Abstract
Purpose
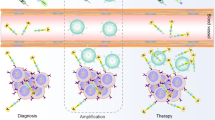
Chimeric antigen receptor (CAR) T cell therapy has achieved great success in treating hematologic malignancies. However, it is yet to prove effective in the treatment of solid tumors. Thus, it is necessary to develop appropriate methodology for the long-term, accurate, and quantitative evaluation of the distribution and activities of CAR T cells in solid tumors. In the present study, we engineered TfR ΔPSMA CAR (CAR-ΔPSMA) T cells, which targeted the transferrin receptor (TfR) expressed by tumor cells and could be tracked in vivo via a reporter gene encoding the truncated prostate specific membrane antigen (ΔPSMA). We then quantitatively monitored these CAR T cells in vitro and in vivo using [68Ga]Ga-PSMA-617 positron emission tomography (PET)/computed tomography (CT).
Methods
The CAR-ΔPSMA T cells were genetically engineered by transducing T cells with a lentiviral vector encoding TfR41BBζ-T2A-ΔPSMA. Firstly, the target expression, activation, and cytotoxicity of CAR-ΔPSMA T cells were validated in vitro. Secondly, the minimum thresholds of CAR-ΔPSMA T cells detection for [68Ga]Ga-PSMA-617 PET/CT were also determined in vitro and in vivo respectively. Lastly, the feasibility of monitoring the biodistribution and infiltration of CAR-ΔPSMA T cells after systematic administration was evaluated in the breast cancer subcutaneous xenograft model.
Results
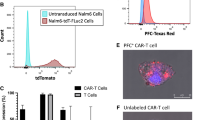
The CAR-ΔPSMA T cells retained activation and tumor killing capacity after transduction of the ΔPSMA-encoding reporter gene. Next, the CAR-ΔPSMA T cells could be reliably tracked by [68Ga]Ga-PSMA-617 PET/CT, the detection sensitivity of which was 250 cells/mm3 in vitro and 100 cells/mm3 in vivo. Next, the sequential imaging assays revealed that [68Ga]Ga-PSMA-617 PET/CT could be used to specifically visualize ΔPSMA+ CAR T cells at the tumor site. The increase in the [68Ga]Ga-PSMA-617 signal intensity over time allowed us to effectively detect CAR T cells in vivo.
Conclusion
Our findings preliminarily confirmed that [68Ga]Ga-PSMA-617 PET/CT could reliably detect CAR-ΔPSMA T cells in vitro and in vivo in solid tumors, laying the foundation for the monitoring CAR T cell therapy in the future.






Similar content being viewed by others
Data availability
Data and materials are available from the corresponding authors upon reasonable request.
References
Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. 2021;21:345–59. https://doi.org/10.1038/s41568-021-00347-z.
June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–5. https://doi.org/10.1126/science.aar6711.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019;380:45–56. https://doi.org/10.1056/NEJMoa1804980.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377:2531–44. https://doi.org/10.1056/NEJMoa1707447.
Shah BD, Ghobadi A, Oluwole OO, Logan AC, Boissel N, Cassaday RD, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398:491–502. https://doi.org/10.1016/S0140-6736(21)01222-8.
Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–52. https://doi.org/10.1016/S0140-6736(20)31366-0.
Munshi NC, Anderson LD Jr, Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N Engl J Med. 2021;384:705–16. https://doi.org/10.1056/NEJMoa2024850.
Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398:314–24. https://doi.org/10.1016/S0140-6736(21)00933-8.
Newick K, O’Brien S, Moon E, Albelda SM. CAR T Cell Therapy for Solid Tumors. Annu Rev Med. 2017;68:139–52. https://doi.org/10.1146/annurev-med-062315-120245.
Martinez M, Moon EK. CAR T Cells for Solid Tumors: New Strategies for Finding, Infiltrating, and Surviving in the Tumor Microenvironment. Front Immunol. 2019;10:128. https://doi.org/10.3389/fimmu.2019.00128.
Shimabukuro-Vornhagen A, Böll B, Schellongowski P, Valade S, Metaxa V, Azoulay E, et al. Critical care management of chimeric antigen receptor T-cell therapy recipients. CA Cancer J Clin. 2022;72:78–93. https://doi.org/10.3322/caac.21702.
Azoulay É, Castro P, Maamar A, Metaxa V, de Moraes AG, Voigt L, et al. Outcomes in patients treated with chimeric antigen receptor T-cell therapy who were admitted to intensive care (CARTTAS): an international, multicentre, observational cohort study. Lancet Haematol. 2021;8:e355-64. https://doi.org/10.1016/S2352-3026(21)00060-0.
Shao F, Long Y, Ji H, Jiang D, Lei P, Lan X. Radionuclide-based molecular imaging allows CAR-T cellular visualization and therapeutic monitoring. Theranostics. 2021;11:6800–17. https://doi.org/10.7150/thno.56989.
Minn I, Rowe SP, Pomper MG. Enhancing CAR T-cell therapy through cellular imaging and radiotherapy. Lancet Oncol. 2019;20:e443–51. https://doi.org/10.1016/S1470-2045(19)30461-9.
Kaur T, Upadhyay J, Pukale S, Mathur A, Ansari MN. Investigation of Trends in the Research on Transferrin Receptor-Mediated Drug Delivery via a Bibliometric and Thematic Analysis. Pharmaceutics. 2022;14:2574. https://doi.org/10.3390/pharmaceutics14122574.
Guo Z, Zhang Y, Fu M, Zhao L, Wang Z, Xu Z, et al. The Transferrin Receptor-Directed CAR for the Therapy of Hematologic Malignancies. Front Immunol. 2021;12:652924. https://doi.org/10.3389/fimmu.2021.652924.
Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, et al. Breast Cancer Statistics, 2022. CA Cancer J Clin. 2022;72:524–41. https://doi.org/10.3322/caac.21754.
Walker RA, Day SJ. Transferrin receptor expression in non-malignant and malignant human breast tissue. TJ Pathol. 1986;148:217–24. https://doi.org/10.1002/path.1711480305.
Habashy HO, Powe DG, Staka CM, Rakha EA, Ball G, Green AR, et al. Transferrin receptor (CD71) is a marker of poor prognosis in breast cancer and can predict response to tamoxifen. Breast Cancer Res Treat. 2010;119:283–93. https://doi.org/10.1007/s10549-009-0345-x.
Ling Y, Wang W, Hao L, Wu X, Liang J, Zhang H, et al. Self-Amplifying Iridium(III) Photosensitizer for Ferroptosis-Mediated Immunotherapy Against Transferrin Receptor-Overexpressing Cancer. Small. 2022;18:e2203659. https://doi.org/10.1002/smll.202203659.
Fu M, He Q, Guo Z, Zhou X, Li H, Zhao L, et al. Therapeutic Bispecific T-Cell Engager Antibody Targeting the Transferrin Receptor. Front Immunol. 2019;10:1396. https://doi.org/10.3389/fimmu.2019.01396.
Wang G, Kumar A, Ding W, Korangath P, Bera T, Wei J, et al. Intraductal administration of transferrin receptor-targeted immunotoxin clears ductal carcinoma in situ in mouse models of breast cancer-a preclinical study. Proc Natl Acad Sci U S A. 2022;119:e2200200119. https://doi.org/10.1073/pnas.2200200119.
James ML, Gambhir SS. A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev. 2012;92:897–965. https://doi.org/10.1152/physrev.00049.2010.
Rowe SP, Pomper MG. Molecular imaging in oncology: Current impact and future directions. CA Cancer J Clin. 2022;72:333–52. https://doi.org/10.3322/caac.21713.
Sakemura R, Can I, Siegler EL, Kenderian SS. In vivo CART cell imaging: Paving the way for success in CART cell therapy. Mol Ther Oncolytics. 2021;20:625–33. https://doi.org/10.1016/j.omto.2021.03.003.
Minn I, Huss DJ, Ahn HH, Chinn TM, Park A, Jones J, et al. Imaging CAR T cell therapy with PSMA-targeted positron emission tomography. Sci Adv. 2019;5:eaaw5096. https://doi.org/10.1126/sciadv.aaw5096.
Vedvyas Y, Shevlin E, Zaman M, Min IM, Amor-Coarasa A, Park S, et al. Longitudinal PET imaging demonstrates biphasic CAR T cell responses in survivors. JCI Insight. 2016;1:e90064. https://doi.org/10.1172/jci.insight.90064.
Moroz MA, Zhang H, Lee J, Moroz E, Zurita J, Shenker L, et al. Comparative Analysis of T Cell Imaging with Human Nuclear Reporter Genes. J Nucl Med. 2015;56:1055–60. https://doi.org/10.2967/jnumed.115.159855.
Sellmyer MA, Richman SA, Lohith K, Hou C, Weng CC, Mach RH, et al. Imaging CAR T Cell Trafficking with eDHFR as a PET Reporter Gene. Mol Ther. 2020;28:42–51. https://doi.org/10.1016/j.ymthe.2019.10.007.
Keu KV, Witney TH, Yaghoubi S, Rosenberg J, Kurien A, Magnusson R, et al. Reporter gene imaging of targeted T cell immunotherapy in recurrent glioma. Sci Transl Med. 2017;9:eaag2196. https://doi.org/10.1126/scitranslmed.aag2196.
Volpe A, Lang C, Lim L, Man F, Kurtys E, Ashmore-Harris C, et al. Spatiotemporal PET Imaging Reveals Differences in CAR-T Tumor Retention in Triple-Negative Breast Cancer Models. Mol Ther. 2020;28:2271–85. https://doi.org/10.1016/j.ymthe.2020.06.028.
Krebs S, Ahad A, Carter LM, Eyquem J, Brand C, Bell M, et al. Antibody with Infinite Affinity for In Vivo Tracking of Genetically Engineered Lymphocytes. J Nucl Med. 2018;59:1894–900. https://doi.org/10.2967/jnumed.118.208041.
Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–5.
Boulch M, Cazaux M, Loe-Mie Y, Thibaut R, Corre B, Lemaître F, et al. A cross-talk between CAR T cell subsets and the tumor microenvironment is essential for sustained cytotoxic activity. Sci Immunol. 2021;6:eabd4344. https://doi.org/10.1126/sciimmunol.abd4344.
Larson RC, Kann MC, Bailey SR, Haradhvala NJ, Llopis PM, Bouffard AA, et al. CAR T cell killing requires the IFNγR pathway in solid but not liquid tumours. Nature. 2022;604:563–70. https://doi.org/10.1038/s41586-022-04585-5.
Moatti A, Cohen JL. The TNF-α/TNFR2 Pathway: Targeting a Brake to Release the Anti-tumor Immune Response. Front Cell Dev Biol. 2021;9:725473. https://doi.org/10.3389/fcell.2021.725473.
Wang XY, Wang Y, Wu Q, Liu JJ, Liu Y, Pan DH, et al. Feasibility study of 68Ga-labeled CAR T cells for in vivo tracking using micro-positron emission tomography imaging. Acta Pharmacol Sin. 2021;42:824–31. https://doi.org/10.1038/s41401-020-00511-5.
Volpe A, Adusumilli PS, Schöder H, Ponomarev V. Imaging cellular immunotherapies and immune cell biomarkers: from preclinical studies to patients. J Immunother Cancer. 2022;10:e004902. https://doi.org/10.1136/jitc-2022-004902.
Benešová M, Schäfer M, Bauder-Wüst U, Afshar-Oromieh A, Kratochwil C, Mier W, et al. Preclinical Evaluation of a Tailor-Made DOTA-Conjugated PSMA Inhibitor with Optimized Linker Moiety for Imaging and Endoradiotherapy of Prostate Cancer. J Nucl Med. 2015;56:914–20. https://doi.org/10.2967/jnumed.114.147413.
Afshar-Oromieh A, Hetzheim H, Kratochwil C, Benesova M, Eder M, Neels OC, et al. The Theranostic PSMA Ligand PSMA-617 in the Diagnosis of Prostate Cancer by PET/CT: Biodistribution in Humans, Radiation Dosimetry, and First Evaluation of Tumor Lesions. J Nucl Med. 2015;56:1697–705. https://doi.org/10.2967/jnumed.115.161299.
Gühne F, Radke S, Winkens T, Kühnel C, Greiser J, Seifert P, et al. Differences in Distribution and Detection Rate of the [68Ga]Ga-PSMA Ligands PSMA-617, -I&T and -11-Inter-Individual Comparison in Patients with Biochemical Relapse of Prostate Cancer. Pharmaceuticals (Basel). 2021;15:9. https://doi.org/10.3390/ph15010009.
Singh AP, Chen W, Zheng X, Mody H, Carpenter TJ, Zong A, et al. Bench-to-bedside translation of chimeric antigen receptor (CAR) T cells using a multiscale systems pharmacokinetic-pharmacodynamic model: A case study with anti-BCMA CAR-T. CPT Pharmacometrics Syst Pharmacol. 2021;10:362–76. https://doi.org/10.1002/psp4.12598.
Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15:81–94. https://doi.org/10.1038/nrclinonc.2017.166.
Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143-52. https://doi.org/10.1016/S1470-2045(17)30074-8.
Espie D, Donnadieu E. CAR T-cell behavior and function revealed by real-time imaging. Semin Immunopathol. 2023;45:229–39. https://doi.org/10.1007/s00281-023-00983-7.
Schwenck J, Sonanini D, Cotton JM, Rammensee HG, la Fougère C, Zender L, et al. Advances in PET imaging of cancer. Nat Rev Cancer. 2023;23:474–90. https://doi.org/10.1038/s41568-023-00576-4.
Funding
This work was financially supported by National Natural Science Foundation of China (grants 82030052), Hubei Province Science and Technology Innovation Team ([2022] No. 72) and Key Project of Hubei Province Natural Science Foundation (No. 2021CFA008).
Author information
Authors and Affiliations
Contributions
X. L., L. P and D. J.: study design; X. S. and Y. Z.: manuscript writing; X. S. and Y. G.: tracer synthesis and identification; Y. Z. and Z. X.: Preparation of CAR T cells; X. S., X. L. and Y. L.: image acquisition and interpretation; X. L., L. P and D. J.: manuscript revision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experimental schemes were performed under the guidance and approved by Union Hospital, Tongji Medical College, Huazhong University of Science and Technology and Institutional Animal Care and Use Committee of Tongji Medical College of Huazhong University of Science and Technology.
Conflict of interest
All authors have no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, X., Zhang, Y., Lv, X. et al. Noninvasive longitudinal PET/CT imaging of CAR T cells using PSMA reporter gene. Eur J Nucl Med Mol Imaging 51, 965–977 (2024). https://doi.org/10.1007/s00259-023-06508-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06508-6