Abstract
Purpose
Standardized uptake value (SUV) has been prevalently used to measure [68 Ga]Ga-PSMA-11 activity in prostate cancer, but it is susceptible to multiple factors. Parametric imaging allows for absolute quantification of tracer uptake and provides a better diagnostic accuracy that is crucial for lesion detection. However, the clinical significance of total-body parametric imaging of [68 Ga]Ga-PSMA-11 remains to be fully assessed. Therefore, the aim of our study is to delve into the diagnostic implications of total-body parametric imaging of [68 Ga]Ga-PSMA-11 PET/CT for patients with prostate cancer.
Methods
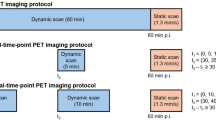
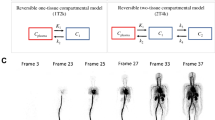
Twenty prostate cancer patients were included and underwent a dynamic total-body [68 Ga]Ga-PSMA-11 PET/CT scan. An irreversible two-tissue compartment model (2T3k) was fitted for each tissue time-to-activity curve, and the net influx rate (Ki) was obtained. The image quality and semi-quantitative analysis of lesion-to-background ratio (LBR), signal-to-noise ratio (SNR), and contrast-to-noise ratio (CNR) were compared between parametric images and SUV images.
Results
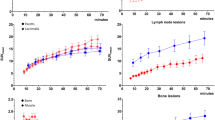
Kinetic modeling using 2T3k demonstrated favorable model fitting in both normal organs and lesions. All of the lesions detected on SUV images (55–60 min) could be detected on Ki images. The correlation between Ki, SUVmean, and SUVmax in both normal organs and pathological lesions was found to be positive and statistically significant. Conversely, a moderate positive correlations were found between Ki and K1 (R = 0.69, P < 0.001; R = 0.61, P < 0.001) and Ki and k3 (R = 0.69, P < 0.001; R = 0.62, P < 0.001), in normal organs and pathological lesions, respectively. Visual assessment in Ki images showed less image noise and higher lesions conspicuity compared to SUV images. Ki image-derived LBR, SNR, and CBR of pathological lesions including primary tumors (PTs), lymph node metastases (LNMs) and bone metastases (BMs), exhibited remarkably higher folds (1.4–3.6 folds) compared to those derived from SUV of corresponding lesions.
Conclusions
Total-body parametric imaging of [68 Ga]Ga-PSMA-11 enhanced lesion contrast and improved lesion detectability compared to SUV images. This may potentially serve as an imaging biomarker and theranostic tool for precise diagnosis and treatment evaluation in prostate cancer patients.






Similar content being viewed by others
Data availability
The data could be obtained from the corresponding author upon request.
References
Adams MC, Turkington TG, Wilson JM, Wong TZ. A systematic review of the factors affecting accuracy of SUV measurements. AJR Am J Roentgenol. 2010;195:310–20. https://doi.org/10.2214/AJR.10.4923.
Bertoldo A, Rizzo G, Veronese M. Deriving physiological information from PET images: from SUV to compartmental modelling. Clin Translat Imaging. 2014;2:239–51. https://doi.org/10.1007/s40336-014-0067-x.
Dimitrakopoulou-Strauss A, Pan L, Sachpekidis C. Kinetic modeling and parametric imaging with dynamic PET for oncological applications: general considerations, current clinical applications, and future perspectives. Eur J Nucl Med Mol Imaging. 2021;48:21–39. https://doi.org/10.1007/s00259-020-04843-6.
Wang G, Rahmim A, Gunn RN. PET Parametric Imaging: Past, Present, and Future. IEEE Transact Radiat Plasma Med Sci. 2020;4:663–75. https://doi.org/10.1109/TRPMS.2020.3025086.
Rahmim A, Lodge MA, Karakatsanis NA, Panin VY, Zhou Y, McMillan A, et al. Dynamic whole-body PET imaging: principles, potentials and applications. Eur J Nucl Med Mol Imaging. 2019;46:501–18. https://doi.org/10.1007/s00259-018-4153-6.
Zhang X, Cherry SR, Xie Z, Shi H, Badawi RD, Qi J. Subsecond total-body imaging using ultrasensitive positron emission tomography. Proc Natl Acad Sci U S A. 2020;117:2265–7. https://doi.org/10.1073/pnas.1917379117.
Zhang X, Xie Z, Berg E, Judenhofer MS, Liu W, Xu T, et al. Total-Body Dynamic Reconstruction and Parametric Imaging on the uEXPLORER. J Nucl Med. 2020;61:285–91. https://doi.org/10.2967/jnumed.119.230565.
Zhang YQ, Hu PC, Wu RZ, Gu YS, Chen SG, Yu HJ, et al. The image quality, lesion detectability, and acquisition time of (18)F-FDG total-body PET/CT in oncological patients. Eur J Nucl Med Mol Imaging. 2020;47:2507–15. https://doi.org/10.1007/s00259-020-04823-w.
Wen J, Zhu Y, Li L, Liu J, Chen Y, Chen R. Determination of optimal 68 Ga-PSMA PET/CT imaging time in prostate cancers by total-body dynamic PET/CT. Eur J Nucl Med Mol Imaging. 2022;49:2086–95. https://doi.org/10.1007/s00259-021-05659-8.
Chen R, Wang Y, Zhu Y, Shi Y, Xu L, Huang G, et al. The Added Value of (18)F-FDG PET/CT Compared with (68)Ga-PSMA PET/CT in Patients with Castration-Resistant Prostate Cancer. J Nucl Med. 2022;63:69–75. https://doi.org/10.2967/jnumed.120.262250.
Yu H, Gu Y, Fan W, Gao Y, Wang M, Zhu X, et al. Expert consensus on oncological [(18)F]FDG total-body PET/CT imaging (version 1). Eur Radiol. 2023;33:615–26. https://doi.org/10.1007/s00330-022-08960-8.
Eiber M, Herrmann K, Calais J, Hadaschik B, Giesel FL, Hartenbach M, et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): Proposed miTNM Classification for the Interpretation of PSMA-Ligand PET/CT. J Nucl Med. 2018;59:469–78. https://doi.org/10.2967/jnumed.117.198119.
Wang Y, Li E, Cherry SR, Wang G. Total-Body PET Kinetic Modeling and Potential Opportunities Using Deep Learning. PET Clin. 2021;16:613–25. https://doi.org/10.1016/j.cpet.2021.06.009.
Ringheim A, Campos Neto GC, Anazodo U, Cui L, da Cunha ML, Vitor T, et al. Kinetic modeling of (68)Ga-PSMA-11 and validation of simplified methods for quantification in primary prostate cancer patients. EJNMMI Res. 2020;10:12. https://doi.org/10.1186/s13550-020-0594-6.
Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7. https://doi.org/10.1038/jcbfm.1983.1.
Sachpekidis C, Kopka K, Eder M, Hadaschik BA, Freitag MT, Pan L, et al. 68Ga-PSMA-11 Dynamic PET/CT Imaging in Primary Prostate Cancer. Clin Nucl Med. 2016;41:e473–9. https://doi.org/10.1097/RLU.0000000000001349.
Sachpekidis C, Eder M, Kopka K, Mier W, Hadaschik BA, Haberkorn U, et al. (68)Ga-PSMA-11 dynamic PET/CT imaging in biochemical relapse of prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:1288–99. https://doi.org/10.1007/s00259-015-3302-4.
Huang X, Zhou Y, Bao S, Huang SC. Clustering-based linear least square fitting method for generation of parametric images in dynamic FDG PET studies. Int J Biomed Imaging. 2007;2007:65641. https://doi.org/10.1155/2007/65641.
Blomqvist G. On the construction of functional maps in positron emission tomography. J Cereb Blood Flow Metab. 1984;4:629–32. https://doi.org/10.1038/jcbfm.1984.89.
Zhou Y, Flores S, Mansor S, Hornbeck RC, Tu Z, Perlmutter JS, et al. Spatially constrained kinetic modeling with dual reference tissues improves (18)F-flortaucipir PET in studies of Alzheimer disease. Eur J Nucl Med Mol Imaging. 2021;48:3172–86. https://doi.org/10.1007/s00259-020-05134-w.
Gjedde A. High- and low-affinity transport of D-glucose from blood to brain. J Neurochem. 1981;36:1463–71. https://doi.org/10.1111/j.1471-4159.1981.tb00587.x.
Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations J Cereb Blood Flow Metab. 1985;5:584–90. https://doi.org/10.1038/jcbfm.1985.87.
Wong DF, Gjedde A, Wagner HN Jr. Quantification of neuroreceptors in the living human brain. I. Irreversible binding of ligands. J Cereb Blood Flow Metab. 1986;6:137–46. https://doi.org/10.1038/jcbfm.1986.27.
Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics. 1977;33:363–74. https://doi.org/10.2307/2529786.
Strauss DS, Sachpekidis C, Kopka K, Pan L, Haberkorn U, Dimitrakopoulou-Strauss A. Pharmacokinetic studies of [(68) Ga]Ga-PSMA-11 in patients with biochemical recurrence of prostate cancer: detection, differences in temporal distribution and kinetic modelling by tissue type. Eur J Nucl Med Mol Imaging. 2021;48:4472–82. https://doi.org/10.1007/s00259-021-05420-1.
Sari H, Mingels C, Alberts I, Hu J, Buesser D, Shah V, et al. First results on kinetic modelling and parametric imaging of dynamic (18)F-FDG datasets from a long axial FOV PET scanner in oncological patients. Eur J Nucl Med Mol Imaging. 2022;49:1997–2009. https://doi.org/10.1007/s00259-021-05623-6.
Sari H, Eriksson L, Mingels C, Alberts I, Casey ME, Afshar-Oromieh A, et al. Feasibility of using abbreviated scan protocols with population-based input functions for accurate kinetic modeling of [(18)F]-FDG datasets from a long axial FOV PET scanner. Eur J Nucl Med Mol Imaging. 2023;50:257–65. https://doi.org/10.1007/s00259-022-05983-7.
Dias AH, Jochumsen MR, Zacho HD, Munk OL, Gormsen LC. Multiparametric dynamic whole-body PSMA PET/CT using [(68)Ga]Ga-PSMA-11 and [(18)F]PSMA-1007. EJNMMI Res. 2023;13:31. https://doi.org/10.1186/s13550-023-00981-8.
Lu M, Lindenberg L, Mena E, Turkbey B, Seidel J, Ton A, et al. A Pilot Study of Dynamic (18)F-DCFPyL PET/CT Imaging of Prostate Adenocarcinoma in High-Risk Primary Prostate Cancer Patients. Mol Imaging Biol. 2022;24:444–52. https://doi.org/10.1007/s11307-021-01670-5.
Prasad V, Steffen IG, Diederichs G, Makowski MR, Wust P, Brenner W. Biodistribution of [(68)Ga]PSMA-HBED-CC in Patients with Prostate Cancer: Characterization of Uptake in Normal Organs and Tumour Lesions. Mol Imaging Biol. 2016;18:428–36. https://doi.org/10.1007/s11307-016-0945-x.
Hofman MS, Hicks RJ, Maurer T, Eiber M. Prostate-specific Membrane Antigen PET: Clinical Utility in Prostate Cancer, Normal Patterns, Pearls, and Pitfalls. Radiographics. 2018;38:200–17. https://doi.org/10.1148/rg.2018170108.
Rosar F, Wenner F, Khreish F, Dewes S, Wagenpfeil G, Hoffmann MA, et al. Early molecular imaging response assessment based on determination of total viable tumor burden in [68Ga]Ga-PSMA-11 PET/CT independently predicts overall survival in [177Lu]Lu-PSMA-617 radioligand therapy. Eur J Nucl Med Mol Imaging. 2022;49:1584–94. https://doi.org/10.1007/s00259-021-05594-8.
Seifert R, Kessel K, Schlack K, Weber M, Herrmann K, Spanke M, et al. PSMA PET total tumor volume predicts outcome of patients with advanced prostate cancer receiving [(177)Lu]Lu-PSMA-617 radioligand therapy in a bicentric analysis. Eur J Nucl Med Mol Imaging. 2021;48:1200–10. https://doi.org/10.1007/s00259-020-05040-1.
Unterrainer LM, Beyer L, Zacherl MJ, Gildehaus FJ, Todica A, Kunte SC, et al. Total Tumor Volume on (18)F-PSMA-1007 PET as Additional Imaging Biomarker in mCRPC Patients Undergoing PSMA-Targeted Alpha Therapy with (225)Ac-PSMA-I&T. Biomedicines. 2022;10:946. https://doi.org/10.3390/biomedicines10050946.
Cunningham VJ, Jones T. Spectral analysis of dynamic PET studies. J Cereb Blood Flow Metab. 1993;13:15–23. https://doi.org/10.1038/jcbfm.1993.5.
Acknowledgements
We express our appreciation to Wenjian Gu (Central Research Institute, UIH Group) for his assistance in preparing the code for the generating parametric image presented in this manuscript.
Funding
The study was supported by National Natural Science Foundation of China (No. 92259103, 82171972); National Key R&D Program of China (No. 2021YFA0910004); Clinical Research Project of Health Industry of Shanghai Municipal Health Commission (20214Y0438); Nurture projects for the Youth Medical Talents-Medical Imaging Practitioners Program (SHWRS(2021)_099).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Consent for publication
Not applicable.
Ethical approval
The study involving human participants was in line with the principles of the ethics committee in Renji hospital and the declaration of Helsinki in 1964. Animal-based research was not included in this study.
Consent to participate
The informed consent document was signed by all participants prior to their voluntary enrollment in this study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, R., Ng, Y.L., Yang, X. et al. Comparison of parametric imaging and SUV imaging with [68 Ga]Ga-PSMA-11 using dynamic total-body PET/CT in prostate cancer. Eur J Nucl Med Mol Imaging 51, 568–580 (2024). https://doi.org/10.1007/s00259-023-06456-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06456-1