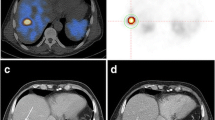
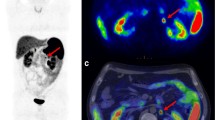
Abstract
Purpose
To evaluate the role of 68Ga-DOTATOC PET parameters in predicting DAXX/ATRX loss of expression in patients with Pancreatic neuroendocrine tumors (PanNET) candidate to surgery.
Methods
This retrospective study included 72 consecutive patients with PanNET (January 2018–March 2022) who underwent to 68Ga-DOTATOC PET for preoperative staging. Image analysis: qualitative assessment and extraction of SUVmax, SUV mean, somatostatin receptor density (SRD), and total lesion somatostatin receptor density (TLSRD) from primary PanNET. Radiological diameter and biopsy information (grade, Ki67) were collected. Loss of expression (LoE) of DAXX/ATRX was assessed by immunohistochemistry on surgical specimen. Student t-test, univariate and multivariate logistic regression and ROC curves have been used to investigate the predictive value of PET parameters on DAXX/ATRX LoE.
Results
Forty-two/72 patients had a G1, 28/72 a G2, and 2/72 a G3 PanNET. Seven/72 patients had DAXX LoE, 10/72 ATRX LoE, and 2/72 DAXX/ATRX LoE. SRD and TLSRD could predict DAXX LoE (p = 0.002, p = 0.018, respectively). By evaluating SRD in combination with radiological diameter, only SRD maintained statistical significance (multivariate logistic regression: p = 0.020, OR = 1.05), providing the best prediction (AUC-ROC = 79.01%; cut-off = 46.96; sensitivity = 77.78%; specificity = 88.89%). In the sub-analysis performed on 55 patients with biopsy availability, SRD demonstrated its role in providing useful and additional information (multivariate logistic regression: SRD p = 0.007; grade p = 0.040).
Conclusion
SRD has a predictive role on DAXX LoE in PanNETs, with higher probability of LoE at increasing SRD values. SRD provides complementary/additional information to grade assessed on biopsy material, and the combined use of these approaches might support patients’ management by preoperatively identifying subjects with more aggressive diseases.





Similar content being viewed by others
Data Availability
Data will be made available upon request to the corresponding author.
References
Lee MR, Harris C, Baeg KJ, Aronson A, Wisnivesky JP, Kim MK. Incidence trends of gastroenteropancreatic neuroendocrine tumors in the United States. Clin Gastroenterol Hepatol. 2019;17:2212-7 e1. https://doi.org/10.1016/j.cgh.2018.12.017.
Partelli S, Bartsch DK, Capdevila J, Chen J, Knigge U, Niederle B, et al. ENETS Consensus guidelines for standard of care in neuroendocrine tumours: surgery for small intestinal and pancreatic neuroendocrine tumours. Neuroendocrinology. 2017;105:255–65. https://doi.org/10.1159/000464292.
Falconi M, Bartsch DK, Eriksson B, Kloppel G, Lopes JM, O’Connor JM, et al. ENETS consensus guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: well-differentiated pancreatic non-functioning tumors. Neuroendocrinology. 2012;95:120–34. https://doi.org/10.1159/000335587.
Partelli S, Muffatti F, Andreasi V, Giannone F, Rossi G, Palumbo D, et al. A single-center prospective observational study investigating the accuracy of preoperative diagnostic procedures in the assessment of lymph node metastases in nonfunctioning pancreatic neuroendocrine tumors. Ann Surg. 2022. https://doi.org/10.1097/SLA.0000000000005615.
Cives M, Partelli S, Palmirotta R, Lovero D, Mandriani B, Quaresmini D, et al. DAXX mutations as potential genomic markers of malignant evolution in small nonfunctioning pancreatic neuroendocrine tumors. Sci Rep. 2019;9:18614. https://doi.org/10.1038/s41598-019-55156-0.
Kim JY, Brosnan-Cashman JA, An S, Kim SJ, Song KB, Kim MS, et al. Alternative lengthening of telomeres in primary pancreatic neuroendocrine tumors is associated with aggressive clinical behavior and poor survival. Clin Cancer Res. 2017;23:1598–606. https://doi.org/10.1158/1078-0432.CCR-16-1147.
Marinoni I, Kurrer AS, Vassella E, Dettmer M, Rudolph T, Banz V, et al. Loss of DAXX and ATRX are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors. Gastroenterology. 2014;146:453-60 e5. https://doi.org/10.1053/j.gastro.2013.10.020.
Scarpa A, Chang DK, Nones K, Corbo V, Patch AM, Bailey P, et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature. 2017;543:65–71. https://doi.org/10.1038/nature21063.
Singhi AD, Liu TC, Roncaioli JL, Cao D, Zeh HJ, Zureikat AH, et al. Alternative lengthening of telomeres and loss of DAXX/ATRX expression predicts metastatic disease and poor survival in patients with pancreatic neuroendocrine tumors. Clin Cancer Res. 2017;23:600–9. https://doi.org/10.1158/1078-0432.CCR-16-1113.
Marinoni I. Prognostic value of DAXX/ATRX loss of expression and ALT activation in PanNETs: is it time for clinical implementation? Gut. 2022;71:847–8. https://doi.org/10.1136/gutjnl-2021-324664.
Takumi K, Fukukura Y, Higashi M, Ideue J, Umanodan T, Hakamada H, et al. Pancreatic neuroendocrine tumors: correlation between the contrast-enhanced computed tomography features and the pathological tumor grade. Eur J Radiol. 2015;84:1436–43. https://doi.org/10.1016/j.ejrad.2015.05.005.
Tacelli M, Petrone MC, Capurso G, Muffatti F, Andreasi V, Partelli S, et al. Diagnostic accuracy of EUS-FNA in the evaluation of pancreatic neuroendocrine neoplasms grading: possible clinical impact of misclassification. Endosc Ultrasound. 2021;10:372–80. https://doi.org/10.4103/EUS-D-20-00261.
Hofman MS, Kong G, Neels OC, Eu P, Hong E, Hicks RJ. High management impact of Ga-68 DOTATATE (GaTate) PET/CT for imaging neuroendocrine and other somatostatin expressing tumours. J Med Imaging Radiat Oncol. 2012;56:40–7. https://doi.org/10.1111/j.1754-9485.2011.02327.x.
Mapelli P, Partelli S, Salgarello M, Doraku J, Pasetto S, Rancoita PMV, et al. Dual tracer 68Ga-DOTATOC and 18F-FDG PET/computed tomography radiomics in pancreatic neuroendocrine neoplasms: an endearing tool for preoperative risk assessment. Nucl Med Commun. 2020;41:896–905. https://doi.org/10.1097/MNM.0000000000001236.
Mapelli P, Partelli S, Salgarello M, Doraku J, Muffatti F, Schiavo Lena M, et al. Dual tracer 68Ga-DOTATOC and 18F-FDG PET improve preoperative evaluation of aggressiveness in resectable pancreatic neuroendocrine neoplasms. Diagnostics (Basel). 2021;11. https://doi.org/10.3390/diagnostics11020192.
Mapelli P, Bezzi C, Palumbo D, Canevari C, Ghezzo S, SamanesGajate AM, et al. (68)Ga-DOTATOC PET/MR imaging and radiomic parameters in predicting histopathological prognostic factors in patients with pancreatic neuroendocrine well-differentiated tumours. Eur J Nucl Med Mol Imaging. 2022;49:2352–63. https://doi.org/10.1007/s00259-022-05677-0.
Abdulrezzak U, Kurt YK, Kula M, Tutus A. Combined imaging with 68Ga-DOTA-TATE and 18F-FDG PET/CT on the basis of volumetric parameters in neuroendocrine tumors. Nucl Med Commun. 2016;37:874–81. https://doi.org/10.1097/MNM.0000000000000522.
Toriihara A, Baratto L, Nobashi T, Park S, Hatami N, Davidzon G, et al. Prognostic value of somatostatin receptor expressing tumor volume calculated from (68)Ga-DOTATATE PET/CT in patients with well-differentiated neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2019;46:2244–51. https://doi.org/10.1007/s00259-019-04455-9.
IARC. Digestive system tumours. WHO classification of Tumours, 5th Edition, Volume 1, WHO Classification of Tumours Editorial Board. 2019: ISBN-13 978-92-832-4499-8 https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Digestive-System-Tumours-2019
Orlhac F, Eertink JJ, Cottereau AS, Zijlstra JM, Thieblemont C, Meignan M, et al. A guide to ComBat harmonization of imaging biomarkers in multicenter studies. J Nucl Med. 2022;63:172–9. https://doi.org/10.2967/jnumed.121.262464.
Demsar JCT, Erjavec A, Gorup C, Hocevar T, Milutinovic M. Mozina M, Polajnar M, Toplak M, Staric A, Stajdohar M, Umek L, Zagar L, Zbontar J, Zitnik M, Zupan B, Orange: data mining toolbox in python. J Mach 2013.
Arakelyan J, Zohrabyan D, Philip PA. Molecular profile of pancreatic neuroendocrine neoplasms (PanNENs): opportunities for personalized therapies. Cancer. 2021;127:345–53. https://doi.org/10.1002/cncr.33354.
Hackeng WM, Brosens LAA, Kim JY, O’Sullivan R, Sung YN, Liu TC, et al. Non-functional pancreatic neuroendocrine tumours: ATRX/DAXX and alternative lengthening of telomeres (ALT) are prognostically independent from ARX/PDX1 expression and tumour size. Gut. 2022;71:961–73. https://doi.org/10.1136/gutjnl-2020-322595.
Pea A, Yu J, Marchionni L, Noe M, Luchini C, Pulvirenti A, et al. Genetic analysis of small well-differentiated pancreatic neuroendocrine tumors identifies subgroups with differing risks of liver metastases. Ann Surg. 2020;271:566–73. https://doi.org/10.1097/SLA.0000000000003022.
Wang F, Xu X, Ye Z, Qin Y, Yu X, Ji S. Prognostic significance of altered ATRX/DAXX gene in pancreatic neuroendocrine tumors: a meta-analysis. Front Endocrinol. 2021; 12:691557. https://doi.org/10.3389/fendo.2021.691557.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
All patients gave their informed consent to participate in the study.
Conflict of interest
Prof. Massimo Falconi was an Advisory Board Member of Advanced Accelerator Application (AAA). All the other authors have no conflicts of interest related to the present paper to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology—Digestive tract
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mapelli, P., Bezzi, C., Muffatti, F. et al. Somatostatin receptor activity assessed by 68Ga-DOTATOC PET can preoperatively predict DAXX/ATRX loss of expression in well-differentiated pancreatic neuroendocrine tumors. Eur J Nucl Med Mol Imaging 50, 2818–2829 (2023). https://doi.org/10.1007/s00259-023-06210-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06210-7