;16:e9610. https://doi.org/10.15252/msb.20209610.
Acknowledgements
The authors thank Susan Cohrs and Fan Sozzi-Guo for technical assistance with the experiments at PSI and Annette Krämer, Henrik Mikael Portmann and Bruno Mancosu for technical assistance with the experiments at ETH Zurich. They thank Luisa M. Deberle for general support of the project at ETH Zurich and the Molecular and Biomolecular Analysis Service (MoBiAS) in the Department of Chemistry and Applied Biosciences at ETH Zurich for mass spectrometry. The authors thank Mara Wieser for support in protein production and crystallization at PSI, and Matthew Rodrigues for advice and support on ligand geometry refinement in the crystal structure. The authors thank Niels Rupp (Theragnostic Tumor Pathology Laboratory, University of Zurich) and Benjamin Hunkeler (PSI) for the immunohistochemical studies performed in the Department of Pathology and Molecular Pathology at the University Hospital Zurich, Switzerland.
Funding
Open Access funding provided by Lib4RI – Library for the Research Institutes within the ETH Domain: Eawag, Empa, PSI & WSL. The entire project and, in particular, Darja Beyer, Jinling Wang, Christian Vaccarin and Yingfang He were supported by the Swiss National Science Foundation (SNSF), as a part of the National Research Program 78 (NRP78: Grant N° 4078P0_198274). Darja Beyer was also funded by the Swiss National Science Foundation (Grant N° 310030_188978). Christian Vaccarin was also funded by the Swiss National Science Foundation (Grant N° 210456). The work of Roger Benoit was supported by the SNSF (Spark: Grant N° CRSK-3_190414) and by Promedica Foundation, Chur, Switzerland (Grant N° 1401/M).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was performed in agreement with the national law and PSI-internal guidelines of radiation safety protection. In vivo experiments were approved by the local veterinarian department and ethics committee and conducted in accordance with the Swiss law of animal protection.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Institutional review board statement
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. In particular, all animal experiments were carried out according to the guidelines of the Swiss Regulations for Animal Welfare. The preclinical studies have been ethically approved by the Cantonal Committee of Animal Experimentation and permitted by the responsible cantonal authorities (License N° 75743).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, J., Beyer, D., Vaccarin, C. et al. Development of radiofluorinated MLN-4760 derivatives for PET imaging of the SARS-CoV-2 entry receptor ACE2. Eur J Nucl Med Mol Imaging (2024). https://doi.org/10.1007/s00259-024-06831-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00259-024-06831-6