Abstract
Purpose
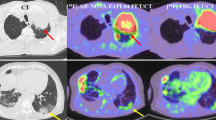
In vivo CXCR4 receptor quantification in different lung cancer (LC) sub-types using [68Ga]Ga-Pentixafor PET/CT and to study correlation with quantitative CXCR4 receptors’ tissue density by immunochemistry analyses.
Methods
[68Ga]Ga-Pentixafor PET/CT imaging was performed prospectively in 94 (77 M: 17F, mean age 60.1 ± 10.1 years) LC patients. CXCR4 receptors’ expression on lung mass in all the patients was estimated by immunohistochemistry (IHC) and fluorescence-activated cell sorting (FACS) analyses. SUVmax on PET, intensity score on IHC, and mean fluorescence index (MFI) on FACS analyses were measured.
Results
A total of 75/94 (79.8%) cases had non-small cell lung cancer (NSCLC), 14 (14.9%) had small cell lung cancer (SCLC), and 5 (5.3%) had lung neuroendocrine neoplasm (NEN). All LC types showed increased CXCR4 expression on PET (SUVmax) and FACS (MFI). However, both these parameters (mean SUVmax = 10.3 ± 5.0; mean MFI = 349.0 ± 99.0) were significantly (p = 0.005) higher in SCLC as compared to those in NSCLC and lung NEN. The mean SUVmax in adenocarcinoma (n = 16) was 8.0 ± 1.9 which was significantly (p = 0.003) higher than in squamous cell carcinoma (n = 54; 6.2 ± 2.1) and in not-otherwise specified (NOS) sub-types (n = 5; 5.8 ± 1.5) of NSCLC. A significant correlation (r = 0.697; p = 001) was seen between SUVmax and MFI values in squamous cell NSCLC as well as in NSCLC adenocarcinoma (r = 0.538, p = 0.031) which supports the specific in vivo uptake of [68Ga]Ga-Pentixafor by CXCR4 receptors. However, this correlation was not significant in SCLC (r = 0.435, p = 0.121) and NEN (r = 0.747, p = 0.147) which may be due to the small sample size. [68Ga]Ga-Pentixafor PET/CT provided good sensitivity (85.7%) and specificity (78.1%) for differentiating SCLC from NSCLC (ROC cutoff SUVmax = 7.2). This technique presented similar sensitivity (87.5%) and specificity (71.4%) (ROC cutoff SUVmax = 6.7) for differentiating adenocarcinoma and squamous cell variants of NSCLC.
Conclusion
The high sensitivity and specificity of [68Ga]Ga-Pentixafor PET/CT for in vivo targeting of CXCR4 receptors in lung cancer can thus be used effectively for the response assessment and development of CXCR4-based radioligand therapies in LC.





Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding authoron reasonable request.
References
AL-Jahdali H, Khan AN, Loutfi S, Al-Harbi AS. Guidelines for the role of FDG-PET/CT in lung cancer management. Journal of Infection and Public Health [Internet]. King Saud Bin Abdulaziz University for Health Sciences; 2012;5:S35–40. Available from: https://doi.org/10.1016/j.jiph.2012.09.003.
Baum RP, Świȩtaszczyk C, Prasad V. FDG-PET/CT in lung cancer: an update. Front Radiat Ther Oncol. 2010;42:15–45.
Kandathil A, Kay FU, Butt YM, Wachsmann JW, Subramaniam RM. Role of FDG PET/CT in the eighth edition of TNM staging of non–small cell lung cancer. Radiographics. 2018;38:2134–49.
Ambrosini V, Nicolini S, Caroli P, Nanni C, Massaro A, Marzola MC, et al. PET/CT imaging in different types of lung cancer: an overview. Eur J Radiol [Internet]. Elsevier Ireland Ltd; 2012;81:988–1001. Available from: https://doi.org/10.1016/j.ejrad.2011.03.020.
Manoharan P, Salem A, Mistry H, Gornall M, Harden S, Julyan P, et al. 18F-Fludeoxyglucose PET/CT in SCLC: analysis of the CONVERT Randomized Controlled Trial. J Thorac Oncol [Internet]. Elsevier Inc; 2019 [cited 2022 Oct 1];14:1296–305. Available from: http://www.jto.org/article/S1556086419302825/fulltext.
Araz M, Soydal C, Özkan E, Sen E, Nak D, Kucuk ON, et al. Prognostic value of metabolic parameters on baseline 18F-FDG PET/CT in small cell lung cancer. Q J Nucl Med Mol Imaging. 2022;66:61–6. https://doi.org/10.23736/S1824-4785.19.03169-8.
Xiao M, Ma X, Ma F, Li Y, Zhang G, Qiang J. Whole-tumor histogram analysis of apparent diffusion coefficient for differentiating adenosquamous carcinoma and adenocarcinoma from squamous cell carcinoma in patients with cervical cancer. Acta Radiol [Internet]. SAGE Publications; 2021;63:1415–24. Available from: https://doi.org/10.1177/02841851211035915.
William Strauss H, Mariani G, Volterrani D, Larson SM. Nuclear oncology: pathophysiology and clinical applications. Nuclear oncology: pathophysiology and clinical applications. 2013.
Park H, Sholl LM, Hatabu H, Awad MM, Nishino M. Imaging of precision therapy for lung cancer: current state of the art. Radiology. 2019;293:15–29.
Sarvaiya PJ, Guo D, Ulasov I, Gabikian P, Lesniak MS. Chemokines in tumor progression and metastasis. Oncotarget. 2013;4:2171–85.
Burger JA, Stewart DJ, Wald O, Peled A. Potential of CXCR4 antagonists for the treatment of metastatic lung cancer. Expert Rev Anticancer Ther [Internet]. Taylor & Francis; 2011;11:621–30. Available from: https://doi.org/10.1586/era.11.11.
Domanska UM, Kruizinga RC, Nagengast WB, Timmer-Bosscha H, Huls G, de Vries EGE, et al. A review on CXCR4/CXCL12 axis in oncology: no place to hide. Eur J Cancer [Internet]. Elsevier Ltd; 2013;49:219–30. Available from: https://doi.org/10.1016/j.ejca.2012.05.005.
Demmer O, Gourni E, Schumacher U, Kessler H, Wester H-JJ. PET imaging of CXCR4 receptors in cancer by a new optimized ligand. ChemMedChem [Internet]. 2011/07/20. WILEY-VCH Verlag; 2011;6:1789–91. Available from: https://pubmed.ncbi.nlm.nih.gov/21780290.
Demmer O, Dijkgraaf I, Schumacher U, Marinelli L, Cosconati S, Gourni E, et al. Design, synthesis, and functionalization of dimeric peptides targeting chemokine receptor CXCR4. J Med Chem [Internet]. American Chemical Society; 2011;54:7648–62. Available from: https://doi.org/10.1021/jm2009716.
Gourni E, Demmer O, Schottelius M, D’Alessandria C, Schulz S, Dijkgraaf I, et al. PET of CXCR4 expression by a 68Ga-labeled highly specific targeted contrast agent. J Nucl Med [Internet]. 2011;52:1803–10. Available from: http://jnm.snmjournals.org/cgi/doi/10.2967/jnumed.111.098798. Accessed 11 Apr 2016.
Vag T, Gerngross C, Herhaus P, Eiber M, Philipp-Abbrederis K, Graner F-P, et al. First experience with chemokine receptor CXCR4-targeted PET imaging of patients with solid cancers. Journal of Nuclear Medicine [Internet]. 2016;57:741–6. Available from: http://jnm.snmjournals.org/cgi/doi/10.2967/jnumed.115.161034. Accessed 21 Aug 2016.
Watts A, Singh B, Basher R, Singh H, Bal A, Kapoor R, et al. 68Ga-Pentixafor PET/CT demonstrating higher CXCR4 density in small cell lung carcinoma than in non-small cell variant. Eur J Nucl Med Mol Imaging. 2017;44:909–10.
Watts A, Arora D, Kumar N, Thakur S, Basher R, Radotra B, et al. 68Ga-Pentixafor PET/CT offers high contrast image for the detection of CXCR4 expression in recurrent glioma. J Nucl Med [Internet]. 2019;60:491. Available from: http://jnm.snmjournals.org/content/60/supplement_1/491.abstract.
Watts A, Chutani S, Arora D, Madivanane V, Thakur S, Kamboj M, et al. Automated radiosynthesis, quality control, and biodistribution of Ga-68 Pentixafor: first Indian experience. Indian J Nucl Med. Wolters Kluwer Medknow Publications; 2021;36:237–44.
Singh N, Aggarwal AN, Gupta D, Behera D, Jindal SK. Unchanging clinico-epidemiological profile of lung cancer in North India over three decades. Cancer Epidemiol. 2010;34:101–4.
Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16:2927–31.
Duda DG, Kozin SV, Kirkpatrick ND, Xu L, Fukumura D, Jain RK. CXCL12 (SDF1α)-CXCR4/CXCR7 pathway inhibition: an emerging sensitizer for anticancer therapies? Clin Cancer Res. 2011;17:2074–80.
Buck AK, Haug A, Dreher N, Lambertini A, Higuchi T, Lapa C, et al. Imaging of C-X-C motif chemokine receptor 4 expression in 690 patients with solid or hematologic neoplasms using 68 Ga-PentixaFor PET. J Nucl Med. Society of Nuclear Medicine; 2022;jnumed.121.263693.
Herrmann K, Schottelius M, Lapa C, Osl T, Poschenrieder A, Hanscheid H, et al. First-in-human experience of CXCR4-directed endoradiotherapy with 177Lu- and 90Y-labeled Pentixather in advanced-stage multiple myeloma with extensive intra- and extramedullary disease. J Nucl Med [Internet]. 2016;57:248–51. Available from: http://jnm.snmjournals.org/cgi/doi/10.2967/jnumed.115.167361. Accessed 11 Apr 2016.
Lapa C, Herrmann K, Schirbel A, Hänscheid H, Lückerath K, Schottelius M, et al. CXCR4-directed endoradiotherapy induces high response rates in extramedullary relapsed multiple myeloma. Theranostics [Internet]. Ivyspring International Publisher; 2017;7:1589–97. Available from: http://www.thno.org/v07p1589.htm. Accessed 8 Aug 2022.
Lapa C, Hänscheid H, Kircher M, Schirbel A, Wunderlich G, Werner RA, et al. Feasibility of CXCR4-directed radioligand therapy in advanced diffuse large B-cell lymphoma. J Nucl Med. Society of Nuclear Medicine Inc.; 2019;60:60–4.
Habringer S, Lapa C, Herhaus P, Schottelius M, Istvanffy R, Steiger K, et al. Dual targeting of acute leukemia and supporting niche by CXCR4-directed theranostics. Theranostics. Ivyspring International Publisher; 2018;8:369–83.
Maurer S, Herhaus P, Lippenmeyer R, Hänscheid H, Kircher M, Schirbel A, et al. Side effects of CXC-chemokine receptor 4-directed endoradiotherapy with Pentixather before hematopoietic stem cell transplantation. J Nucl Med. Society of Nuclear Medicine Inc.; 2019;60:1399–405.
Werner RA, Kircher S, Higuchi T, Kircher M, Schirbel A, Wester HJ, et al. CXCR4-directed imaging in solid tumors. Front Oncol. Frontiers Media S.A.; 2019;9.
Furusato B, Rhim JS. CXCR4 and cancer. In: Amy M. Fulton, editor. Chemokine receptors in cancer. Humana Press; 2009. p. 31–45. Available from: https://doi.org/10.1007/978-1-60327-267-4_2.
Shekhawat AS, Singh B, Malhotra P, Watts A, Basher R, Kaur H, et al. Imaging CXCR4 receptors expression for staging multiple myeloma by using 68Ga-Pentixafor PET/CT: comparison with 18F-FDG PET/CT. Br J Radiol. British Institute of Radiology; 2022; 1;95(1136):20211272. https://doi.org/10.1259/bjr.20211272.
Liang J-XX, Gao W, Liang Y, Zhou X-MM. Chemokine receptor CXCR4 expression and lung cancer prognosis: a meta-analysis. Int J Clin Exp Med [Internet]. e-Century Publishing Corporation; 2015;8:5163–74. Available from: https://pubmed.ncbi.nlm.nih.gov/26131090. Accessed 07 May 2020.
Spano JP, Andre F, Morat L, Sabatier L, Besse B, Combadiere C, et al. Chemokine receptor CXCR4 and early-stage non-small cell lung cancer: pattern of expression and correlation with outcome. Ann Oncol. 2004;15:613–7.
Buck AK, Serfling SE, Lindner T, Hänscheid H, Schirbel A, Hahner S, et al. CXCR4-targeted theranostics in oncology. Eur J Nucl Med Mol Imaging. Springer Science and Business Media LLC; 2022.
Buck AK, Grigoleit GU, Kraus SK, Schirbel A, Heinsch M, Dreher N, et al. C-X-C motif chemokine receptor 4-targeted radioligand therapy in patients with advanced T-cell lymphoma. J Nucl Med [Internet]. 2022;jnumed.122.264207. http://jnm.snmjournals.org/content/early/2022/06/23/jnumed.122.264207.abstract. Accessed 8 Aug 2022.
Oronsky B, Reid TR, Oronsky A, Carter CA. What’s new in SCLC? A review. Neoplasia (United States) [Internet]. The Authors; 2017;19:842–7. Available from: https://doi.org/10.1016/j.neo.2017.07.007.
Burger M, Glodek A, Hartmann T, Schmitt-Gräff A, Silberstein LE, Fujii N, et al. Functional expression of CXCR4 (CD184) on small-cell lung cancer cells mediates migration, integrin activation, and adhesion to stromal cells. Oncogene. 2003;22:8093–101.
Bunyaviroch T, Coleman RE. PET evaluation of lung cancer. J Nucl Med. 2006;47:451–69.
Chlipala EA, Bendzinski CM, Dorner C, Sartan R, Copeland K, Pearce R, et al. An image analysis solution for quantification and determination of immunohistochemistry staining reproducibility. Appl Immunohistochem Mol Morphol [Internet]. 2020;28. Available from: https://journals.lww.com/appliedimmunohist/Fulltext/2020/07000/An_Image_Analysis_Solution_For_Quantification_and.3.aspx. Accessed 01 Oct 2022.
Burger JA, Stewart DJ. CXCR4 chemokine receptor antagonists: perspectives in SCLC. Expert Opin Investig Drugs. 2009;18:481–90.
Watts A, Singh B, Bal A, Kapoor R, Arora S, Mittal B, et al. 68Ga-Pentixafor PET/CT for imaging CXCR4/CXCL12 receptor-ligand axis in lung cancer - a head-to-head comparison with 18F-FDG PET/CT. J Nucl Med [Internet]. 2022;63:2266. Available from: http://jnm.snmjournals.org/content/63/supplement_2/2266.abstract.
Wester HJ, Keller U, Schottelius M, Beer A, Philipp-Abbrederis K, Hoffmann F, et al. Disclosing the CXCR4 expression in lymphoproliferative diseases by targeted molecular imaging. Theranostics. 2015;5:618–30.
Philipp-Abbrederis K, Herrmann K, Knop S, Schottelius M, Eiber M, Lückerath K, et al. In vivo molecular imaging of chemokine receptor CXCR4 expression in patients with advanced multiple myeloma. EMBO Mol Med [Internet]. BlackWell Publishing Ltd; 2015;7:477–87. Available from: http://embomolmed.embopress.org/cgi/doi/10.15252/emmm.201404698. Accessed 07 May 2020.
Lapa C, Schreder M, Schirbel A, Samnick S, Kortüm KM, Herrmann K, et al. [68Ga]Pentixafor-PET/CT for imaging of chemokine receptor CXCR4 expression in multiple myeloma - comparison to [18F]FDG and laboratory values. Theranostics. Ivyspring International Publisher; 2017;7:205–12.
Herhaus P, Habringer S, Philipp-Abbrederis K, Vag T, Gerngross C, Schottelius M, et al. Targeted positron emission tomography imaging of CXCR4 expression in patients with acute myeloid leukemia. Haematologica. Ferrata Storti Foundation; 2016;101:932–40.
Mayerhoefer ME, Jaeger U, Staber P, Raderer M, Wadsak W, Pfaff S, et al. [68Ga]Ga-Pentixafor PET/MRI for CXCR4 imaging of chronic lymphocytic leukemia: preliminary results. Investigative Radiology [Internet]. 2018;53. Available from: https://journals.lww.com/investigativeradiology/Fulltext/2018/07000/_68Ga_Ga_Pentixafor_PET_MRI_for_CXCR4_Imaging_of.4.aspx. Accessed 21 May 2020.
Schottelius M, Osl T, Poschenrieder A, Hoffmann F, Beykan S, Hänscheid H, et al. [(177)Lu]Pentixather: comprehensive preclinical characterization of a first CXCR4-directed endoradiotherapeutic agent. Theranostics [Internet]. Ivyspring International Publisher; 2017;7:2350–62. Available from: https://pubmed.ncbi.nlm.nih.gov/28744319. Accessed 21 May 2020.
Watts A, Singh B, Singh H, Kaur H, Bal A, Vohra M, et al. Gallium-68-Pentixafor PET/CT demonstrating in vivo CXCR4 receptors’ overexpression in rare lung malignancies: correlation with the histological and histochemical findings. J Nucl Med Technol [Internet]. 2022;jnmt.122.264141. Available from: http://tech.snmjournals.org/content/early/2022/05/24/jnmt.122.264141.abstract.
Singh B, Kaur H, Parihar AS, Watts A, Prasad V. Precision radiomolecular oncology: challenging the classical statistical evidence-based medicine. In: Sobti RC, Dhalla NS, editors. Biomedical translational research: drug design and discovery [Internet]. Singapore: Springer Nature Singapore; 2022. p. 97–110. Available from: https://doi.org/10.1007/978-981-16-9232-1_7.
Serfling SE, Lapa C, Dreher N, Hartrampf PE, Rowe SP, Higuchi T, et al. Impact of tumor burden on normal organ distribution in patients imaged with CXCR4-targeted [68Ga]Ga-PentixaFor PET/CT. Mol Imaging Biol. Springer Science and Business Media Deutschland GmbH; 2022.
Werner RA, Weich A, Higuchi T, Schmid JS, Schirbel A, Lassmann M, et al. Imaging of chemokine receptor 4 expression in neuroendocrine tumors - a triple tracer comparative approach. Theranostics. 2017;7:1489–98.
Funding
The research work was carried out using the grant by the Department of Science and Technology (DST), Government of India, under the DST-FIST program (grant SR/FST/LSI-548/2012) for setting up the infrastructure (automated chemistry module and other accessories and chemicals) required for the present work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Ankit Watts: radiopharmaceutical synthesis, quality control testing, PET/CT imaging, image reconstruction, image quantification, IHC/FACS analysis, sample preparation, data curation, manuscript writing, and statistical analysis. Baljinder Singh: study conceptualization, manuscript editing, grant/funding to carry out the research work, supervision, project administration. Harmandeep Singh: data interpretation, PET biopsy, manuscript editing. Bhagwant R. Mittal: study designing, data interpretation, formal analysis, PET biopsy, manuscript editing, supervision. Amanjit Bal: histopathological and immunohistochemical analyses of the lung tissues, manuscript editing. Harneet Kaur: manuscript writing/editing and statistical analysis. Ninjit Dhanota: sample preparation, FACS analysis, and data interpretation. Sunil K. Arora: sample preparation, FACS analysis and data interpretation, formal analysis, supervision. Digambar Behera: study designing, patients’ enrolment, clinical examination, bronchoscopy/biopsy, supervision. The first draft of the manuscript was written by AW and BS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was (vide letter No. INT/IEC/2017/194 dated 23.08.2017) granted to the study as PhD project of the first author by the Institute Ethics Committee (IEC) of the Postgraduate Institute of Medical Education & Research (PGIMER), Chandigarh, India.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology - Chest
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Watts, A., Singh, B., Singh, H. et al. [68Ga]Ga-Pentixafor PET/CT imaging for in vivo CXCR4 receptor mapping in different lung cancer histologic sub-types: correlation with quantitative receptors’ density by immunochemistry techniques. Eur J Nucl Med Mol Imaging 50, 1216–1227 (2023). https://doi.org/10.1007/s00259-022-06059-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-06059-2