Abstract
Purpose
To develop and validate the predictive value of an 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) model for breast cancer neoadjuvant chemotherapy (NAC) efficacy based on the tumor-to-liver ratio (TLR) radiomic features and multiple data pre-processing methods.
Methods
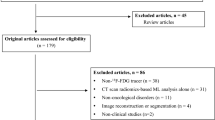
One hundred and ninety-three breast cancer patients from multiple centers were retrospectively included in this study. According to the endpoint of NAC, we divided the patients into pathological complete remission (pCR) and non-pCR groups. All patients underwent 18F-FDG PET/CT imaging before NAC treatment, and CT and PET images volume of interest (VOI) segmentation by manual segmentation and semi-automated absolute threshold segmentation, respectively. Then, feature extraction of VOI was performed with the pyradiomics package. A total of 630 models were created based on the source of radiomic features, the elimination of the batch effect approach, and the discretization method. The differences in data pre-processing approaches were compared and analyzed to identify the best-performing model, which was further tested by the permutation test.
Results
A variety of data pre-processing methods contributed in varying degrees to the improvement of model effects. Among them, TLR radiomic features and Combat and Limma methods that eliminate batch effects could enhance the model prediction overall, and data discretization could be used as a potential method that can further optimize the model. A total of seven excellent models were selected and then based on the AUC of each model in the four test sets and their standard deviations, we selected the optimal model. The optimal model predicted AUC between 0.7 and 0.77 for the four test groups, with p-values less than 0.05 for the permutation test.
Conclusion
It is necessary to enhance the predictive effect of the model by eliminating confounding factors through data pre-processing. The model developed in this way is effective in predicting the efficacy of NAC for breast cancer.






Similar content being viewed by others
Data availability
The data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. https://doi.org/10.3322/caac.21492.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. https://doi.org/10.3322/caac.21654.
Antunovic L, De Sanctis R, Cozzi L, Kirienko M, Sagona A, Torrisi R, et al. PET/CT radiomics in breast cancer: promising tool for prediction of pathological response to neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2019;46:1468–77. https://doi.org/10.1007/s00259-019-04313-8.
Mao N, Shi Y, Lian C, Wang Z, Zhang K, Xie H, et al. Intratumoral and peritumoral radiomics for preoperative prediction of neoadjuvant chemotherapy effect in breast cancer based on contrast-enhanced spectral mammography. Eur Radiol. 2022;32:3207–19. https://doi.org/10.1007/s00330-021-08414-7.
Jiang M, Li CL, Luo XM, Chuan ZR, Lv WZ, Li X, et al. Ultrasound-based deep learning radiomics in the assessment of pathological complete response to neoadjuvant chemotherapy in locally advanced breast cancer. Eur J Cancer. 2021;147:95–105. https://doi.org/10.1016/j.ejca.2021.01.028.
Liu Z, Li Z, Qu J, Zhang R, Zhou X, Li L, et al. Radiomics of multiparametric MRI for pretreatment prediction of pathologic complete response to neoadjuvant chemotherapy in breast cancer: a multicenter study. Clin Cancer Res. 2019;25:3538–47. https://doi.org/10.1158/1078-0432.CCR-18-3190.
Montemezzi S, Benetti G, Bisighin MV, Camera L, Zerbato C, Caumo F, et al. 3T DCE-MRI radiomics improves predictive models of complete response to neoadjuvant chemotherapy in breast cancer. Front Oncol. 2021;11:630780. https://doi.org/10.3389/fonc.2021.630780.
Krajnc D, Papp L, Nakuz TS, Magometschnigg HF, Grahovac M, Spielvogel CP, et al. Breast tumor characterization using [(18)F]FDG-PET/CT imaging combined with data preprocessing and radiomics. Cancers (Basel). 2021; 13. https://doi.org/10.3390/cancers13061249.
Fonti R, Conson M, Del Vecchio S. PET/CT in radiation oncology. Semin Oncol. 2019;46:202–9. https://doi.org/10.1053/j.seminoncol.2019.07.001.
Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, et al. NCCN Guidelines® insights: breast cancer, version 4.2021. J Natl Compr Cancer Netw JNCCN. 2021;19:484–93. https://doi.org/10.6004/jnccn.2021.0023.
Sun XY, Chen TX, Chang C, Teng HH, Xie C, Ruan MM, et al. SUVmax of (18)FDG PET/CT predicts histological grade of lung adenocarcinoma. Acad Radiol. 2021;28:49–57. https://doi.org/10.1016/j.acra.2020.01.030.
Lim JH, Choi JY, Im Y, Yoo H, Jhun BW, Jeong BH, et al. Prognostic value of SUVmax on 18F-fluorodeoxyglucose PET/CT scan in patients with malignant pleural mesothelioma. PLoS One. 2020;15:e0229299. https://doi.org/10.1371/journal.pone.0229299.
O’Rourke C, Welaratne I, Cournane S, McLoughlin LC, Reynolds JV, Johnston C, et al. Diagnostic accuracy of SUVmax in predicting malignancy of supraclavicular lymph nodes from primary oesophageal cancer. Eur J Radiol. 2020;125:108860. https://doi.org/10.1016/j.ejrad.2020.108860.
Onner H, Canaz F, Dincer M, Isiksoy S, Sivrikoz IA, Entok E, et al. Which of the fluorine-18 fluorodeoxyglucose positron emission tomography/computerized tomography parameters are better associated with prognostic factors in breast cancer? Medicine (Baltimore). 2019;98:15925. https://doi.org/10.1097/MD.0000000000015925.
Wang C, Zhao K, Hu S, Huang Y, Ma L, Li M, et al. The PET-derived tumor-to-liver standard uptake ratio (SUV TLR) is superior to tumor SUVmax in predicting tumor response and survival after chemoradiotherapy in patients with locally advanced esophageal cancer. Front Oncol. 2020;10:1630. https://doi.org/10.3389/fonc.2020.01630.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S-S150. https://doi.org/10.2967/jnumed.108.057307.
Huang J, Huang L, Zhou J, Duan Y, Zhang Z, Wang X, et al. Elevated tumor-to-liver uptake ratio (TLR) from (18)F-FDG-PET/CT predicts poor prognosis in stage IIA colorectal cancer following curative resection. Eur J Nucl Med Mol Imaging. 2017;44:1958–68. https://doi.org/10.1007/s00259-017-3779-0.
Mayerhoefer ME, Materka A, Langs G, Haggstrom I, Szczypinski P, Gibbs P, et al. Introduction to radiomics. J Nucl Med. 2020;61:488–95. https://doi.org/10.2967/jnumed.118.222893.
Manafi-Farid R, Askari E, Shiri I, Pirich C, Asadi M, Khateri M, et al. [(18)F]FDG-PET/CT radiomics and artificial intelligence in lung cancer: technical aspects and potential clinical applications. Semin Nucl Med. 2022;52:759–80. https://doi.org/10.1053/j.semnuclmed.2022.04.004.
Anan N, Zainon R, Tamal M. A review on advances in (18)F-FDG PET/CT radiomics standardisation and application in lung disease management. Insights Imaging. 2022;13:22. https://doi.org/10.1186/s13244-021-01153-9.
Jiang H, Li A, Ji Z, Tian M, Zhang H. Role of radiomics-based baseline PET/CT imaging in lymphoma: diagnosis, prognosis, and response assessment. Mol Imag Biol. 2022;24:537–49. https://doi.org/10.1007/s11307-022-01703-7.
Ha S, Park S, Bang JI, Kim EK, Lee HY. Metabolic radiomics for pretreatment (18)F-FDG PET/CT to characterize locally advanced breast cancer: histopathologic characteristics, response to neoadjuvant chemotherapy, and prognosis. Sci Rep. 2017;7:1556. https://doi.org/10.1038/s41598-017-01524-7.
Shiri I, Rahmim A, Ghaffarian P, Geramifar P, Abdollahi H, Bitarafan-Rajabi A. The impact of image reconstruction settings on 18F-FDG PET radiomic features: multi-scanner phantom and patient studies. Eur Radiol. 2017;27:4498–509. https://doi.org/10.1007/s00330-017-4859-z.
Shafiq-Ul-Hassan M, Zhang GG, Latifi K, Ullah G, Hunt DC, Balagurunathan Y, et al. Intrinsic dependencies of CT radiomic features on voxel size and number of gray levels. Med Phys. 2017;44:1050–62. https://doi.org/10.1002/mp.12123.
Leijenaar RT, Nalbantov G, Carvalho S, van Elmpt WJ, Troost EG, Boellaard R, et al. The effect of SUV discretization in quantitative FDG-PET Radiomics: the need for standardized methodology in tumor texture analysis. Sci Rep. 2015;5:11075. https://doi.org/10.1038/srep11075.
Whybra P, Parkinson C, Foley K, Staffurth J, Spezi E. Assessing radiomic feature robustness to interpolation in (18)F-FDG PET imaging. Sci Rep. 2019;9:9649. https://doi.org/10.1038/s41598-019-46030-0.
Ferreira M, Lovinfosse P, Hermesse J, Decuypere M, Rousseau C, Lucia F, et al. [(18)F]FDG PET radiomics to predict disease-free survival in cervical cancer: a multi-scanner/center study with external validation. Eur J Nucl Med Mol Imaging. 2021;48:3432–43. https://doi.org/10.1007/s00259-021-05303-5.
Clark K, Vendt B, Smith K, Freymann J, Kirby J, Koppel P, et al. The Cancer Imaging Archive (TCIA): maintaining and operating a public information repository. J Digit Imaging. 2013;26:1045–57. https://doi.org/10.1007/s10278-013-9622-7.
Yin G, Song Y, Li X, Zhu L, Su Q, Dai D, et al. Prediction of mediastinal lymph node metastasis based on (18)F-FDG PET/CT imaging using support vector machine in non-small cell lung cancer. Eur Radiol. 2021;31:3983–92. https://doi.org/10.1007/s00330-020-07466-5.
Kim J, Yoo SW, Kang SR, Cho SG, Oh JR, Chong A, et al. Prognostic significance of metabolic tumor volume measured by (18)F-FDG PET/CT in operable primary breast cancer. Nucl Med Mol Imaging. 2012;46:278–85. https://doi.org/10.1007/s13139-012-0161-9.
Chen K, Yin G, Xu W. Predictive value of (18)F-FDG PET/CT-based radiomics model for occult axillary lymph node metastasis in clinically node-negative breast cancer. Diagnostics (Basel). 2022;12. https://doi.org/10.3390/diagnostics12040997.
Haider SP, Mahajan A, Zeevi T, Baumeister P, Reichel C, Sharaf K, et al. PET/CT radiomics signature of human papilloma virus association in oropharyngeal squamous cell carcinoma. Eur J Nucl Med Mol Imaging. 2020;47:2978–91. https://doi.org/10.1007/s00259-020-04839-2.
Mayerhoefer ME, Riedl CC, Kumar A, Dogan A, Gibbs P, Weber M, et al. [18F]FDG-PET/CT radiomics for prediction of bone marrow involvement in mantle cell lymphoma: a retrospective study in 97 patients. Cancers (Basel). 2020;12. https://doi.org/10.3390/cancers12051138.
Forouzannezhad P, Maes D, Hippe DS, Thammasorn P, Iranzad R, Han J, et al. Multitask learning radiomics on longitudinal imaging to predict survival outcomes following risk-adaptive chemoradiation for non-small cell lung cancer. Cancers (Basel). 2022;14. https://doi.org/10.3390/cancers14051228.
Peng H, Dong D, Fang MJ, Li L, Tang LL, Chen L, et al. Prognostic value of deep learning PET/CT-based radiomics: potential role for future individual induction chemotherapy in advanced nasopharyngeal carcinoma. Clin Cancer Res. 2019;25:4271–9. https://doi.org/10.1158/1078-0432.CCR-18-3065.
Mu W, Jiang L, Shi Y, Tunali I, Gray JE, Katsoulakis E, et al. Non-invasive measurement of PD-L1 status and prediction of immunotherapy response using deep learning of PET/CT images. J Immunother Cancer. 2021;9. https://doi.org/10.1136/jitc-2020-002118.
Montemurro F, Nuzzolese I, Ponzone R. Neoadjuvant or adjuvant chemotherapy in early breast cancer? Expert Opin Pharmacother. 2020;21:1071–82. https://doi.org/10.1080/14656566.2020.1746273.
See SHC, Siziopikou KP. Pathologic evaluation of specimens after neoadjuvant chemotherapy in breast cancer: current recommendations and challenges. Pathol Res Pract. 2022;230:153753. https://doi.org/10.1016/j.prp.2021.153753.
Qi TH, Hian OH, Kumaran AM, Tan TJ, Cong TRY, Su-Xin GL, et al. Multi-center evaluation of artificial intelligent imaging and clinical models for predicting neoadjuvant chemotherapy response in breast cancer. Breast Cancer Res Treat. 2022;193:121–38. https://doi.org/10.1007/s10549-022-06521-7.
Eshaghzadeh Torbati M, Minhas DS, Ahmad G, O’Connor EE, Muschelli J, Laymon CM, et al. A multi-scanner neuroimaging data harmonization using RAVEL and ComBat. NeuroImage. 2021;245:118703. https://doi.org/10.1016/j.neuroimage.2021.118703.
Bell TK, Godfrey KJ, Ware AL, Yeates KO, Harris AD. Harmonization of multi-site MRS data with ComBat. NeuroImage. 2022;257:119330. https://doi.org/10.1016/j.neuroimage.2022.119330.
van Timmeren JE, Carvalho S, Leijenaar RTH, Troost EGC, van Elmpt W, de Ruysscher D, et al. Challenges and caveats of a multi-center retrospective radiomics study: an example of early treatment response assessment for NSCLC patients using FDG-PET/CT radiomics. PLoS One. 2019;14:e0217536. https://doi.org/10.1371/journal.pone.0217536.
Orlhac F, Frouin F, Nioche C, Ayache N, Buvat I. Validation of a method to compensate multicenter effects affecting CT radiomics. Radiology. 2019;291:53–9. https://doi.org/10.1148/radiol.2019182023.
Orlhac F, Boughdad S, Philippe C, Stalla-Bourdillon H, Nioche C, Champion L, et al. A postreconstruction harmonization method for multicenter radiomic studies in PET. J Nucl Med. 2018;59:1321–8. https://doi.org/10.2967/jnumed.117.199935.
Mahon RN, Ghita M, Hugo GD, Weiss E. ComBat harmonization for radiomic features in independent phantom and lung cancer patient computed tomography datasets. Phys Med Biol. 2020;65:015010. https://doi.org/10.1088/1361-6560/ab6177.
Garcia-Dias R, Scarpazza C, Baecker L, Vieira S, Pinaya WHL, Corvin A, et al. Neuroharmony: a new tool for harmonizing volumetric MRI data from unseen scanners. NeuroImage. 2020;220:117127. https://doi.org/10.1016/j.neuroimage.2020.117127.
Pfaehler E, Beukinga RJ, de Jong JR, Slart R, Slump CH, Dierckx R, et al. Repeatability of (18) F-FDG PET radiomic features: a phantom study to explore sensitivity to image reconstruction settings, noise, and delineation method. Med Phys. 2019;46:665–78. https://doi.org/10.1002/mp.13322.
Desseroit MC, Tixier F, Weber WA, Siegel BA, Cheze Le Rest C, Visvikis D, et al. Reliability of PET/CT shape and heterogeneity features in functional and morphologic components of non-small cell lung cancer tumors: a repeatability analysis in a prospective multicenter cohort. J Nucl Med. 2017;58:406–11. https://doi.org/10.2967/jnumed.116.180919.
Pfaehler E, van Sluis J, Merema BBJ, van Ooijen P, Berendsen RCM, van Velden FHP, et al. Experimental multicenter and multivendor evaluation of the performance of PET radiomic features using 3-dimensionally printed phantom inserts. J Nucl Med. 2020;61:469–76. https://doi.org/10.2967/jnumed.119.229724.
Song B-I. A machine learning-based radiomics model for the prediction of axillary lymph-node metastasis in breast cancer. Breast Cancer. 2021;28:664–71. https://doi.org/10.1007/s12282-020-01202-z.
Chang C, Zhou S, Yu H, Zhao W, Ge Y, Duan S, et al. A clinically practical radiomics-clinical combined model based on PET/CT data and nomogram predicts EGFR mutation in lung adenocarcinoma. Eur Radiol. 2021;31:6259–68. https://doi.org/10.1007/s00330-020-07676-x.
Eertink JJ, Zwezerijnen GJC, Cysouw MCF, Wiegers SE, Pfaehler EAG, Lugtenburg PJ, et al. Comparing lesion and feature selections to predict progression in newly diagnosed DLBCL patients with FDG PET/CT radiomics features. Eur J Nucl Med Mol Imaging. 2022;49:4642–51. https://doi.org/10.1007/s00259-022-05916-4.
Eertink JJ, van de Brug T, Wiegers SE, Zwezerijnen GJC, Pfaehler EAG, Lugtenburg PJ, et al. (18)F-FDG PET baseline radiomics features improve the prediction of treatment outcome in diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging. 2022;49:932–42. https://doi.org/10.1007/s00259-021-05480-3.
Hwang HW, Jung H, Hyeon J, Park YH, Ahn JS, Im YH, et al. A nomogram to predict pathologic complete response (pCR) and the value of tumor-infiltrating lymphocytes (TILs) for prediction of response to neoadjuvant chemotherapy (NAC) in breast cancer patients. Breast Cancer Res Treat. 2019;173:255–66. https://doi.org/10.1007/s10549-018-4981-x.
Akimoto E, Kadoya T, Kajitani K, Emi A, Shigematsu H, Ohara M, et al. Role of (18)F-PET/CT in predicting prognosis of patients with breast cancer after neoadjuvant chemotherapy. Clin Breast Cancer. 2018;18:45–52. https://doi.org/10.1016/j.clbc.2017.09.006.
Acknowledgements
We gratefully appreciate the award that supported collection and sharing of TCIA dataset (U01 CA142565, PI Thomas E. Yankeelov).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation were performed by Kun Chen, Jian Wang, and Shuai Li; data collection and analysis were performed by Kun Chen and Jian Wang. The first draft of the manuscript was written by Kun Chen, and the manuscript was reviewed and edited by Jian Wang, Wengui Xu, and Wen Zhou. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures in studies involving human participants were conducted by the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Ethics Committee of the Tianjin Medical University Cancer Institute and Hospital approved this study (registration number bc2022176; 12 July 2022).
Consent to participate and publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kun Chen and Jian Wang contributed equally to this work.
This article is part of the Topical Collection on Advanced Image Analyses (Radiomics and Artificial Intelligence).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, K., Wang, J., Li, S. et al. Predictive value of 18F-FDG PET/CT-based radiomics model for neoadjuvant chemotherapy efficacy in breast cancer: a multi-scanner/center study with external validation. Eur J Nucl Med Mol Imaging 50, 1869–1880 (2023). https://doi.org/10.1007/s00259-023-06150-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06150-2