Abstract
Purpose
Positron emission tomography (PET) with O-(2-[18F]fluoroethyl)-L-tyrosine ([18F]FET) is a well-established tool for non-invasive assessment of adult central nervous system (CNS) tumors. However, data on its diagnostic utility and impact on clinical management in children and adolescents are limited.
Methods
Twenty-one children and young adults (13 males; mean age, 8.6 ± 5.2 years; range, 1–19 at initial diagnosis) with either newly diagnosed (n = 5) or pretreated (n = 16) CNS tumors were retrospectively analyzed. All patients had previously undergone neuro-oncological work-up including cranial magnetic resonance imaging. In all cases, [18F]FET-PET was indicated in a multidisciplinary team conference. The impact of PET imaging on clinical decision-making was assessed. Histopathology (n = 12) and/or clinical and imaging follow-up (n = 9) served as the standard of reference.
Results
The addition of [18F]FET-PET to the available information had an impact on further patient management in 14 out of 21 subjects, with avoidance of invasive surgery or biopsy in four patients, biopsy guidance in four patients, change of further treatment in another five patients, and confirmation of diagnosis in one patient.
Conclusion
[18F]FET-PET may provide important additional information for treatment guidance in pediatric and adolescent patients with CNS tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary tumors of the central nervous system (CNS) are the most common solid malignancies in childhood and one of the main causes for cancer-related deaths in children, with 5-year overall survival rates of up to 75% depending on risk group and histology [1,2,3]. Since childhood brain tumors comprise various biological entities (astrocytoma, medulloblastoma, and ependymoma being the most common) with rather heterogeneous presentation (depending on the tumor location and the age of the child), early diagnosis remains challenging [4]. Cranial magnetic resonance imaging (cMRI) is considered the standard of care for neuroimaging. It provides detailed anatomical and structural information as well as characteristic features of different tumor entities. Although advanced cMRI techniques (such as diffusion tensor imaging, perfusion imaging, or spectroscopy) are able to deliver additional information, it remains a diagnostic challenge to specify tumor grading and differentiate between true progression and therapy-related findings [5].
Positron emission tomography (PET) using O-(2-[18F]fluoroethyl)-L-tyrosine ([18F]FET) as a marker of amino acid transport can offer supplemental information regarding tumor biology. It is an established tool in adult brain tumor imaging, where it has proven valuable in prognostication [6,7,8], treatment monitoring [9,10,11], and differentiation of non-specific post-therapeutic changes (pseudoprogression) from tumor recurrence [12,13,14]. To date, only very few studies have focused on the use and utility of [18F]FET-PET in pediatric brain tumors. This study aimed at investigating the additional benefit of [18F]FET-PET in children and adolescents with primary CNS tumors in a real-world scenario. To this end, we retrospectively analyzed the clinical impact of amino acid PET in cases requiring a complex clinical process of decision-making (i.e., due to insufficient or equivocal information gained from cMRI).
Material and methods
Subjects
This retrospective analysis included 21 consecutive pediatric and adolescent patients with primary CNS tumors (mean age, 8.6 ± 5.2 years; age range, 1–19 at initial diagnosis; and mean age, 12.6 ± 5.6; age range, 2–23 at PET scan date) who underwent [18F]FET-PET for further diagnostic work-up between July 2010 and October 2018 at the Department of Nuclear Medicine at University Hospital Würzburg, Germany. The general cut-off for patient age was 18 at initial diagnosis. One older patient was included as she was diagnosed with a typical pediatric brain tumor (pilomyxoid astrocytoma of the optic pathway) at the age of 19 (patient #11). Five patients presented with newly diagnosed gliomas, while the remaining 16 subjects with primary brain tumors were referred due to equivocal MRI diagnosis.
All subjects had previously undergone comprehensive neuro-oncologic work-up as described in more detail in sections “cMRI” and “Standard of reference.” In these select cases, [18F]FET-PET was also clinically indicated at a weekly multidisciplinary team conference in pediatric neuro-oncology for the confirmation of diagnosis or determination of further treatment planning (e.g., extent of irradiation or biopsy guidance). This was conducted due to difficulties in the clinical decision-making based on the available information (all clinical and histopathological data in addition to current MR imaging) alone, and the expert panel opted for additional PET imaging in order to gain clinical confidence.
Written informed consent was provided by all legal guardians or patients, respectively. The study adhered to the standards established in the Declaration of Helsinki. Given the retrospective nature of this analysis of routinely acquired data, the local ethics committee of the University of Würzburg waived the need for further approval.
Preparation of [18F]FET and PET imaging
[18F]FET was synthetized in-house at the Interdisciplinary PET Centre of the University Hospital of Würzburg using a GE TRACERlab FX-FN synthesis module (GE Medical Systems, Uppsala, Sweden) as previously described [13, 15].
All patients fasted for at least 12 h prior to PET imaging. Twenty minutes after intravenous injection of [18F]FET (156 ± 69 MBq), patients were scanned on an integrated PET/CT scanner (Biograph mCT 64, Siemens Healthineers, Knoxville, TN). Static PET emission data were collected in three-dimensional mode using a 200 × 200 matrix for 10 min. Subsequent CT scans for attenuation correction were acquired using a low-dose protocol (CARE Dose 4D; 80 mAs; 120 kV; matrix, 512 × 512; 3 mm slice thickness; increment, 30 mm/s; rotation time, 0.5 s; pitch index, 0.8). PET images were reconstructed iteratively (TrueX; 3 iterations; 24 subsets; Gaussian filtering, 2 mm; decay, attenuation and scatter correction) using dedicated manufacturer software (syngo MI.PET/CT; Siemens Healthineers).
cMRI
All patients underwent a cranial MRI prior to [18F]FET-PET, with a median interval of 18 days between PET and cMRI (range, 5–70 days). cMRI was performed in-house for 18 out of 21 patients. A total of 10/18 patients were scanned on a 1.5 T MRI (Magnetom Symphony or Magnetom Aera, both Siemens Healthcare, Erlangen, Germany) and 8/18 patients on a 3 T MRI (Magnetom Trio Tim, Siemens Healthcare, Erlangen, Germany). The remaining 3 patients presented with cMRI studies from other hospitals.
Basic cMRI, including T2-weighted images (T2WI), fluid-attenuated inversion recovery (FLAIR) (in 18/21 patients; proton density images in the remaining cases), T1-weighted images without (T1WI) as well as with contrast enhancement (T1WI + CE), and diffusion weighted images (DWI) were obtained for all patients but one (patient #20, missing diffusion weighted imaging). Additional spectroscopy was available in 5/21 subjects.
High-grade gliomas with high cellularity were defined by a low signal on T2WI and restricted diffusion. In contrast, low-grade gliomas displayed signs of low cellularity with a high signal on T2WI and high apparent diffusion coefficient values. Details of the cMRI examinations are summarized in Supplemental Table 1.
PET image analysis
All [18F]FET-PET scans were evaluated independently by one very experienced investigator (rater 1, AKB, more than 15 years of experience) and one with intermediate experience (rater 2, OK, more than 5 years of experience). Distinction between tumor and non-specific tracer uptake was based on combined analysis of [18F]FET-PET and MR, previous imaging, and clinical history and as previously described [16].
First, a visual inspection of scans for tumor uptake was performed. Then, on the axial slice presenting the maximum tumor uptake, regions of interest (ROI) were selected. Standardized uptake values for maximal (SUVmax) and mean tumor uptake (SUVmean) were derived by placing a 10-mm circular region of interest over the area with the peak activity. For assessment of background activity, normal-appearing brain on the contralateral hemisphere (SUVBKG) was selected, and data evaluation including calculation of tumor-to-background ratios (TBR) was performed as previously described [17,18,19]. Subsequently, mean and maximum tumor-to-background ratios (TBRmean; TBRmax) were calculated. For the differentiation of vital tumor from unspecific changes, validated TBR cut-off values were used [13, 18].
The radiotracer concentration in the ROIs was normalized to the injected dose per kilogram of patient’s body weight to derive the SUVs.
Assessment of the clinical impact of [18F]FET-PET
Individual patient cases, including cMRI and [18F]FET-PET scans, were subsequently discussed by a multidisciplinary team. The clinical impact of [18F]FET-PET on the treatment was rated in a multidisciplinary consensus by an experienced pediatric neurosurgeon (JK), experienced pediatric oncologist (PGS), and two nuclear medicine specialists (AKB, CL) who had access to all clinical data but were blinded to future treatment decisions and outcomes. Impact on clinical decision-making due to addition of [18F]FET-PET to the diagnostic algorithm was rated with a two-sided score (yes versus no), with impact being defined as a direct influence on patient management by the addition of relevant new, treatment-guiding information (e.g., detection of residual tumor, differentiation of true tumor progression versus treatment-related changes, change in definition of tumor extent, or change of biopsy location).
Standard of reference
The reference standard was biopsy or resection, if feasible (n = 12). Otherwise, a combination of clinical and radiological follow-up was used (n = 9). Twelve patients underwent either serial stereotactic biopsy or surgery for histopathological analysis. Histological classification, molecular genetic analysis, and tumor grading were accomplished by an experienced neuropathologist (CMM). The biopsy samples/surgical specimens were fixed in formalin and embedded in paraffin. All samples (3–4 μm sections stained with hematoxylin and eosin) were histologically assessed and graded according to the respective current WHO criteria [20, 21].
Nine patients received serial follow-up cMRI that served as radiological standard of reference. Pre- and untreated low-grade tumors with stable tumor lesions during follow-up were rated as remnant tumor. For high-grade tumors, stable lesions for 6 months without treatment were classified as non-tumors.
Statistical analysis
Most of the data provided are descriptive. Descriptive statistics for patient characteristics were reported as mean ± standard deviation (SD), median, and range.
Results
Patient characteristics
A total of 21 pediatric and adolescent patients with primary CNS tumors were included (mean age, 8.6 ± 5.2 years; median age 8; range, 1–19 at initial diagnosis; and mean age, 12.6 ± 5.6; median age 14; range, 2–23 at PET scan date). PET and MRI scan were performed within a median of 14 days (range, 5–70 days). Five patients presented with newly diagnosed gliomas, and sixteen children/adolescents were referred with pretreated brain tumors (3 patients with surgery alone, 7 patients with combined surgery and radiochemotherapy, 2 patients with combined surgery and chemotherapy, 3 patients with radiochemotherapy, and 1 patient with chemotherapy alone). In patients who had received combined radiochemotherapy, [18F]FET-PET scans were performed more than 12 weeks from cessation of radiotherapy (median, 31 months; range, 3–141 months).
Six patients presented with low-grade gliomas (pretreated WHO III anaplastic astrocytoma, at the time point of imaging graded as WHO I pilocytic astrocytoma, n = 1; WHO grade I pilocytic astrocytoma, n = 2; WHO grade II pilomyxoid astrocytoma/optic pathway glioma, n = 1; radiological diagnosis of low-grade glioma, n = 2) and 15 subjects with high-grade tumors, distributed as follows: WHO grade III anaplastic astrocytoma (n = 4, one of them as second malignancy after initial treatment of a germ cell tumor), WHO grade III anaplastic ependymoma (n = 1), WHO grade IV diffuse intrinsic pontine glioma (DIPG, n = 2) (neuroradiological diagnosis, one as a second malignancy after initial treatment of a medulloblastoma), WHO grade IV CNS primitive neuroectodermal tumor (n = 1), WHO grade IV ependymoblastoma (n = 2), and WHO grade IV glioblastoma (n = 5). Detailed patient characteristics are summarized in Table 1.
PET findings
Fourteen out of 21 patients were PET-positive (3/5 with newly diagnosed brain tumors, 11/16 with pretreated lesions).
In the patients with newly diagnosed CNS tumors, SUVmean and SUVmax ranged from 1.1 to 3.6 and from 1.3 to 5.0; TBRmean and TBRmax ranged from 0.8 to 3.8 and from 1.0 to 5.1, respectively. All patients were correctly classified as high-grade (n = 3/3) or low-grade glioma (n = 2/2).
In the patients with concern of tumor recurrence or persistence of vital tumor, SUVmean and SUVmax ranged from 1.5 to 5.4 and from 1.7 to 6.1, respectively. While TBRmean and TBRmax ranged from 1.1 to 3.9 and from 1.2 to 4.7, respectively. The diagnostic accuracy of [18F]FET-PET in this sub-cohort was 88% (n = 14/16) with a sensitivity of 100% (n = 11/11) and a specificity of 60% (n = 3/5). Individual imaging results are presented in Supplemental Table 2.
Impact of additional [18F]FET-PET imaging on clinical decisions
[18F]FET-PET had an impact on further treatment decisions in a total of 14 out of 21 patients. Invasive surgery or biopsy was avoided in four patients, and PET was crucial to biopsy or surgery guidance in another four patients. In three patients, [18F]FET-PET directly changed further therapy: one patient received chemotherapy instead of radiotherapy (patient #2), chemotherapy was changed to another regimen in another patient (patient #5), and one patient received no further radiotherapy (patient #10). In one patient (patient #15), [18F]FET-PET confirmed the suspicion on true tumor progression and thus prompted subsequent surgery. A detailed overview of the individual clinical impact of [18F]FET-PET is presented in Table 2. Respective examples are given in Figure 1.
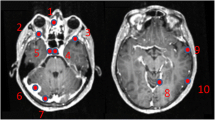
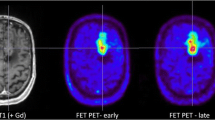
Example of two patients with concordant imaging results (A) and incremental diagnostic value of [18F]FET-PET (B). A Example of a patient (#10) with an anaplastic astrocytoma WHO grade III who was treated with combined radiochemotherapy prior to PET. Axial T2WI and T1WI + CE depict the tumor in the left thalamus and insular region (red arrows); axial PET shows high uptake of [18F] FET in these regions (white arrows). Both MRI and PET identified correctly the tumor recurrence. B Example of a patient (#19) with a pilocytic astrocytoma who was treated with surgery and chemotherapy prior to PET. Axial T2WI and T1WI + CE show cystic as well as contrast enhancing lesions next to the surgical cavity consistent with both unspecific changes and tumor recurrence (red asterisk); in contrast, axial PET demonstrates focal high uptake of [18F]FET (white asterisk)
Discussion
The value of [18F]FET-PET as an easy-to-read and robust tool in imaging for adults with gliomas has been established and has repeatedly been demonstrated over many years. Only a few studies have focused on its usefulness in and value for pediatric and adolescent patients with primary CNS tumors [22,23,24,25,26].
A recent prospective Danish study reported significantly increased diagnostic accuracy and clinical impact in 8% of scans when PET imaging was added to cMRI [25]. Notably, in cases deemed clinically indicated due to difficult decision-making on cMRI alone, its impact was as high as 33%.
Our study further supports these findings. Our real-world data suggest that [18F]FET-PET is a useful adjunct in challenging pediatric settings. In our cohort, inclusion of amino acid PET in the diagnostic algorithm impacted clinical management in two thirds of patients, either by avoiding surgery or biopsy, guiding targeted biopsy or surgery, changing further therapy management, or prompting alternative surgery. Consistent with the vast body of the literature available for adults, it reliably distinguished low-grade from high-grade glioma [27,28,29] and unspecific changes (e.g., (late) pseudoprogression, radiation necrosis) from true tumor recurrence [12, 14, 30].
cMRI remains the standard imaging modality in pediatric brain tumors, including medulloblastoma, atypical teratoid rhabdoid tumor, ependymoma, and primitive neuroectodermal tumor displaying characteristic imaging features. In times of molecular pathology, the diagnosis of diffuse intrinsic pontine glioma, optic pathway glioma, and tectal glioma is still based on neuroradiolological criteria. Additionally, in patients with DIPG and optic pathway glioma, it is still legitimate to begin therapy without previous biopsy if typical imaging criteria are fulfilled. T2W- and DW-imaging provides information about cellularity as a sign of aggressiveness in order to differentiate low-grade from high-grade gliomas. MR-based imaging in pediatric patients with central nervous system tumors is also useful for assessment of tumor dynamics (especially in low-grade gliomas, in order to initiate therapy), as well as treatment response monitoring and patient follow-up, due to the fact that residual tumor growth is exclusively detectable by sequential scans. However, despite the broad range of utility of this imaging modality, there remain challenges when attempting to differentiate treatment-related changes from recurrent tumor. In addition to basic MRI sequences, advanced MRI techniques, such as spectroscopic imaging and MR perfusion, may provide additional physiological information and should be considered in all equivocal situations. However, their interpretation can be more challenging due to a lack of standardization of image acquisition and processing (despite upcoming guidelines such as the European Society of Pediatric Oncology (SIOPE) Imaging Guideline), difficulties in interpretation (as a result of methodological problems), and a higher sensitivity to artifacts [31, 32].
In contrast, [18F]FET-PET, as an easy-to-read tool, might be particularly appealing to clinicians. It could substantively support clinical decisions, especially in cases where cMRI imaging is ambiguous.
Our retrospective study suffers from various limitations. It includes only 21 pediatric and adolescent patients with various tumor entities in different clinical settings. Thus, numbers are too small to differentiate among tumor types or clinical situations in which [18F]FET-PET might be of particular clinical value and large(r) multi-center studies are highly warranted. However, our cohort represents an authentic, real-world scenario encountered in daily routine.
PET-based tumor volumetry or a comparison of volumetry between PET and MR imaging was not performed. Additionally, no dynamic PET acquisitions which could have provided valuable diagnostic information [33,34,35] were performed. However, the reduction in acquisition time gained by static imaging must be acknowledged, as scans in this young patient group are often performed under general anesthesia or sedation in order to reduce motion artifacts. Single-session hybrid PET/MRI for the acquisition of both structural data and metabolic information should be considered for these patients, as the extra risk of additional anesthesia can be mitigated while obtaining optimized data co-registration.
Another limitation is the lack of stringent histopathological confirmation of imaging results, as tissue samples could not be obtained in all patients.
Nevertheless, the interpretation of [18F]FET-PET imaging is simple and robust, even in pediatric patients with CNS tumors for which pathophysiology and molecular pathogenesis are still not fully understood (and may differ from adult primary brain tumors). In this setting, a close collaboration with colleagues from pediatric oncology, surgery, neuroradiology, and neuropathology in multidisciplinary teams is of utmost importance.
Whereas cMRI remains the standard neuroimaging modality in pediatric patients with CNS tumors, the addition of amino acid PET may provide further information and subsequently influence treatment management, particularly in those select cases where standard neuro-oncological work-up is ambiguous.
Conclusion
[18F]FET-PET is a robust imaging tool, which provides important additional information for treatment decisions in pediatric and adolescent patients with CNS tumors, especially in clinically challenging situations.
Data Availability
All data and materials are available upon request.
References
Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S. (1992–2004). Cancer. 2008;112:416–32. https://doi.org/10.1002/cncr.23169.
Ostrom QT, de Blank PM, Kruchko C, Petersen CM, Liao P, Finlay JL, et al. Alex’s lemonade stand foundation infant and childhood primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2015;16(Suppl 10):x1–36. https://doi.org/10.1093/neuonc/nou327.
Frühwald MC, Rutkowski S. Tumors of the central nervous system in children and adolescents. Dtsch Arztebl Int. 2011;108:390–7. https://doi.org/10.3238/arztebl.2011.0390.
Nejat F, El Khashab M, Rutka JT. Initial management of childhood brain tumors: neurosurgical considerations. J Child Neurol. 2008;23:1136–48. https://doi.org/10.1177/0883073808321768.
Goo HW, Ra Y-S. Advanced MRI for pediatric brain tumors with emphasis on clinical benefits. Korean J Radiol. 2017;18:194–207. https://doi.org/10.3348/kjr.2017.18.1.194.
Jansen NL, Suchorska B, Wenter V, Schmid-Tannwald C, Todica A, Eigenbrod S, et al. Prognostic significance of dynamic 18F-FET PET in newly diagnosed astrocytic high-grade glioma. J Nucl Med Off Publ Soc Nucl Med. 2015;56:9–15. https://doi.org/10.2967/jnumed.114.144675.
Suchorska B, Jansen NL, Linn J, Kretzschmar H, Janssen H, Eigenbrod S, et al. Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology. 2015;84:710–9. https://doi.org/10.1212/WNL.0000000000001262.
Unterrainer M, Schweisthal F, Suchorska B, Wenter V, Schmid-Tannwald C, Fendler WP, et al. Serial 18F-FET PET imaging of primarily 18F-FET-negative glioma: does it make sense? J Nucl Med Off Publ Soc Nucl Med. 2016;57:1177–82. https://doi.org/10.2967/jnumed.115.171033.
Galldiks N, Langen KJ, Holy R, Pinkawa M, Stoffels G, Nolte KW, et al. Assessment of treatment response in patients with glioblastoma using O-(2–18F-fluoroethyl)-L-tyrosine PET in comparison to MRI. J Nucl Med Off Publ Soc Nucl Med. 2012;53:1048–57. https://doi.org/10.2967/jnumed.111.098590.
Hutterer M, Nowosielski M, Putzer D, Waitz D, Tinkhauser G, Kostron H, et al. O-(2–18F-fluoroethyl)-L-tyrosine PET predicts failure of antiangiogenic treatment in patients with recurrent high-grade glioma. J Nucl Med Off Publ Soc Nucl Med. 2011;52:856–64. https://doi.org/10.2967/jnumed.110.086645.
Ceccon G, Lazaridis L, Stoffels G, Rapp M, Weber M, Blau T, et al. Use of FET PET in glioblastoma patients undergoing neurooncological treatment including tumour-treating fields: initial experience. Eur J Nucl Med Mol Imaging. 2018. https://doi.org/10.1007/s00259-018-3992-5.
Kebir S, Fimmers R, Galldiks N, Schafer N, Mack F, Schaub C, et al. Late pseudoprogression in glioblastoma: diagnostic value of dynamic O-(2-[18F]fluoroethyl)-L-tyrosine PET. Clin Cancer Res Off J Am Assoc Cancer Res. 2016;22:2190–6. https://doi.org/10.1158/1078-0432.CCR-15-1334.
Mihovilovic MI, Kertels O, Hanscheid H, Lohr M, Monoranu CM, Kleinlein I, et al. O-(2-((18)F)fluoroethyl)-L-tyrosine PET for the differentiation of tumour recurrence from late pseudoprogression in glioblastoma. J Neurol Neurosurg Psychiatry. 2018. https://doi.org/10.1136/jnnp-2017-317155.
Galldiks N, Dunkl V, Stoffels G, Hutterer M, Rapp M, Sabel M, et al. Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-L-tyrosine PET. Eur J Nucl Med Mol Imaging. 2015;42:685–95. https://doi.org/10.1007/s00259-014-2959-4.
Bourdier T, Greguric I, Roselt P, Jackson T, Faragalla J, Katsifis A. Fully automated one-pot radiosynthesis of O-(2-[18F]fluoroethyl)-L-tyrosine on the TracerLab FX(FN) module. Nucl Med Biol. 2011;38:645–51. https://doi.org/10.1016/j.nucmedbio.2011.01.001.
Piccardo A, Albert NL, Borgwardt L, Fahey FH, Hargrave D, Galldiks N, et al. Joint EANM/SIOPE/RAPNO practice guidelines/SNMMI procedure standards for imaging of paediatric gliomas using PET with radiolabelled amino acids and [(18)F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2022;49:3852–69. https://doi.org/10.1007/s00259-022-05817-6.
Law I, Albert NL, Arbizu J, Boellaard R, Drzezga A, Galldiks N, et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [(18)F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2019;46:540–57. https://doi.org/10.1007/s00259-018-4207-9.
Kertels O, Mihovilovic MI, Linsenmann T, Kessler AF, Tran-Gia J, Kircher M, et al. Clinical utility of different approaches for detection of late pseudoprogression in glioblastoma with O-(2-[18F]fluoroethyl)-L-tyrosine PET. Clin Nucl Med. 2019;44:695–701. https://doi.org/10.1097/rlu.0000000000002652.
Lapa C, Linsenmann T, Monoranu CM, Samnick S, Buck AK, Bluemel C, et al. Comparison of the amino acid tracers 18F-FET and 18F-DOPA in high-grade glioma patients. J Nucl Med. 2014;55:1611–6. https://doi.org/10.2967/jnumed.114.140608.
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. https://doi.org/10.1007/s00401-007-0243-4.
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–20. https://doi.org/10.1007/s00401-016-1545-1.
Marner L, Nysom K, Sehested A, Borgwardt L, Mathiasen R, Henriksen OM, et al. Early postoperative (18)F-FET PET/MRI for pediatric brain and spinal cord tumors. J Nucl Med. 2019;60:1053–8. https://doi.org/10.2967/jnumed.118.220293.
Dunkl V, Cleff C, Stoffels G, Judov N, Sarikaya-Seiwert S, Law I, et al. The usefulness of dynamic O-(2–18F-fluoroethyl)-L-tyrosine PET in the clinical evaluation of brain tumors in children and adolescents. J Nucl Med. 2015;56:88–92. https://doi.org/10.2967/jnumed.114.148734.
Misch M, Guggemos A, Driever PH, Koch A, Grosse F, Steffen IG, et al. (18)F-FET-PET guided surgical biopsy and resection in children and adolescence with brain tumors. Childs Nerv Syst. 2015;31:261–7. https://doi.org/10.1007/s00381-014-2552-y.
Marner L, Lundemann M, Sehested A, Nysom K, Borgwardt L, Mathiasen R, et al. Diagnostic accuracy and clinical impact of [18F]FET PET in childhood CNS tumors. Neuro Oncol. 2021;23:2107–16. https://doi.org/10.1093/neuonc/noab096.
Grosse F, Wedel F, Thomale UW, Steffen I, Koch A, Brenner W, et al. Benefit of static FET PET in pretreated pediatric brain tumor patients with equivocal conventional MRI results. Klin Padiatr. 2021;233:127–34. https://doi.org/10.1055/a-1335-4844.
Verger A, Filss CP, Lohmann P, Stoffels G, Sabel M, Wittsack HJ, et al. Comparison of (18)F-FET PET and perfusion-weighted MRI for glioma grading: a hybrid PET/MR study. Eur J Nucl Med Mol Imaging. 2017;44:2257–65. https://doi.org/10.1007/s00259-017-3812-3.
Vettermann F, Suchorska B, Unterrainer M, Nelwan D, Forbrig R, Ruf V, et al. Non-invasive prediction of IDH-wildtype genotype in gliomas using dynamic (18)F-FET PET. Eur J Nucl Med Mol Imaging. 2019;46:2581–9. https://doi.org/10.1007/s00259-019-04477-3.
Popperl G, Kreth FW, Mehrkens JH, Herms J, Seelos K, Koch W, et al. FET PET for the evaluation of untreated gliomas: correlation of FET uptake and uptake kinetics with tumour grading. Eur J Nucl Med Mol Imaging. 2007;34:1933–42. https://doi.org/10.1007/s00259-007-0534-y.
Popperl G, Gotz C, Rachinger W, Gildehaus FJ, Tonn JC, Tatsch K. Value of O-(2-[18F]fluoroethyl)-L-tyrosine PET for the diagnosis of recurrent glioma. Eur J Nucl Med Mol Imaging. 2004;31:1464–70. https://doi.org/10.1007/s00259-004-1590-1.
Langen K-J, Galldiks N, Hattingen E, Shah NJ. Advances in neuro-oncology imaging. Nat Rev Neurol. 2017;13:279–89. https://doi.org/10.1038/nrneurol.2017.44.
Avula S, Peet A, Morana G, Morgan P, Warmuth-Metz M, Jaspan T. European Society for Paediatric Oncology (SIOPE) MRI guidelines for imaging patients with central nervous system tumours. Childs Nerv Syst. 2021;37:2497–508. https://doi.org/10.1007/s00381-021-05199-4.
Galldiks N, Stoffels G, Filss C, Rapp M, Blau T, Tscherpel C, et al. The use of dynamic O-(2–18F-fluoroethyl)-l-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro Oncol. 2015;17:1293–300. https://doi.org/10.1093/neuonc/nov088.
Jansen NL, Graute V, Armbruster L, Suchorska B, Lutz J, Eigenbrod S, et al. MRI-suspected low-grade glioma: is there a need to perform dynamic FET PET? Eur J Nucl Med Mol Imaging. 2012;39:1021–9. https://doi.org/10.1007/s00259-012-2109-9.
Rohrich M, Huang K, Schrimpf D, Albert NL, Hielscher T, von Deimling A, et al. Integrated analysis of dynamic FET PET/CT parameters, histology, and methylation profiling of 44 gliomas. Eur J Nucl Med Mol Imaging. 2018;45:1573–84. https://doi.org/10.1007/s00259-018-4009-0.
Funding
Open Access funding enabled and organized by Projekt DEAL. The Neuroradiological Reference Center for pediatric brain tumor (HIT) studies is supported by the German Childhood Cancer Foundation (Deutsche Kinderkrebsstiftung). The Pediatric Neuro-Oncology Program for High Grad Glioma Würzburg (M.E.) is supported by a grant from the German Childhood Cancer Foundation (Deutsche Kinderkrebsstiftung).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Olivia Kertels, Jürgen Krauß, Brigitte Bison, and Constantin Lapa. The first draft of the manuscript was written by Olivia Kertels, Brigitte Bison, and Constantin Lapa. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Ethical approval was waived by the local Ethics Committee of the University of Würzburg in view of the retrospective nature of the study, and all the procedures being performed were part of the routine care.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The preliminary data of this study was presented during the Annual Congress of the European Association of Nuclear Medicine (EANM’20): Featured Session: Molecular Brain Tumor Imaging (OP-358).
This article is part of the Topical Collection on Oncology—Brain.
Brigitte Bison and Constantin Lapa contributed equally to this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kertels, O., Krauß, J., Monoranu, C.M. et al. [18F]FET-PET in children and adolescents with central nervous system tumors: does it support difficult clinical decision-making?. Eur J Nucl Med Mol Imaging 50, 1699–1708 (2023). https://doi.org/10.1007/s00259-023-06114-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06114-6