Abstract
Purpose
Toludesvenlafaxine is a recently developed antidepressant that belongs to the triple reuptake inhibitor class. Despite the in vitro evidence that toludesvenlafaxine inhibits the reuptake of serotonin (5-HT), norepinephrine (NE) and dopamine (DA), there is no in vivo evidence that toludesvenlafaxine binds to DAT and increases DA level, a mechanism thought to contribute to its favorable clinical performance.
Methods
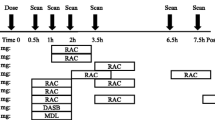
Positron emission tomography/computed tomography (PET/CT) was used to examine the DAT binding capacity in healthy rats and human subjects and microdialysis was used to examine the striatal DA level in rats. [18F]FECNT and [11C]CFT were used as PET/CT radioactive tracer for rat and human studies, respectively.
Results
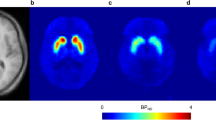
In rats, 9 mg/kg of toludesvenlafaxine hydrochloride (i.v.) followed by an infusion of 3 mg/kg via minipump led to the binding rate to striatum DAT at 3.7 – 32.41% and to hypothalamus DAT at 5.91 – 17.52% during the 45 min scanning period. 32 mg/kg oral administration with toludesvenlafaxine hydrochloride significantly increased the striatal DA level with the AUC0 − 180 min increased by 63.9%. In healthy volunteers, 160 mg daily toludesvenlafaxine hydrochloride sustained-release tablets for 4 days led to an average occupancy rates of DAT at 8.04% ± 7.75% and 8.09% ± 7.00%, respectively, in basal ganglion 6 h and 10 h postdose.
Conclusion
These results represent the first to confirm the binding of toludesvenlafaxine to DAT in both rats and humans using PET/CT, and its elevation of brain DA level, which may help understand the unique pharmacological and functional effects of triple reuptake inhibitors such as toludesvenlafaxine.
ClinicalTrials.gov identifiers
: NCT05905120. Registered 14 June 2023. (retrospectively registered).






Similar content being viewed by others
Data availability
The data are available upon request at the corresponding author’s address.
References
WHO, Depressive disorder. (depression). https://www.who.int/news-room/fact-sheets/detail/depression.
Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, et al. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. https://doi.org/10.1038/nrdp.2016.65.
Fasipe OJ. Neuropharmacological classification of antidepressant agents based on their mechanisms of action. Archives Med Health Sci. 2018;6(1):81–94. https://doi.org/10.4103/amhs.amhs_7_18.
Gartlehner G, Thieda P, Hansen RA, Gaynes BN, Deveaugh-Geiss A, Krebs EE. Comparative risk for harms of second-generation antidepressants: a systematic review and meta-aNElysis. Drug Saf. 2008;31:851–65. https://doi.org/10.2165/00002018-200831100-00004.
Shelton RC. Serotonin and norepinephrine reuptake inhibitors. Handb Exp Pharmacol. 2019;250:145–80. https://doi.org/10.1007/164_2018_164.
Label of SPRAVATO®. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211243s004lbl.pdf.
Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64(3):327–37. https://doi.org/10.1001/archpsyc.64.3.327.
Guiard BP, Chenu F, El Mansari M, Blier P. Characterization of the electrophysiological properties of triple reuptake inhibitors on monoaminergic neurons. Int J Neuropsychopharmacol. 2011;14(2):211–23. https://doi.org/10.1017/S1461145710000076.
Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, et al. STAR*D study team. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243–52. https://doi.org/10.1056/NEJMoa052964.
Zisook S, Rush AJ, Haight BR, Clines DC, Rockett CB. Use of bupropion in combination with serotonin reuptake inhibitors. Biol Psychiatry. 2006;59(3):203–10. https://doi.org/10.1016/j.biopsych.2005.06.027.
Kamijima K, Kimura M, Kuwahara K, Kitayama Y, Tadori Y. Randomized, double-blind comparison of aripiprazole/sertraline combination and placebo/sertraline combination in patients with major depressive disorder. Psychiatry Clin Neurosci. 2018;72(8):591–601. https://doi.org/10.1111/pcn.12663.
Chen Z, Skolnick P. Triple uptake inhibitors: therapeutic potential in depression and beyond. Expert Opin Investig Drugs. 2007;16(9):1365–77. https://doi.org/10.1517/13543784.16.9.1365.
Sharma H, Santra S, Dutta A. Triple reuptake inhibitors as potential next-generation antidepressants: a new hope? Future Med Chem. 2015;7(17):2385–406. https://doi.org/10.4155/fmc.15.134.
Vasiliu O, Efficacy. Tolerability, and Safety of Toludesvenlafaxine for the Treatment of Major Depressive Disorder-A Narrative Review. Pharmaceuticals (Basel). 2023;16(3):411. https://doi.org/10.3390/ph16030411. Published 2023 Mar 8.
Zhu H, Wang W, Sha C, Guo W, Li C, Zhao F, et al. Pharmacological characterization of Toludesvenlafaxine as a triple reuptake inhibitor. Front Pharmacol. 2021;12:741794. https://doi.org/10.3389/fphar.2021.741794.
Mi W, Di X, Wang Y, Li H, Xu X, Li L, et al. A phase 3, multicenter, double-blind, randomized, placebo-controlled clinical trial to verify the efficacy and safety of ansofaxine (LY03005) for major depressive disorder. Transl Psychiatry. 2023;13(1):163. https://doi.org/10.1038/s41398-023-02435-0.
NMPA. https://www.nmpa.gov.cn/zhuanti/cxylqx/cxypxx/20221103083801194.html. (Chinese).
Murali D, Barnhart TE, Vandehey NT, Christian BT, Nickles RJ, Converse AK, et al. An efficient synthesis of dopamine transporter tracer [18F]FECNT. Appl Radiat Isot. 2013;72:128–32. https://doi.org/10.1016/j.apradiso.2012.10.010.
Goodman MM, Kilts CD, Keil R, Shi B, Martarello L, Xing D, et al. 18F-labeled FECNT: a selective radioligand for PET imaging of brain dopamine transporters. Nucl Med Biol. 2000;27(1):1–12. https://doi.org/10.1016/s0969-8051(99)00080-3.
Wong JM, Malec PA, Mabrouk OS, Ro J, Dus M, Kennedy RT. Benzoyl chloride derivatization with liquid chromatography-mass spectrometry for targeted metabolomics of neurochemicals in biological samples. J Chromatogr A. 2016;1446:78–90. https://doi.org/10.1016/j.chroma.2016.04.006.
Wu L, Liu J, Feng X, Dong J, Qin W, Liu Y, et al. 11 C-CFT-PET in Presymptomatic FTDP-17: a potential Biomarker Predicting Onset. J Alzheimers Dis. 2018;61(2):613–8. https://doi.org/10.3233/JAD-170561.
Zhang YF, Wang XY, Cao L, Guo QY, Wang XM. Effects of hypoxic-ischemic brain injury on striatal dopamine transporter in newborn piglets: evaluation of 11 C-CFT PET/CT for DAT quantification. Nucl Med Biol. 2011;38(8):1205–12. https://doi.org/10.1016/j.nucmedbio.2011.05.001.
Shang Y, Gibbs MA, Marek GJ, Stiger T, Burstein AH, Marek K, et al. Displacement of serotonin and dopamine transporters by venlafaxine extended release capsule at steady state: a [123I]2beta-carbomethoxy-3beta-(4-iodophenyl)-tropane single photon emission computed tomography imaging study. J Clin Psychopharmacol. 2007;27(1):71–5. https://doi.org/10.1097/JCP.0b013e31802e0017.
Kugaya A, Seneca NM, Snyder PJ, Williams SA, Malison RT, Baldwin RM, et al. Changes in human in vivo serotonin and dopamine transporter availabilities during chronic antidepressant administration. Neuropsychopharmacology. 2003;28(2):413–20. https://doi.org/10.1038/sj.npp.1300036.
De Win MM, Habraken JB, Reneman L, van den Brink W, den Heeten GJ, Booij J. Validation of [(123)I]beta-CIT SPECT to assess serotonin transporters in vivo in humans: a double-blind, placebo-controlled, crossover study with the selective serotonin reuptake inhibitor citalopram. Neuropsychopharmacology. 2005;30(5):996–1005. https://doi.org/10.1038/sj.npp.1300683.
Meyer JH, Wilson AA, Sagrati S, Hussey D, Carella A, Potter WZ, et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11 C]DASB Positron emission tomography study. Am J Psychiatry. 2004;161(5):826–35. https://doi.org/10.1176/appi.ajp.161.5.826.
Zhou FC, Lesch KP, Murphy DL. Serotonin uptake into dopamine neurons via dopamine transporters: a compensatory alternative. Brain Res. 2002;942(1–2):109–19. https://doi.org/10.1016/s0006-8993(02)02709-9.
Meyer JH, Goulding VS, Wilson AA, Hussey D, Christensen BK, Houle S. Bupropion occupancy of the dopamine transporter is low during clinical treatment. Psychopharmacology. 2002;163(1):102–5. https://doi.org/10.1007/s00213-002-1166-3.
McCabe C, Cowen PJ, Harmer CJ. Neural representation of reward in recovered depressed patients. Psychopharmacology. 2009;205(4):667–77. https://doi.org/10.1007/s00213-009-1573-9.
Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35(3):537–55. https://doi.org/10.1016/j.neubiorev.2010.06.006.
Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35(1):68–77. https://doi.org/10.1016/j.tins.2011.11.005.
Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80(1):1–27. https://doi.org/10.1152/jn.1998.80.1.1.
Salamone JD, Correa M, Mingote S, Weber SM. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J Pharmacol Exp Ther. 2003;305(1):1–8. https://doi.org/10.1124/jpet.102.035063.
Szczypiński JJ, Gola M. Dopamine dysregulation hypothesis: the common basis for motivational anhedonia in major depressive disorder and schizophrenia? Rev Neurosci. 2018;29(7):727–44. https://doi.org/10.1515/revneuro-2017-0091.
Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166(1):64–73. https://doi.org/10.1176/appi.ajp.2008.07081336.
Argyropoulos SV, Nutt DJ. Anhedonia revisited: is there a role for dopamine-targeting drugs for depression? J Psychopharmacol. 2013;27(10):869–77. https://doi.org/10.1177/0269881113494104.
Zhu XN, Li J, Qiu GL, Wang L, Lu C, Guo YG, et al. Propofol exerts anti-anhedonia effects via inhibiting the dopamine transporter. Neuron. 2023;111(10):1626–e16366. https://doi.org/10.1016/j.neuron.2023.02.017.
Higgins A, Nash M, Lynch AM. Antidepressant-associated sexual dysfunction: impact, effects, and treatment. Drug Healthc Patient Saf. 2010;2:141–50. https://doi.org/10.2147/DHPS.S7634.
Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol. 2009;29(3):259–66. https://doi.org/10.1097/JCP.0b013e3181a5233f.
Lyons DJ, Ammari R, Hellysaz A, Broberger C. Serotonin and antidepressant SSRIs inhibit rat neuroendocrine dopamine neurons: parallel actions in the Lactotrophic Axis. J Neurosc. 2016;36(28):7392–406. https://doi.org/10.1523/JNEUROSCI.4061-15.2016.
Bijlsma EY, Chan JS, Olivier B, Veening JG, Millan MJ, Waldinger MD, et al. Sexual side effects of serotonergic antidepressants: mediated by inhibition of serotonin on central dopamine release? Pharmacol Biochem Behav. 2014;121:88–101. https://doi.org/10.1016/j.pbb.2013.10.004.
Marek GJ, Carpenter LL, McDougle CJ, Price LH. Synergistic action of 5-HT2A antagonists and selective serotonin reuptake inhibitors in neuropsychiatric disorders. Neuropsychopharmacology. 2003;28(2):402–12. https://doi.org/10.1038/sj.npp.1300057.
Giuliano F, Allard J. Dopamine and male sexual function. Eur Urol. 2001;40(6):601–8. https://doi.org/10.1159/000049844.
Pereira VM, Arias-Carrión O, Machado S, Nardi AE, Silva AC. Bupropion in the depression-related sexual dysfunction: a systematic review. CNS Neurol Disord Drug Targets. 2014;13(6):1079–88. https://doi.org/10.2174/1871527313666140612112630.
Clayton AH, Baker RA, Sheehan JJ, Cain ZJ, Forbes RA, Marler SV, et al. Comparison of adjunctive use of aripiprazole with bupropion or selective serotonin reuptake inhibitors/serotonin-norepinephrine reuptake inhibitors: analysis of patients beginning adjunctive treatment in a 52-week, open-label study. BMC Res Notes. 2014;7:459. https://doi.org/10.1186/1756-0500-7-459.
Wang W, Zhang C, Fan Y, Yue S, Yang Y, Liu R, et al. Dopamine receptor agonist rotigotine-loaded microspheres ameliorates sexual function deteriorated by fluoxetine in depression rats. ASN Neuro. 2021;13:17590914211052862. https://doi.org/10.1177/17590914211052862.
Philipp M, Tiller JW, Baier D, Kohnen R. Comparison of moclobemide with selective serotonin reuptake inhibitors (SSRIs) on sexual function in depressed adults. The Australian and German study groups. Eur Neuropsychopharmacol. 2000;10(5):305–14. https://doi.org/10.1016/s0924-977x(00)00085-7.
Clayton AH, Tourian KA, Focht K, Hwang E, Cheng RF, Thase ME. Desvenlafaxine 50 and 100 mg/d versus placebo for the treatment of major depressive disorder: a phase 4, randomized controlled trial. J Clin Psychiatry. 2015;76(5):562–9. https://doi.org/10.4088/JCP.13m08978.
Gelenberg AJ, McGahuey C, Laukes C, Okayli G, Moreno F, Zentner L, et al. Mirtazapine substitution in SSRI-induced sexual dysfunction. J Clin Psychiatry. 2000;61(5):356–60. https://doi.org/10.4088/jcp.v61n0506.
Fowler JS, Volkow ND, Wang GJ, Gatley SJ, Logan J. [(11)]Cocaine: PET studies of cocaine pharmacokinetics, dopamine transporter availability and dopamine transporter occupancy. Nucl Med Biol. 2001;28(5):561–72. https://doi.org/10.1016/s0969-8051(01)00211-6.
Alexis Revet François, Montastruc A, Roussin, et al. Antidepressants and movement disorders: a postmarketing study in the world pharmacovigilance database. BMC Psychiatry. 2020;20:308. https://doi.org/10.1186/s12888-020-02711-z.
Chen P-H, Chung K-H. Bupropion-Induced Dyskinesia in a young adult patient with bipolar disorder. Prim Care Companion CNS Disord. 2017;19(6):17l02128. https://doi.org/10.4088/PCC.17l02128.
Taha Can Tuman Uğur, Çakır O. Tardive Dyskinesia Associated with Bupropion. Clin Psychopharmacol Neurosci. 2017;15(2):194–6. https://doi.org/10.9758/cpn.2017.15.2.194.
Francisco, Grandas. Lydia López-Manzanares. Bupropion-induced parkinsonism. Mov Disord. 2007;22(12):1830–1. https://doi.org/10.1002/mds.21425.
Ido Lurie O, Ganor GM. Bupropion-Related Exacerbation of Tic Disorder in an adult: a Case Report. Clin Neuropharmacol. 2019 Jan/Feb;42(1):19. https://doi.org/10.1097/WNF.0000000000000312.
Funding
This study was supported by Collaborative Innovation Center Project of Translational Medicine, Shanghai Jiaotong University School of Medicine, TM202116PT (2021–2023) and STI2030-Major Projects (2022ZD0213800).
Author information
Authors and Affiliations
Contributions
Fang Xie and Yifeng Shen contributed to the study conception and design. Material preparation, data collection and analysis were performed by Zhiwei Huang, Junhao Wu, Yihui Guan and Yumei Wei. The first draft of the manuscript was written by Zhiwei Huang and Junhao Wu. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
The Shanghai Mental Health Center ethics committee approved the study protocol and informed consent form (2022-90). All subjects signed written informed consent. The study adhered to the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice (ICH GCP) guidelines.
Conflict of interest
All authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Z., Wu, J., Guan, Y. et al. PET/CT study of dopamine transporter (DAT) binding with the triple reuptake inhibitor toludesvenlafaxine in rats and humans. Eur J Nucl Med Mol Imaging (2024). https://doi.org/10.1007/s00259-024-06700-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00259-024-06700-2