Abstract
Purpose
We aimed to evaluate whether [68 Ga]Ga-FAPI-04 PET/CT could characterize the early stages of cardiac fibrosis in pressure overload heart failure.
Methods
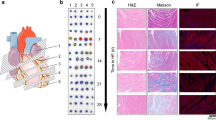
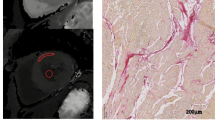
Sprague–Dawley rats underwent abdominal aortic constriction (AAC) (n = 12) and sham surgery (n = 10). All rats were scanned with [68 Ga]Ga-FAPI-04 PET/CT at 2, 4, and 8 weeks after surgery. The expression of fibroblast activation protein (FAP) in the myocardium was detected by immunohistochemistry. [68 Ga]Ga-FAPI-04 PET signal and FAP expression were compared between two groups.
Results
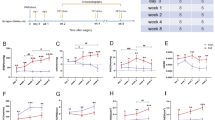
Compared with the sham group, the AAC group presented with decreased ejection fraction (EF) and fractional shortening (FS) and increased left ventricular internal dimensions in diastole (LVIDd) and systole (LVIDs) at 4 and 8 weeks (all p < 0.01). The AAC group showed higher [68 Ga]Ga-FAPI-04 accumulation in the heart than the sham group at 2, 4, and 8 weeks, and FAPI increased significantly from 2 to 8 weeks (all p < 0.001). Immunohistochemistry confirmed the higher density of the FAP+ area in the AAC group. The intensity of the [68 Ga]Ga-FAPI-04 correlated with the density of the FAP+ area (p < 0.001). The expression of the [68 Ga]Ga-FAPI-04 at 4 weeks correlated with the deterioration of cardiac function at 8 weeks (EF: R = − 0.87; FS: R = − 0.72; LVIDd: R = 0.77; LVIDs: R = 0.79; all p < 0.001). The AAC group also showed an increased [68 Ga]Ga-FAPI-04 signal in the liver, peaking at 4 weeks and then declining. Cardiac and liver PET signals correlated at 4 weeks in the AAC group (R = 0.69, p = 0.0010), suggesting an early fibrotic link between organs. A combination of the [68 Ga]Ga-FAPI-04 intensity in the heart and liver at 4 weeks better predicted the deterioration of cardiac function at 8 weeks.
Conclusions
The activated fibroblasts in the heart and liver after pressure overload can be monitored by [68 Ga]Ga-FAPI-04 PET/CT, which reveals an early fibrotic link in cardio-liver interactions and could better predict nonischemic heart failure prognosis.







Similar content being viewed by others
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123:255–78. https://doi.org/10.1016/j.pharmthera.2009.05.002.
Rai V, Sharma P, Agrawal S, Agrawal DK. Relevance of mouse models of cardiac fibrosis and hypertrophy in cardiac research. Mol Cell Biochem. 2017;424:123–45. https://doi.org/10.1007/s11010-016-2849-0.
Ivey MJ, Tallquist MD. Defining the cardiac fibroblast. Circ J. 2016;80:2269–76. https://doi.org/10.1253/circj.CJ-16-1003.
Loktev A, Lindner T, Mier W, Debus J, Altmann A, Jager D, et al. A tumor-imaging method targeting cancer-associated fibroblasts. J Nucl Med. 2018;59:1423–9. https://doi.org/10.2967/jnumed.118.210435.
Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. (68)Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. 2019;60:801–5. https://doi.org/10.2967/jnumed.119.227967.
Varasteh Z, Mohanta S, Robu S, Braeuer M, Li Y, Omidvari N, et al. Molecular Imaging of fibroblast activity after myocardial infarction using a (68)Ga-labeled fibroblast activation protein inhibitor, FAPI-04. J Nucl Med. 2019;60:1743–9. https://doi.org/10.2967/jnumed.119.226993.
Notohamiprodjo S, Nekolla SG, Robu S, Villagran Asiares A, Kupatt C, Ibrahim T, et al. Imaging of cardiac fibroblast activation in a patient after acute myocardial infarction using (68)Ga-FAPI-04. J Nucl Cardiol. 2021. https://doi.org/10.1007/s12350-021-02603-z.
Aghajanian H, Kimura T, Rurik JG, Hancock AS, Leibowitz MS, Li L, et al. Targeting cardiac fibrosis with engineered T cells. Nature. 2019;573:430–3. https://doi.org/10.1038/s41586-019-1546-z.
Lin K, Chen X, Xue Q, Yao S, Miao W. Diffuse uptake of [(68)Ga]Ga-FAPI in the left heart in a patient with hypertensive heart disease by PET/CT. J Nucl Cardiol. 2021. https://doi.org/10.1007/s12350-021-02646-2.
Finke D, Heckmann MB, Herpel E, Katus HA, Haberkorn U, Leuschner F, et al. Early detection of checkpoint inhibitor-associated myocarditis using (68)Ga-FAPI PET/CT. Front Cardiovasc Med. 2021;8:614997. https://doi.org/10.3389/fcvm.2021.614997.
Wang L, Zhang Z, Zhao Z, Yan C, Fang W. (68)Ga-FAPI right heart uptake in a patient with idiopathic pulmonary arterial hypertension. J Nucl Cardiol. 2020. https://doi.org/10.1007/s12350-020-02407-7.
Ku HC, Lee SY, Wu YA, Yang KC, Su MJ. A model of cardiac remodeling through constriction of the abdominal aorta in rats. J Vis Exp. 2016. https://doi.org/10.3791/54818.
Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, Debus J, et al. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med. 2018;59:1415–22. https://doi.org/10.2967/jnumed.118.210443.
Yang F, Liu S, Gu Y, Yan Y, Ding X, Zou L, et al. MicroRNA-22 promoted osteogenic differentiation of valvular interstitial cells by inhibiting CAB39 expression during aortic valve calcification. Cell Mol Life Sci. 2022;79:146. https://doi.org/10.1007/s00018-022-04177-6.
Gonzalez A, Schelbert EB, Diez J, Butler J. Myocardial interstitial fibrosis in heart failure: biological and translational perspectives. J Am Coll Cardiol. 2018;71:1696–706. https://doi.org/10.1016/j.jacc.2018.02.021.
Thackeray JT, Bengel FM. Molecular imaging of myocardial inflammation with positron emission tomography post-ischemia: a determinant of subsequent remodeling or recovery. JACC Cardiovasc Imaging. 2018;11:1340–55. https://doi.org/10.1016/j.jcmg.2018.05.026.
Glasenapp A, Derlin K, Wang Y, Bankstahl M, Meier M, Wollert KC, et al. Multimodality imaging of inflammation and ventricular remodeling in pressure-overload heart failure. J Nucl Med. 2020;61:590–6. https://doi.org/10.2967/jnumed.119.232488.
Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14:591–602. https://doi.org/10.1038/nrcardio.2017.65.
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;101161CIR0000000000001063. https://doi.org/10.1161/CIR.0000000000001063.
Goto K, Schauer A, Augstein A, Methawasin M, Granzier H, Halle M, et al. Muscular changes in animal models of heart failure with preserved ejection fraction: what comes closest to the patient? ESC Heart Fail. 2021;8:139–50. https://doi.org/10.1002/ehf2.13142.
Lopez B, Ravassa S, Gonzalez A, Zubillaga E, Bonavila C, Berges M, et al. Myocardial collagen cross-linking is associated with heart failure hospitalization in patients with hypertensive heart failure. J Am Coll Cardiol. 2016;67:251–60. https://doi.org/10.1016/j.jacc.2015.10.063.
Azevedo CF, Nigri M, Higuchi ML, Pomerantzeff PM, Spina GS, Sampaio RO, et al. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol. 2010;56:278–87. https://doi.org/10.1016/j.jacc.2009.12.074.
Xanthopoulos A, Starling RC, Kitai T, Triposkiadis F. Heart failure and liver disease: cardiohepatic interactions. JACC Heart Fail. 2019;7:87–97. https://doi.org/10.1016/j.jchf.2018.10.007.
Pirasteh A, Periyasamy S, Meudt JJ, Liu Y, Lee LM, Schachtschneider KM, et al. Staging liver fibrosis by fibroblast activation protein inhibitor positron emission tomography in a human-sized swine model. J Nucl Med. 2022. https://doi.org/10.2967/jnumed.121.263736.
Ostovaneh MR, Ambale-Venkatesh B, Fuji T, Bakhshi H, Shah R, Murthy VL, et al. Association of liver fibrosis with cardiovascular diseases in the general population: the Multi-Ethnic Study of Atherosclerosis (MESA). Circ Cardiovasc Imaging. 2018;11:e007241. https://doi.org/10.1161/CIRCIMAGING.117.007241.
Funding
This work was financially supported by the “234 Discipline Climbing Plan” of the First Affiliated Hospital of Naval Medical University (2019YPT002, 2020YPT002), and the Shanghai Science and Technology Innovation Action Plan “Science and Technology Support Project in Biomedical Science” (21S11906000).
Author information
Authors and Affiliations
Contributions
All authors’ material preparation and animal procedures were performed by Guokun Wang and Qinqin Yang. Data collection and analysis were performed by Shengyong Wu, Xudong Xu, Xiao Li, Siyu Liang and Guixia Pan. Changjing Zuo, Xianxian Zhao, Chao Cheng and Suxuan Liu contributed to the study conception and design. Guokun Wang, Qinqin Yang and Shengyong Wu wrote the draft of the manuscript. Chao Cheng and Suxuan Liu conceived the study and interpreted the results. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors do not have any conflicts of interest to declare.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiology.
Supplementary Information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, G., Yang, Q., Wu, S. et al. Molecular imaging of fibroblast activity in pressure overload heart failure using [68 Ga]Ga-FAPI-04 PET/CT. Eur J Nucl Med Mol Imaging 50, 465–474 (2023). https://doi.org/10.1007/s00259-022-05984-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05984-6