Abstract
Purpose
225Ac-PSMA-617 has demonstrated good anti-tumor effect as a treatment option for metastatic castration-resistant prostate cancer (mCRPC) patients. No study has previously assessed treatment outcome and survival following 225Ac-PSMA-617 treatment of de novo metastatic hormone-sensitive prostate carcinoma (mHSPC) patients. Based on the potential side effects that are known and explained to the patients by the oncologist, some of the patients refused the standard treatment and are seeking alternative therapies. Thus, we report our preliminary findings in a retrospective series of 21 mHSPC patients that refused standard treatment options and were treated with 225Ac-PSMA-617.
Methods
We retrospectively reviewed patients with histologically confirmed de novo treatment-naïve bone ± visceral mHSPC that were treated with 225Ac-PSMA-617 radioligand therapy (RLT). Inclusion criteria included an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2, treatment-naive bone ± visceral mHSPC, and patients refusal for ADT ± docetaxel, abiraterone acetate, or enzalutamide. We evaluated the response to treatment using prostate-specific antigen (PSA) response and the progression-free survival (PFS) and overall survival (OS) as well as the toxicities.
Results
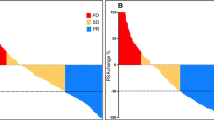
Twenty-one mHSPC patients were included in this preliminary work. Following treatment, twenty patients (95%) had any decline in PSA and eighteen patients (86%) presented with a PSA decline of ≥ 50% including 4 patients in whom PSA became undetectable. A lower percentage decrease in PSA following treatment was associated with increased mortality and shorter progression-free survival. Overall, administration of 225Ac-PSMA-617 was well tolerated. The commonest toxicity seen was grade I/II dry mouth observed in 94% of patients.
Conclusions
Given these favorable results, randomized prospective multicenter trials assessing the clinical value of 225Ac-PSMA-617 as a therapeutic agent for mHSPC administered either as monotherapy or administered concomitant with ADT are of interest.
Similar content being viewed by others
Introduction
Prostate carcinoma is the most frequent malignancy and the second leading cause of cancer-related death in men [1].
At the time of diagnosis, the vast majority of newly diagnosed prostate carcinomas is either confined to the prostate or extends loco-regionally into surrounding structures or surrounding lymph nodes [2]. An estimated 5% of all prostate carcinoma patients, however, presents with de novo metastatic disease or metastatic hormone-sensitive prostate carcinoma (mHSPC) at the time of diagnosis [3]. According to current clinical guidelines based on randomized clinical trials, patients with high-volume mHSPC should be treated with standard androgen deprivation therapy (ADT) and abiraterone acetate (AA) with prednisone (P) or docetaxel (DOC) given adding either AA or DOC to ADT treatment was shown to significantly improve overall survival of mHSPC, as apalutamide and local RT for low-volume mHSPC [2, 4,5,6]. In the absence of a prospective randomized head-to-head comparison demonstrating superiority of one approach over the other, selection of either treatment option, ADT + AA+P versus ADT +DOC, is currently based taking into consideration disease burden, quality of life, duration of therapies, underlying comorbidities, wide respect to side effects, and patient preferences for treatment. While ADT + AA+P or ADT +DOC are usually well tolerated by fit and motivated patients, this is not the case in elderly, frail men presenting with important comorbidities. Accordingly, for the latter patients in whom mono-ADT is currently the treatment of choice, novel less toxic treatment options are warranted.
225Ac-PSMA-617 is a novel treatment option for prostate carcinoma patients and preliminary results obtained in patients with advanced prostate carcinoma have shown its therapeutic potential based on its high linear energy transfer (LET)-value related to its decay scheme, with 6 daughter products 221-Francium, 217-Astatine, 213-Bismuth, 213-Polonium, 209-Lead, and 209-Thallium (221Fr, 217At, 213Bi, 213Po, 209Pb and 209Tl) with several α- and β-decays [7,8,9,10,11,12,13,14]. In the studies reported to date using this agent, toxicities encountered using this agent are grade I or II xerostomia, observed in 85% of patients, anemia encountered in 37% of patients (predominantly grade I and II and no grade IV), and renal impairment encountered in 32% of patients (grade IV in 3% of patients only) [7,8,9,10,11,12,13,14,15,16]. Overall, side effects proved more prevalent among patients with a super-scan pattern.
Here, we report preliminary findings in a series of 21 bone ± visceral mHSPC patients (stage M1b or M1c) that refused standard treatment options and were treated with 225Ac-PSMA-617.
Methods
This is a retrospective review of patients with histologically confirmed de novo treatment naïve bone ± visceral mHSPC that were treated with 225Ac-PSMA-617 radioligand therapy (RLT). Inclusion criteria included an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2, treatment-naive bone ± visceral mHSPC, and patients refusal for ADT ± docetaxel, abiraterone acetate, or enzalutamide. Patients were aware that treatment of choice is widely available mono-ADT as well as the possibility of optional radiotherapy to the primary prostate tumor in low-volume patients [5, 6]. Unfortunately, except for ADT, the university hospital does not offer apalutamide, abiraterone acetate, or enzalutamide due to budgetary constraints. Exclusion criteria included a glomerular filtration rate below 60 mL/min, urinary tract obstruction as determined by 99mTc-MAG renal scintigraphy, and bone marrow suppression. The decision to treat patients with 225Ac-PSMA-617 was made by the local interdisciplinary tumor board. Based on the potential side effects that are known and explained to the patients by the oncologist, some of the patients refused the standard treatment and are seeking alternative therapies. Patients were informed upfront of the fact that 225Ac-PSMA-617 treatment is not approved in South Africa as well as of the possible adverse events related to the treatment, respectively, xerostomia, bone marrow suppression, renal impairment, and potential currently unknown side effects. All patients gave written informed consent to undergo treatment with 225Ac-PSMA-617. The Human Research Ethics Committee of the Faculty of Health Sciences, University of Pretoria, approved this study (Ethics Reference Number: 173/2021).
Patient preparation
Eligible patients had available baseline 68Ga-PSMA-11 PET/CT scan, clinical laboratory assessments which included urea and creatinine, electrolytes, liver function tests, and full blood count before 225Ac-PSMA-617 radioligand therapy. 225Ac-PSMA-617 treatments were administered every 8 weeks with activities being progressively decreased (de-escalation) from 8 MBq the first time to 6 or 4 MBq subsequently based on response to earlier administered treatments.
Preparation and administration of 225Ac-PSMA-617
225Ac-PSMA-617 was radiolabelled as described previously. The initial administered activity was 8 MBq. For subsequent treatment cycles, administered activity was de-escalated to 7, 6, or 4 MBq based on response to earlier administered treatment. Treatments were repeated every 8 weeks. 68Ga-PSMA-11 PET/CT scan was repeated after each subsequent treatment cycle to determine the burden of residual tumor to guide dose de-escalation. The decision was based on response on the clinical, biochemical, and the on repeat 68Ga-PSMA-11 PET/CT as previously described [10]. This strategy is established on the principle of tumor sink effect in which more radioligand is available for binding in normal organs with reducing tumor bulk induced by successful treatment [17].
Safety
Repeat clinical laboratory assessments which included urea and creatinine, electrolytes, liver function tests, full blood count, and glomerular filtration rate (GFR) were done at baseline, prior to each therapy cycle, 4 weeks after each cycle, and in 12-week intervals throughout follow-up. Severity of hematologic adverse events was graded based on Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. Grade ≥ 3 toxicities were characterized as significant.
Treatment response evaluation
PSA response was assessed according the Prostate Cancer Working Group 3 (PCWG3) criteria; that is, a PSA decline > 50% of the baseline was deemed clinically significant [18]. Follow-up 68Ga-PSMA-11 PET/CT (performed at baseline, prior to each treatment cycle, and every 12 weeks following treatment completion until disease progression) was used to define resolution of initially identified metastatic lesions on the baseline PET/CT scan.
Statistical analysis
Statistical analysis was performed using SPSS, version 28.0 (IBM SPSS). Based on the results of the Kolmogorov-Smirnov test, the appropriate tests were used to compare means (paired Student t test and ANOVA if normally distributed and Mann-Whitney test and Kruskal-Wallis test if not normally distributed). Univariate and regression analysis was performed on data dichotomized according the median value for continuous variables. For quantitative continuous variables, the median was used to obtain two approximately equal-sized groups for logrank testing (10 versus 11 pts). For other variables such as ECOG-score and Gleason score, the cut-off yielding the best equilibrated group sizes was used. For radiological response assessment, patients were dichotomized as follows, CR +PR versus SD + progression. In other cases (negative PSMA PET, PSA decrease > 50%, and M1b versus M1c), it is self-explanatory how two groups for comparison were obtained. The predictive value of dichotomized variables and other clinical risk factors for disease control and OS were estimated by the Kaplan-Meier method and logrank testing. Multivariate analysis was performed using Cox-regression. Finally, the Chi-square test was used to determine differences in proportion when appropriate.
Results
Patient characteristics
Patient characteristics are shown in Table 1. Twenty-one treatment-naive HSPC patients, respectively, 15 M1b and 6 M1c patients, were included in the study; median age was 67 years (range 53–80 years). ECOG scores were as follows, 0 in 8 patients, 1 in 9 patients, 2 in 3 patients, and 3 in 1 patient. Fifteen patients suffered from stage M1b disease and 6 patients from stage M1c disease (in the lungs in 2 patients, in lungs and liver in 1 patient, in the liver in 1 patient, in liver and brain in 1 patient, and finally in the brain in 1 patient). PSA, Hb, and alkaline phosphatase levels, platelet and WBC counts pre-treatment are shown in Table 1.
A total number of 68 cycles were administered (median of 3, range 2–6). Six patients received 2 cycles, 8 patients received 3 cycles, 4 patients received 4 cycles, 2 patients received 5 cycles, and 1 patient received 6 cycles. Eighteen patients (86%) presented with a PSA drop of greater than or equal to 50% and 20 patients (95%) had any drop in PSA (see Fig. 1). PSA became undetectable in 4 patients. 68Ga-PSMA-PET images became negative in 7 patients; that is, avidity was similar to background bloodpool activity. The response assessment decision was based on the clinical, biochemical, and the on repeat 68Ga-PSMA-11 PET/CT as previously described [10], leading to dose de-escalation in the case of good response (see Fig. 2) or maintaining the same dose if the response is not good (see Fig. 3). Although the study numbers are small, these examples seem to support that PSMA tumor expression on PET images appears as one of the predictors of the outcome as suggested by some of the recently published work [19,20,21,22]. All patients had bone metastases and the number was too small to differentiate between various visceral metastases. Hence, the results, OS, did not prove significantly different between M1b and M1c disease (log-rank test, p= 0.478).
A good responder treatment-naïve patient who presented with 68Ga-PSMA-11 (SUV max =22.57) avid extensive bone metastasis at primary diagnosis was discharged after two cycles of 225Ac-PSMA-617 with de-escalating activities of 8/6 MBq. His follow-up 68Ga-PSMA-11 PET/CT scan was negative PFS and his OS was 22 months
A poor responder treatment-naïve patient who presented with some 68Ga-PSMA-11 uptake (SUV max =10.25) extensive bone metastasis at primary diagnosis was discharged after two cycles of 225Ac-PSMA-617 with no de-escalating activities of 8/8 MBq. His follow-up 68Ga-PSMA-11 PET/CT scan was more avid than the baseline and his OS was 5 months
Safety
Administration of 225Ac-PSMA-617 was well tolerated. The commonest toxicity seen was grade I/II dry mouth observed in 94% of patients. There was no reversibility seen on completion; patients tend to adjust to the new lifestyle of living with xerostomia by using over-the-counter saliva substitutes (moisturizing mouth spray) and drinking water frequently. No dry eye was observed in this retrospective analysis. At the group level, hemoglobin levels as well as platelet and white blood cell did not change significantly post-treatment, respectively, p= 0.945, p=0.598, and p=0.841. At the individual level, none of the patients presented with a significant change in platelet or white blood cell count post-treatment when compared to pre-treatment values. The mean pre- and post-hemoglobin level was 10.4 (range, 6.9–15.7 g/dL) versus 9.5 g/dL (range, 5.3–14.2 g/dL); the mean pre- and post-platelet count was 299.5,000 (range, 157,000–687,000/μL) versus 252.5,000/μL (range, 132,000–404,000/μL); the mean pre- and post-white blood cell count was 6.1 (range, 3.82–10.72/μL) versus 6.1/μL (range, 3.69–11.37/μL). However, in ten patients, a significant decrease in Hb level post-treatment was observed. Given the wide range of pre-treatment Hb levels, toxicity assessment according to standard criteria was not deemed meaningful. Thus, we assessed the percentage decrease in HB-levels as compared to baseline values in this patient group. Median decrease in HB-level as compared to baseline values was 25% (range 13–72%).
Overall survival
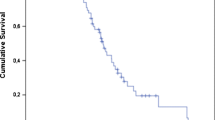
Overall survival of the whole patient population is demonstrated in Fig. 4. Estimated median overall survival for the entire patient population was 31 months (CI 12.8–49.2 months). In univariate analysis, both the age (relatively younger patients lived more) (p= 0.045) and PSA decline (PSA decline > 50%) dichotomized according the median proved to be significantly associated with a favorable OS (p= 0.001) (see Table 2). In multivariate analysis, only PSA decline proved statistical relevant (p=0.003). Median estimated OS for patients with a PSA decline smaller than the median was 11 months (CI 8.1–13.8 months) whereas the median OS of those patients with a PSA decline > the median PSA decline was not yet reached at the date of latest follow-up (36 months).
Progression-free survival
PSA decline proved the only significantly variable in univariate (p=0.013) as well as in multivariate analysis. PFS for patients with a PSA decline > the median was not yet reached at the time of latest follow-up whereas that for patients with a PSA decline < the median was 9.0 months (CI 1.8–16.2 months).
Discussion
A prospective pilot study with 177Lu-PSMA appeared to be a feasible and safe treatment modality in ten patients with low-volume mHSPC in seeking patient alternative therapies. Similar studies have never been done with 225Ac-PSMA [23]. Hence, our retrospective study is the first one to evaluate the preliminary clinical experience using 225Ac-PSMA. In line with previous reports on 225Ac-PSMA-617 treatment, in the series presented, 86% of mHSPC patients (18/21) had a ≥ 50% PSA reduction of their initial PSA-value following 225Ac-PSMA-617 treatment and both baseline PSA values and alkaline phosphatase levels proved unrelated to the percentage PSA reduction confirming the high level of radiobiological effectiveness of 225Ac, related to its 100keV/micron LET, obviating the need of cellular oxygenation for achieving cell death [24, 25].
In the series presented, both the degree of PSA reduction and advanced age at the time of diagnosis proved a significant predictor for overall survival in univariate analysis. An advanced age at diagnosis has been previously linked to a higher likelihood of presenting with de novo metastatic prostate carcinoma and worse prostate cancer-specific survival [26, 27]. In a study by Scosyrev et al. on data from 464,918 patients diagnosed with prostate carcinoma between 1998 and 2007, the authors found that when compared to younger patients (aged < 75 years), older patients were more likely to present with advanced disease and have a greater risk of death from prostate carcinoma despite higher death rates from competing causes and to contribute to more than half of all prostate carcinoma related deaths [28]. Potential causes for more aggressive disease in elderly patients reported in literature include less PSA screening and higher rates of hypogonadism in older men leading to the emergence of cancer less driven by androgens with a more castrate-resistant profile [26, 29, 30]. Low serum levels of testosterone as found in older men have also been previously associated with a higher Gleason score on prostatectomy biopsies [31, 32]. In the series presented, age proved unrelated to the Gleason score of the primary tumor as well as to the baseline PSA levels, reflecting disease extent. However, response to treatment as assessed by the percentage reduction in PSA did prove significantly lower at a higher age and unrelated to the number of treatment cycles administered in favor of a more aggressive character of the underlying prostate carcinoma in these patients.
In multivariate analysis only, the reduction in PSA levels following treatment administration proved predictive for OS as well as the only predictive factor in univariate and multivariate analysis for progression free survival. Given that only 3 patients demonstrated a PSA decline inferior to 50% (cut-off recommended by the PCWG3 group), we used the median of the percentage change in PSA, respectively −98%, to create two equal-sized groups for statistical comparison using the Kaplan-Meier analysis and the log-rank test as customary when applying this test. Using this cut-off, median estimated OS and PFS were respectively 11 months (CI 8.1–13.8 months) and 9.0 months (CI 1.8–16.2 months) for the group with a response inferior to à 98% reduction, whereas for the group that showed a reduction superior or equal to a 98% decrease in PSA levels, median OS and PFS were not yet reached at the time of latest follow-up, respectively 36 months.
Results obtained in this series are difficult to compare directly to those studies that have defined the current standard of care for treatment of de novo mHSPC given the absence of a direct comparison to the current standard of care in our study on the one hand and the specific inclusion of M1b (± M1c) patients included in this trial on the other hand. With the exception of the LATITUDE trial which compared ADT + placebo to ADT + abiraterone acetate (AA) and prednisone (P) in de novo M1b and or M1c prostate carcinoma, all other studies either comparing ADT to ADT +docetaxel (STAMPEDE, CETUG, and CHAARTED trial), ADT to ADT+AA+P (STAMPEDE arm G trial), ADT ± docetaxel to ADT + enzalutimide ± docetexal (ENZAMET trial), and ADT to ADT + apalutamide (TITAN trial) included a mix of patients with the range of newly diagnosed stage M1 prostate carcinoma patients included varying from 48 (STAMPEDE trial) to 78% (TITAN trial) [33,34,35,36,37,38,39]. In the LATITUDE trial, including a similar subset of patients as the ones included in our study, 3-year OS was 66% for the arm receiving ADT+AA+P versus 49% in the control arm (ADT + placebo).
When considering the entire patient cohort in our study, approximately 50% of the patients were still alive at 32 months follow-up (see Fig. 4) suggesting that 225Ac-PSMA-617 treatment may be more or less as efficient when compared to ADT alone but less efficient when compared to ADT+AA+P. However, our study included only a small number of patients and results of randomized clinical trials, including well-selected patients, do not necessarily translate in real-world survival improvement of de novo metastatic prostate carcinoma patients. In this regard, Helgstrand et al. analyzed the incidence and mortality data of patients with de novo metastatic prostate carcinoma included in the SEER database and in the Danish Prostate Cancer Registry. In patients diagnosed between 2000 and 2009, the median OS was 22 months in SEER and 30 months in the Danish registry [40]. More recently, Cattrini et al. analyzed survival data from more than 26,000 patients included in the SEER database. These authors found a survival gain of only 4 months between patients diagnosed in 2011–2014 versus those diagnosed in 2000–2003 and 2004–2010; docetaxel which was approved by the FDA for treatment of mCRPC in 2004, and ARSi which was approved from 2011 onwards [41]. Thus, it appears that overall, our results obtained are in line with those derived from real-world survival analysis. Factors potentially contributing to the discrepant results between real-world results and those derived from randomized trials include patients’ ineligibility or refusal of anticancer treatments (as was the case in the series presented), insurance issues, intrinsic disease aggressiveness, or prior unavailability of drugs in a hormone-sensitive setting (enzalutamide and abiraterone acetate are not readily reimbursed in low-mid–income countries).
In terms to of the safety profile, salivary gland toxicity was noted in majority of our patients (94%), which is slightly more than we reported previously [9, 10, 14], and thus a call for concern to find the solution for xerostomia especially if this treatment is to be considered in the de novo mHSPC cohort. Xerostomia remains a challenge and limiting factor for 225Ac-PSMA-; hence, some approaches continue to be investigated, excluding the suggested dose deescalating scheme from our group. Several workers have investigated the concept of co-administering lower activities 225Ac-PSMA-617 (mean 5.3 MBq) and 177Lu-PSMA-617 (mean 6.9 GBq) in mCRPC patients and found no grade 3 xerostomia and no treatment discontinuation was observed [42,43,44,45]. Another strategy is based on the fact that there might be a severe duct stenosis or obstruction; the study from Rathke et al. have showed a response to sialendoscopic duct dilatation and saline irrigation [46]. Other approaches that have been implemented in some clinics include external cooling [47, 48], orally administered monosodium glutamate [49, 50], and high-dose botulinum toxin injections [51], with preliminary encouraging data on the effectiveness. The hematologic and renal profiles of patients remained safe for the brief period of follow-up as none of the patients demonstrated grade 3/4 hematotoxicities or renal toxicities, similar to our recent report [15].
Unfortunately, there are several limitations to this study. It is a retrospective investigation with a small sample size and related statistical limitations. Additionally, no dosimetry has been performed. Furthermore, a limited worldwide production of 225Ac in general may limit its use in research and subsequently in the clinical practice.
Available data suggest that the incidence of de novo mHSPC is likely to increase in the course of the following years related to the recent trend towards less PSA screening. Because of their higher age and related comorbidities, de novo mHSPC can in some selected cases not receive chemotherapy or do not tolerate ADT± AA well. Accordingly, there is a need for more tolerable and efficacious treatment options of these patients. As suggested by our preliminary findings, 225Ac-PSMA-617 may just be such a treatment. Studies in larger patient populations confirming our results as well as multicenter randomized trials comparing patient outcome following 225Ac-PSMA-617 treatment to that obtained using standard of care treatment in mHSPC are mandatory.
Availability of data and material
All data collected during the conduct of this study are included in this report.
References
Siegel R, Miller K, Fuchs H, Jemal A. Cancer Statistics, 2022. CA: Cancer J Clin. 2022;72:7–33.
Parker C, Castro K, Fizazi A, Heidenreich A, Ost P, Rocopio G, Tombal B, Gillessen S. Clinical practice guidelines- prostate cancer. Ann Oncol. 2020;9:1119–34.
National Cancer Institute. Cancer St facts: prostate cancer. Assessed September 25, 2018. https//seer.cancer.gov/statfacts/html/prost.html.
Parker C, James D, Brawley C, Clarke N, Hoyle A, Ali A, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392:2353–66.
Boeve L, Hulshof M, Vis N, Zwinderman A, Twisk J, Witjes W, et al. Effect on survival of androgen deprivation therapy combined with concurrent radiation therapy to the prostate in patients with primary bone metastatic prostate cancer in a prospective randomised clinical trial: data from the HORRAD trial. Eur Urol. 2019;75:410–8.
Kratochwil C, Bruchertseifer F, Rathke H, Hohenfellner M, Giesel FL, Haberkorn U, Morgenstern A. Targeted alpha therapy of mCRPC with 225Actinium-PSMA-617: swimmer-plot analysis suggest efficacy regarding duration of tumor-control. J Nucl Med. 2018;59:795–802.
Kratochwil C, Bruchertseifer F, Rathke H, Bronzel M, Apostolidis C, Weichert W, et al. Targeted alpha therapy of mCRPC with 225Actinium-PSMA-617: dosimetry estimate and empirical dose finding. J Nucl Med. 2017;58:1624–31.
Sathekge M, Bruchertseifer F, Vorster M, Lawal I, Knoesen O, Mahapane J, et al. Predictors of overall and disease-free survival in metastatic castration-resistant prostate cancer patients receiving 225Ac-PSMA-617 radioligand therapy. J Nucl Med. 2020;61:62–9.
Sathekge M, Bruchertseifer F, Knoesen O, Reyneke F, Lawal I, Lengana T, et al. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: a pilot study. Eur. J. Nucl. Med. Mol. Imaging. 2019;46:129–38.
Yadav MP, Ballal S, Sahoo RK, Tripathi M, Seth A, Bal C. Efficacy and Safety of 225Ac-PSMA-617 targeted alpha therapy in metastatic castration-resistant prostate cancer patients. Theranostics. 2020;23:9364–77.
Feuerecker B, Tauber R, Knorr K, Heck M, Beheshti A, Seidl C, et al. Activity and adverse events of actinium-225-PSMA-617 in advanced metastatic castration-resistant prostate cancer after failure of lutetium-177-PSMA. Eur Urol. 2021;79:343–50.
Zacherl MJ, Gildehaus FJ, Mittlmeier L, Böning G, Gosewisch A, Wenter V, et al. First clinical results for PSMA-targeted α-therapy using 225Ac-PSMA-I&T in advanced-mCRPC patients. J Nucl Med. 2021;62:669–74.
Sathekge M, Bruchertseifer F, Vorster M, Lawal I, Knoesen O, Mahapane J, et al. mCRPC patients receiving 222Ac-PSMA-617 therapy in the post androgen deprivation therapy setting: response to treatment and survival analysis. J Nucl Med. 2022;63:1496–502.
Lawal IO, Morgenstern A, Vorster M, Knoesen O, Mahapane J, Hlongwa KN, et al. Hematologic toxicity profile and efficacy of [225Ac]Ac-PSMA-617 α-radioligand therapy of patients with extensive skeletal metastases of castration-resistant prostate cancer. Eur. J. Nuc.l Med. Mol. Imaging. 2022;49:3581–92.
Filippi L, Chiaravalloti A, Schillaci O, Bagni O. The potential of PSMA-targeted alpha therapy in the management of prostate cancer. Expert Rev Anticancer Ther. 2020;20:823–9.
Gaertner FC, Halabi K, Ahmadzadehfar H, Kürpig S, Eppard E, Kotsikopoulos C, et al. Uptake of PSMA-ligands in normal tissues is dependent on tumor load in patients with prostate cancer. Oncotarget. 2017;8:55094–103.
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016;34:1402–18.
Gafita A, Marcus C, Kostos L, Schuster DM, Calais J, Hofman MS. Predictors and real-world use of prostate-specific radioligand therapy: PSMA and beyond. Am Soc Clin Oncol Educ Book. 2022;42:1–17.
Eiber M, Herrmann K, Calais J, Hadaschik B, Giesel FL, Hartenbach M, et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med. 2018;59:469–78.
Calais J, Czernin J. PSMA expression assessed by PET imaging is a required biomarker for selecting patients for any PSMA-targeted therapy. J Nucl Med. 2021;62:1489–91.
Thang SP, Violet J, Sandhu S, Iravani A, Akhurst T, Kong G, et al. Poor outcomes for patients with metastatic castration-resistant prostate cancer with low prostate-specific membrane antigen (PSMA) expression deemed ineligible for 177Lu-labelled PSMA radioligand therapy. Eur Urol Oncol. 2019;2:670–6.
Privé BM, Peters SMB, Muselaers CHJ, van Oort IM, Janssen MJR, Sedelaar JPM, et al. Lutetium-177-PSMA-617 in low-volume hormone-sensitive metastatic prostate cancer: a prospective pilot study. Clin Cancer Res. 2021;27:3595–601.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics. CA Cancer J Clin. 2022;72:7–33.
Wulbrand C, Seidl C, Gaertner FC, Bruchertseifer F, Morgenstern A, Essler M, et al. Alpha- particle emitting 213Bi-anti-EGFR immuno-conjugates eradicate tumor cells independent of oxygenation. PLOS One. 2013;8:e64730.
Bernard B, Burnett C, Sweeney C, Rider J, Sridhar S. Impact of age at diagnosis of de novo metastatic prostate cancer on survival. Cancer. 2020;126:986–93.
Humphreys M, Fernandes K, Sridhar S. Impact of age at diagnosis on outcomes in men with castrate-resistant prostate cancer (CRPC). J Cancer. 2013;4:304–14.
Scosyrev E, Messing E, Mohile S, Golijanin D, Wu G. Prostate cancer in the elderly: frequency of advanced disease at presentation and disease-specific mortality. Cancer. 2012;118:3062–70.
San Francisco F, Rojas P, De Wolf W, Morgentaler A. Low free testosterone levels predict disease reclassification in men with prostate cancer undergoing active surveillance. BJU Int. 2014;114:229–35.
Chan Y, Knuiman M, Divitini M, Handelsman D, Beilby J, Yeap B. Lower circulating androgens are associated with overall cancer risk and prostate cancer risk in men aged 25-84 years from the Buselton Health Study. Horm Cancer. 2018;9:391–8.
Pichon A, Neuzillet Y, Botto H, Raynaud JP, Radulescu C, Molinié V, et al. Preoperative low serum testosterone is associated with high-grade prostate cancer and an increased Gleason score upgrading. Prostate Cancer Prostatic Dis. 2015;18:382–7.
Muralidhar V, Ziehr DR, Mahal BA, Chen YW, Nezolosky MD, Viswanathan VB, et al. Association between older age and increasing Gleason score. Clin Genitourin Cancer. 2015;13:525–30.
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin AB. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomized, double-blind, phase 3 trial. The. Lancet Oncol. 2019;20:686–700.
James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Eng J Med. 2017;377:338–51.
Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemo-hormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–46.
James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, STAMPEDE investigators, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–77.
Gravis G, Fizazi K, Joly F, Oudard S, Priou F, Esterni B, et al. Androgen deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU15): a randomized, open-label, phase 3 trial. Lancet Oncol. 2013;14:149–58.
Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381:121–31.
Chi KN, Chowdhury S, Bjartell A, Chung BH, de Santana P, Gomes AJ, Given R, et al. Apalutamide in Patients With Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J Clin Oncol. 2021;39:2294–303.
Helgstrand JT, Røder MA, Klemann N, Toft BG, Lichtensztajn DY, Brooks JD, et al. Trends in incidence and 5-year mortality in men with newly diagnosed, metastatic prostate cancer. A population-based analysis of 2 national cohorts. Cancer. 2018;124:2931–8.
Cattrini C, Soldato D, Rubagotti A, Zinoli L, Znaradi E, Barboro P, et al. Epidemiological characteristics and survival in patients with de novo metastatic prostate cancer. Cancer. 2020;12:2855. https://doi.org/10.3390/cancers12102855.
Khreish F, Ebert N, Ries M, Maus S, Rosar F, Bohnenberger H, et al. 225Ac-PSMA-617/177Lu-PSMA-617 tandem therapy of metastatic castration-resistant prostate cancer: pilot experience. Eur J Nucl Med Mol Imaging. 2020;47:721–8.
Rosar F, Hau F, Bartholomä M, Maus S, Stemler T, Linxweiler J, et al. Molecular imaging and biochemical response assessment after a single cycle of [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy in mCRPC patients who have progressed on [177Lu]Lu-PSMA-617 monotherapy. Theranostics. 2021;11:4050–60.
Ling SW, de Blois E, Hooijman E, van der Veldt A, Brabander T. Advances in 177Lu-PSMA and 225Ac-PSMA radionuclide therapy for metastatic castration-resistant prostate cancer. Pharmaceutics. 2022;14:2166. https://doi.org/10.3390/pharmaceutics14102166.
Langbein T, Kulkarni HR, Schuchardt C, Mueller D, Volk GF, Baum RP. Salivary gland toxicity of PSMA-targeted radioligand therapy with 177Lu-PSMA and combined 225Ac- and 177Lu-labeled PSMA ligands (TANDEM-PRLT) in advanced prostate cancer: a single-center systematic investigation. Diagnostics (Basel). 2022;12(8):1926. https://doi.org/10.3390/diagnostics12081926.
Rathke H, Kratochwil C, Hohenberger R, Giesel F.L, Bruchertseifer F, Flechsig P, et al. Initial clinical experience performing sialendoscopy for salivary gland protection in patients undergoing 225Ac-PSMA-617 RLT. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 139–147.
Yilmaz B, Nisli S, Ergul N, Gursu R.U, Acikgoz O, Cermik, T.F. Effect of external cooling on Lu-177 PSMA uptake for parotid glands. J. Nucl. Med 2019, 60, 1388–1393.
Van Kalmthout L.W.M, Lam M.G.E.H, de Keizer B, Krijger G.C, Ververs, T.F.T, De Roos R, Braat A.J.A.T. Impact of external cooling with icepacks on 68Ga-PSMA uptake in salivary glands. EJNMMI Res 2018, 8, 56.
Rousseau E, Lau J, Kuo H.T, Zhang Z, Merken H, Hundal-Jabal N, et al. Monosodium glutamate reduces 68Ga-PSMA-11 uptake in salivary glands and kidneys in a preclinical prostate cancer model. J. Nucl. Med 2018, 59, 1865–1868.
Harsini S, Saprunoff H, Alden T.M, Mohammadi B, Wilson D, Benard F. The effects of monosodium glutamate on PSMA radiotracer uptake in men with recurrent prostate cancer: a prospective, randomized, double-blind, placebo-controlled intraindividual imaging study. J. Nucl. Med 2020, 62, 81–87.
Baum R.P, Langbein T, Singh A, Shahinfar M, Schuchardt C, Volk G.F, Kulkarni H. Injection of botulinum toxin for preventing salivary gland toxicity after PSMA radioligand therapy: an empirical proof of a promising concept. Nucl. Med. Mol. Imaging 2018, 52, 80–81.
Funding
Open access funding provided by University of Pretoria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was performed per the ethical standard of our institutions and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
All patients gave written informed consent to participate in the study.
Consent for publication
All patients provided informed consent to allow for the publication of their anonymized data collected during this study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Theragnostic.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sathekge, M., Bruchertseifer, F., Vorster, M. et al. 225Ac-PSMA-617 radioligand therapy of de novo metastatic hormone-sensitive prostate carcinoma (mHSPC): preliminary clinical findings. Eur J Nucl Med Mol Imaging 50, 2210–2218 (2023). https://doi.org/10.1007/s00259-023-06165-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06165-9