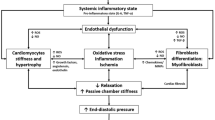
Abstract
Heart disease causing cardiac cell death due to ischemia–reperfusion injury is a major cause of morbidity and mortality in the United States. Coronary heart disease and cardiomyopathies are the major cause for congestive heart failure, and thrombosis of the coronary arteries is the most common cause of myocardial infarction. Cardiac injury is followed by post-injury cardiac remodeling or fibrosis. Cardiac fibrosis is characterized by net accumulation of extracellular matrix proteins in the cardiac interstitium and results in both systolic and diastolic dysfunctions. It has been suggested by both experimental and clinical evidence that fibrotic changes in the heart are reversible. Hence, it is vital to understand the mechanism involved in the initiation, progression, and resolution of cardiac fibrosis to design anti-fibrotic treatment modalities. Animal models are of great importance for cardiovascular research studies. With the developing research field, the choice of selecting an animal model for the proposed research study is crucial for its outcome and translational purpose. Compared to large animal models for cardiac research, the mouse model is preferred by many investigators because of genetic manipulations and easier handling. This critical review is focused to provide insight to young researchers about the various mouse models, advantages and disadvantages, and their use in research pertaining to cardiac fibrosis and hypertrophy.






Similar content being viewed by others
References
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS et al (2013) Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation 127(1):e6–e245
Heron M (2012) Deaths: leading causes for 2008. Natl Vital Stat Rep 60(6):1–94
Yamani M, Massie BM (1993) Congestive heart failure: insights from epidemiology, implications for treatment. Mayo Clin Proc 68(12):1214–1218
Roger VL (2013) Epidemiology of heart failure. Circ Res 113:646–659
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M (2016) Heart disease and stroke statistics-2016 update: a report from the American Heart association. Circulation 133(4):e38–e360
Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D (1993) Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 88(1):107–115
Velagaleti RS, Vasan RS (2007) Heart failure in the twenty-first century: is it a coronary artery disease or hypertension problem? Cardiol Clin 25:487–495
Hasenfuss G (1998) Animal models of human cardiovascular disease, heart failure and hypertrophy. Cardiovasc Res 39(1):60–76
Kwak HB (2013) Aging, exercise, and extracellular matrix in the heart. J Exerc Rehabil 9(3):338–347
Berk BC, Fujiwara K, Lehoux S (2007) ECM remodeling in hypertensive heart disease. J Clin Invest 117(3):568–575
Kong P, Christia P, Frangogiannis NG (2014) The pathogenesis of cardiac fibrosis. Cell Mol Life Sci 71(4):549–574
Frangogiannis NG (2006) Targeting the inflammatory response in healing myocardial infarcts. Curr Med Chem 13(16):1877–1893
Arslan F, de Kleijn DP, Pasterkamp G (2011) Innate immune signaling in cardiac ischemia. Nat Rev Cardiol 8(5):292–300
Bujak M, Frangogiannis NG (2007) The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res 74(2):184–195
Vellaichamy E, Das S, Subramanian U, Maeda N, Pandey KN (2014) Genetically altered mutant mouse models of guanylyl cyclase/natriuretic peptide receptor-A exhibit the cardiac expression of proinflammatory mediators in a gene-dose-dependent manner. Endocrinology 155(3):1045–1056
Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, Wang XF, Frangogiannis NG (2007) Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling. Circulation 116(19):2127–2138
Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE (2011) Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging 4(1):98–108
Biernacka A, Cavalera M, Wang J, Russo I, Shinde A, Kong P, Gonzalez-Quesada C, Rai V, Dobaczewski M, Lee DW, Wang XF, Frangogiannis NG (2015) Smad3 signaling promotes fibrosis while preserving cardiac and aortic geometry in obese diabetic mice. Circ Heart Fail 8(4):788–798
Panek AN, Posch MG, Alenina N, Ghadge SK, Erdmann B, Popova E, Perrot A, Geier C, Dietz R, Morano I, Bader M, Ozcelik C (2009) Connective tissue growth factor overexpression in cardiomyocytes promotes cardiac hypertrophy and protection against pressure overload. PLoS ONE 4(8):e6743
Schneider MD (2002) Serial killer: angiotensin drives cardiac hypertrophy via TGF-Î21. J Clin Investig 109(6):715–716
Hermida N, López B, González A, Dotor J, Lasarte JJ, Sarobe P, Borrás-Cuesta F, Díez J (2009) A synthetic peptide from transforming growth factor-β1 type III receptor prevents myocardial fibrosis in spontaneously hypertensive rats. Cardiovasc Res 81(3):601–609
Yousef ZR, Redwood SR, Marber MS (2000) Postinfarction left ventricular remodelling: where are the theories and trials leading us? Heart 83(1):76–80
Dobaczewski M, Frangogiannis NG (2009) Chemokines and cardiac fibrosis. Front Biosci (Schol Ed). 1:391–405
Frangogiannis NG (2004) Chemokines in the ischemic myocardium: from inflammation to fibrosis. Inflamm Res 53(11):585–595
Saxena A, Chen W, Su Y, Rai V, Uche OU, Li N, Frangogiannis NG (2013) IL-1 induces proinflammatory leukocyte infiltration and regulates fibroblast phenotype in the infarcted myocardium. J Immunol 191(9):4838–4848
Biernacka A, Frangogiannis NG (2011) Aging and cardiac fibrosis. Aging Dis 2(2):158–173
Ljungqvist A, Unge G (1973) The proliferative activity of the myocardial tissue in various forms of experimental cardiac hypertrophy. Acta Pathol Microbiol Scand A 81(3):233–240
Abe R, Donnelly SC, Peng T, Bucala R, Metz CN (2001) Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 166(12):7556–7562
Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS (2010) Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176(1):85–97
Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, Pilling D, Gomer RH, Trial J, Frangogiannis NG, Entman ML (2006) Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci USA 103(48):18284–18289
Kania G, Blyszczuk P, Stein S, Valaperti A, Germano D, Dirnhofer S, Hunziker L, Matter CM, Eriksson U (2009) Heart-infiltrating prominin-1+/CD133+ progenitor cells represent the cellular source of transforming growth factor beta-mediated cardiac fibrosis in experimental autoimmune myocarditis. Circ Res 105(5):462–470
Olivey HE, Mundell NA, Austin AF, Barnett JV (2006) Transforming growth factor-beta stimulates epithelial–mesenchymal transformation in the proepicardium. Dev Dyn 235(1):50–59
Zeisberg EM, Kalluri R (2010) Origins of cardiac fibroblasts. Circ Res 107(11):1304–1312
Lugus JJ, Park C, Ma YD, Choi K (2009) Both primitive and definitive blood cells are derived from Flk-1+ mesoderm. Blood 113(3):563–566
Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R (2007) Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13(8):952–961
Trial J, Cieslik KA, Haudek SB, Duerrschmid C, Entman ML (2013) Th1/M1 conversion to th2/m2 responses in models of inflammation lacking cell death stimulates maturation of monocyte precursors to fibroblasts. Front Immunol 4:287
Weber KT (1989) Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol 13(7):1637–1652
Anderson KR, Sutton MG, Lie JT (1979) Histopathological types of cardiac fibrosis in myocardial disease. J Pathol 128(2):79–85
Isoyama S, Nitta-Komatsubara Y (2002) Acute and chronic adaptation to hemodynamic overload and ischemia in the aged heart. Heart Fail Rev 7(1):63–69
Verheule S, Sato T, Tt Everett, Engle SK, Otten D, Rubart-von der Lohe M, Nakajima HO, Nakajima H, Field LJ, Olgin JE (2004) Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta1. Circ Res 94(11):1458–1465
Ozcan C, Battaglia E, Young R, Suzuki G (2015) LKB1 knockout mouse develops spontaneous atrial fibrillation and provides mechanistic insights into human disease process. J Am Heart Assoc 4(3):e001733
Lin MC, Rockman HA, Chien KR (1995) Heart and lung disease in engineered mice. Nat Med 1:749–751
Gehrmann J, Frantz S, Maguire CT, Vargas M, Ducharme A, Wakimoto H, Lee RT, Berul CI (2001) Electrophysiological characterization of murine myocardial ischemia and infarction. Basic Res Cardiol 96(3):237–250
Kuhlmann MT, Kirchhof P, Klocke R, Hasib L, Stypmann J, Fabritz L, Stelljes M, Tian W, Zwiener M, Mueller M, Kienast J, Breithardt G, Nikol S (2006) G-CSF/SCF reduces inducible arrhythmias in the infarcted heart potentially via increased connexin43 expression and arteriogenesis. J Exp Med 203(1):87–97
Conci E, Pachinger O, Metzler B (2006) Mouse models for myocardial ischaemia/reperfusion. J Kardiol-Austrian J Cardiol 13(7–8):239–244
Tarnavski O, McMullen JR, Schinke M, Nie Q, Kong S, Izumo S (2004) Mouse cardiac surgery: comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol Genomics 16(3):349–360
Zaragoza C, Gomez-Guerrero C, Martin-Ventura JL, Blanco-Colio L, Lavin B, Mallavia B, Tarin C, Mas S, Ortiz A, Egido J (2011) Animal models of cardiovascular diseases. J Biomed Biotechnol 2011:497841. doi:10.1155/2011/497841
Wei H, Campbell W, Vander Heide RS (2006) Heat shock-induced cardioprotection activates cytoskeletal-based cell survival pathways. Am J Physiol Heart Circ Physiol 291(2):H638–H647
Perricone AJ, Bivona BJ, Jackson FR, Vander Heide RS (2013) Conditional knockout of myocyte focal adhesion kinase abrogates ischemic preconditioning in adult murine hearts. J Am Heart Assoc 2(5):e000457
Tsukamoto K, Mani DR, Shi J, Zhang S, Haagensen DE, Otsuka F, Guan J, Smith JD, Weng W, Liao R, Kolodgie FD, Virmani R, Krieger M (2013) Identification of apolipoprotein D as a cardioprotective gene using a mouse model of lethal atherosclerotic coronary artery disease. Proc Nat Acad Sci USA 110(42):17023–17028
Vanhoutte D, Schellings MW, Gotte M, Swinnen M, Herias V, Wild MK, Vestweber D, Chorianopoulos E, Cortés V, Rigotti A, Stepp MA, Van de Werf F, Carmeliet P, Pinto YM, Heymans S (2007) Increased expression of syndecan-1 protects against cardiac dilatation and dysfunction after myocardial infarction. Circulation 115(4):475–482
Xia Y, Lee K, Li N, Corbett D, Mendoza L, Frangogiannis NG (2009) Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem Cell Biol 131(4):471–481
Dewald O, Frangogiannis NG, Zoerlein M, Duerr GD, Klemm C, Knuefermann P, Taffet G, Michael LH, Crapo JD, Welz A, Entman ML (2003) Development of murine ischemic cardiomyopathy is associated with a transient inflammatory reaction and depends on reactive oxygen species. Proc Natl Acad Sci USA 100(5):2700–2705
Irwin MW, Mak S, Mann DL, Qu R, Penninger JM, Yan A, Dawood F, Wen WH, Shou Z, Liu P (1999) Tissue expression and immunolocalization of tumor necrosis factor-alpha in postinfarction dysfunctional myocardium. Circulation 99(11):1492–1498
Kim SC, Boehm O, Meyer R, Hoeft A, Knufermann P, Baumgarten G (2012) A murine closed-chest model of myocardial ischemia and reperfusion. J Vis Exp 65:e3896
Mayr M, Metzler B, Chung YL, McGregor E, Mayr U, Troy H, Hu Y, Leitges M, Pachinger O, Griffiths JR, Dunn MJ, Xu Q (2004) Ischemic preconditioning exaggerates cardiac damage in PKC-delta null mice. Am J Physiol Heart Circ Physiol 287(2):H946–H956
Xu Z, Alloush J, Beck E, Weisleder N (2014) A murine model of myocardial ischemia–reperfusion injury through ligation of the left anterior descending artery. J Vis Exp. doi:10.3791/51329
Michael LH, Entman ML, Hartley CJ, Youker KA, Zhu J, Hall SR, Hawkins HK, Berens K, Ballantyne CM (1995) Myocardial ischemia and reperfusion: a murine model. Am J Physiol 269(6 Pt 2):H2147–H2154
Klocke R, Tian W, Kuhlmann MT, Nikol S (2007) Surgical animal models of heart failure related to coronary heart disease. Cardiovasc Res 74(1):29–38
Vandervelde S, van Amerongen MJ, Tio RA, Petersen AH, van Luyn MJ, Harmsen MC (2006) Increased inflammatory response and neovascularization in reperfused versus non-reperfused murine myocardial infarction. Cardiovasc Pathol 15(2):83–90
Nossuli TO, Lakshminarayanan V, Baumgarten G, Taffet GE, Ballantyne CM, Michael LH, Entman ML (2000) A chronic mouse model of myocardial ischemia–reperfusion: essential in cytokine studies. Am J Physiol Heart Circ Physiol 278(4):H1049–H1055
Gao E, Koch WJ (2013) A novel and efficient model of coronary artery ligation in the mouse. Methods Mol Biol 1037:299–311
Miller DL, Van Winkle DM (1999) Ischemic preconditioning limits infarct size following regional ischemia–reperfusion in in situ mouse hearts. Cardiovasc Res 42(3):680–684
Zhang SH, Reddick RL, Burkey B, Maeda N (1994) Diet-induced atherosclerosis in mice heterozygous and homozygous for apolipoprotein E gene disruption. J Clin Invest 94:937–945
Ishibashi S, Goldstein JL, Brown MS, Herz J, Burns DK (1994) Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J Clin Invest 93(5):1885–1893
Braun A, Trigatti BL, Post MJ, Sato K, Simons M, Edelberg JM, Rosenberg RD, Schrenzel M, Krieger M (2002) Loss of SR-BI expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein E-deficient mice. Circ Res 90(3):270–276
Zhang S, Picard MH, Vasile E, Zhu Y, Raffai RL, Weisgraber KH, Krieger M (2005) Diet-induced occlusive coronary atherosclerosis, myocardial infarction, cardiac dysfunction, and premature death in scavenger receptor class B type I-deficient, hypomorphic apolipoprotein ER61 mice. Circulation 111(25):3457–3464
Nakaoka H, Nakagawa-Toyama Y, Nishida M, Okada T, Kawase R, Yamashita T, Yuasa-Kawase M, Nakatani K, Masuda D, Ohama T, Sonobe T, Shirai M, Komuro I, Yamashita S (2013) Establishment of a novel murine model of ischemic cardiomyopathy with multiple diffuse coronary lesions. PLoS ONE 8(8):e70755
Weinheimer CJ, Lai L, Kelly DP, Kovacs A (2015) Novel mouse model of left ventricular pressure overload and infarction causing predictable ventricular remodelling and progression to heart failure. Clin Exp Pharmacol Physiol 42(1):33–40
Miano JM, Zhu QM, Lowenstein CJ (2016) A CRISPR path to engineering new genetic mouse models for cardiovascular research. Arterioscler Thromb Vasc Biol 36(3):1058–1075
Carroll KJ, Makarewich CA, McAnally J, Anderson DM, Zentilin L, Liu N, Giacca M, Bassel-Duby R, Olson EN (2016) A mouse model for adult cardiac-specific gene deletion with CRISPR/Cas9. Proc Natl Acad Sci USA 113(2):338–343
Davis J, Maillet M, Miano JM, Molkentin JD (2012) Lost in transgenesis: a user’s guide for genetically manipulating the mouse in cardiac research. Circ Res 111(6):761–777
Kong P, Christia P, Saxena A, Su Y, Frangogiannis NG (2013) Lack of specificity of fibroblast-specific protein 1 in cardiac remodeling and fibrosis. Am J Physiol Heart Circ Physiol 305:H1363–H1372
Manabe I, Shindo T, Nagai R (2002) Gene expression in fibroblasts and fibrosis: involvement in cardiac hypertrophy. Circ Res 91:1103–1113
Okamoto Y, Chaves A, Chen J, Kelley R, Jones K et al (2001) Transgenic mice with cardiac-specific expression of activating transcription factor 3, a stress-inducible gene, have conduction abnormalities and contractile dysfunction. Am J Pathol 159:639–650
Rajewsky K, Gu H, Kuhn R, Betz UA, Muller W, Roes J, Schwenk F (1996) Conditional gene targeting. J Clin Invest 98(3):600–603
Rajamannan NM (2006) Models of cardiac fibrosis. Drug Discov Today 3:291–295
Martin TP, Hortigon-Vinagre MP, Findlay JE, Elliott C, Currie S et al (2014) Targeted disruption of the heat shock protein 20-phosphodiesterase 4D (PDE4D) interaction protects against pathological cardiac remodelling in a mouse model of hypertrophy. FEBS Open Bio 4:923–927
Fan D, Takawale A, Basu R, Patel V, Lee J et al (2014) Differential role of TIMP2 and TIMP3 in cardiac hypertrophy, fibrosis, and diastolic dysfunction. Cardiovasc Res 103:268–280
Leong XF, Ng CY, Jaarin K (2015) Animal models in cardiovascular research: hypertension and atherosclerosis. Biomed Res Int 2015:528757. doi:10.1155/2015/528757
deAlmeida AC, van Oort RJ, Wehrens XH (2010) Transverse aortic constriction in mice. J Vis Exp 38:e1729–e1729
Kiefer TL, Bashore TM (2011) Pulmonary hypertension related to left-sided cardiac pathology. Pulm Med 2011:381787
Knight DS, Steeden JA, Moledina S, Jones A, Coghlan JG et al (2015) Left ventricular diastolic dysfunction in pulmonary hypertension predicts functional capacity and clinical worsening: a tissue phase mapping study. J Cardiovasc Magn Reson 17:116
Borgdorff MA, Dickinson MG, Berger RM, Bartelds B (2015) Right ventricular failure due to chronic pressure load: what have we learned in animal models since the NIH working group statement? Heart Fail Rev 20:475–491
Hirata M, Ousaka D, Arai S, Okuyama M, Tarui S et al (2015) Novel model of pulmonary artery banding leading to right heart failure in rats. Biomed Res Int 2015:753210
Furihata T, Kinugawa S, Takada S, Fukushima A, Takahashi M et al (2016) The experimental model of transition from compensated cardiac hypertrophy to failure created by transverse aortic constriction in mice. IJC Heart Vasc 11:24–28
Hutchinson KR, Saripalli C, Chung CS, Granzier H (2015) Increased myocardial stiffness due to cardiac titin isoform switching in a mouse model of volume overload limits eccentric remodeling. J Mol Cell Cardiol 79:104–114
Mohamed BA, Schnelle M, Khadjeh S, Lbik D, Herwig M et al (2016) Molecular and structural transition mechanisms in long-term volume overload. Eur J Heart Fail 18:362–371
Abassi Z, Goltsman I, Karram T, Winaver J, Hoffman A (2011) Aortocaval fistula in rat: a unique model of volume-overload congestive heart failure and cardiac hypertrophy. J Biomed Biotechnol 2011:729497
Houser SR, Margulies KB, Murphy AM, Spinale FG, Francis GS et al (2012) Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res 111:131–150
Patten RD, Hall-Porter MR (2009) Small animal models of heart failure: development of novel therapies, past and present. Circ Heart Fail 2:138–144
Egemnazarov B, Schmidt A, Crnkovic S, Sydykov A, Nagy BM et al (2015) Pressure overload creates right ventricular diastolic dysfunction in a mouse model: assessment by echocardiography. J Am Soc Echocardiogr 28:828–843
Turner NA (2016) Inflammatory and fibrotic responses of cardiac fibroblasts to myocardial damage associated molecular patterns (DAMPs). J Mol Cell Cardiol 94:189–200
Fontes MS, Raaijmakers AJ, van Doorn T, Kok B, Nieuwenhuis S et al (2014) Changes in Cx43 and NaV1.5 expression precede the occurrence of substantial fibrosis in calcineurin-induced murine cardiac hypertrophy. PLoS ONE 9:e87226
Chen TH, Chen MR, Chen TY, Wu TC, Liu SW et al (2016) Cardiac fibrosis in mouse expressing DsRed tetramers involves chronic autophagy and proteasome degradation insufficiency. Oncotarget. doi:10.18632/oncotarget.11026
Heymans S, Schroen B, Vermeersch P, Milting H, Gao F et al (2005) Increased cardiac expression of tissue inhibitor of metalloproteinase-1 and tissue inhibitor of metalloproteinase-2 is related to cardiac fibrosis and dysfunction in the chronic pressure-overloaded human heart. Circulation 112:1136–1144
Arber S, Hunter JJ, Ross J Jr, Hongo M, Sansig G, Borg J, Perriard JC, Chien KR, Caroni P (1997) MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell 88(3):393–403
Iwase M, Uechi M, Vatner DE, Asai K, Shannon RP, Kudej RK, Wagner TE, Wight DC, Patrick TA, Ishikawa Y, Homcy CJ, Vatner SF (1997) Cardiomyopathy induced by cardiac Gs alpha overexpression. Am J Physiol 272(1 Pt 2):H585–H589
Sussman MA, Welch S, Cambon N, Klevitsky R, Hewett TE, Price R, Witt SA, Kimball TR (1998) Myofibril degeneration caused by tropomodulin overexpression leads to dilated cardiomyopathy in juvenile mice. J Clin Invest 101(1):51–61
Zhu F, Li Y, Zhang J, Piao C, Liu T et al (2013) Senescent cardiac fibroblast is critical for cardiac fibrosis after myocardial infarction. PLoS ONE 8:e74535
Moritani T, Iwai M, Kanno H, Nakaoka H, Iwanami J et al (2013) ACE2 deficiency induced perivascular fibrosis and cardiac hypertrophy during postnatal development in mice. J Am Soc Hypertens 7:259–266
Ieronimakis N, Hays A, Prasad A, Janebodin K, Duffield JS et al (2016) PDGFRalpha signalling promotes fibrogenic responses in collagen producing cells in Duchenne muscular dystrophy. J Pathol. doi:10.1002/path.4801
Li Y, Ma J, Zhu H, Singh M, Hill D et al (2011) Targeted inhibition of calpain reduces myocardial hypertrophy and fibrosis in mouse models of type 1 diabetes. Diabetes 60:2985–2994
Rajesh M, Mukhopadhyay P, Batkai S, Patel V, Saito K et al (2010) Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol 56:2115–2125
Cavalera M, Wang J, Frangogiannis NG (2014) Obesity, metabolic dysfunction, and cardiac fibrosis: pathophysiological pathways, molecular mechanisms, and therapeutic opportunities. Transl Res 164:323–335
Hutchinson KR, Lord CK, West TA, Stewart JA Jr (2013) Cardiac fibroblast-dependent extracellular matrix accumulation is associated with diastolic stiffness in type 2 diabetes. PLoS ONE 8:e72080
Jacoby D, McKenna WJ (2012) Genetics of inherited cardiomyopathy. Eur Heart J 33(3):296–304
Eppig JT, Blake JA, Bult CJ, Kadin JA, Richardson JE (2015) The mouse genome database (MGD): facilitating mouse as a model for human biology and disease. Nucleic Acids Res 43:D726–D736
Maron BJ, Maron MS (2013) Hypertrophic cardiomyopathy. Lancet 381(9862):242–255
Geisterfer-Lowrance AA, Christe M, Conner DA, Ingwall JS, Schoen FJ, Seidman CE, Seidman JG (1996) A mouse model of familial hypertrophic cardiomyopathy. Science 272(5262):731–734
Welikson RE, Buck SH, Patel JR, Moss RL, Vikstrom KL, Factor SM, Miyata S, Weinberger HD, Leinwand LA (1999) Cardiac myosin heavy chains lacking the light chain binding domain cause hypertrophic cardiomyopathy in mice. Am J Physiol 276(6 Pt 2):H2148–H2158
Gottshall KR, Hunter JJ, Tanaka N, Dalton N, Becker KD, Ross J Jr, Chien KR (1997) Ras-dependent pathways induce obstructive hypertrophy in echo-selected transgenic mice. Proc Natl Acad Sci USA 94(9):4710–4715
Gruver CL, DeMayo F, Goldstein MA, Means AR (1993) Targeted developmental overexpression of calmodulin induces proliferative and hypertrophic growth of cardiomyocytes in transgenic mice. Endocrinology 133(1):376–388
Hirota H, Yoshida K, Kishimoto T, Taga T (1995) Continuous activation of gp130, a signal-transducing receptor component for interleukin 6-related cytokines, causes myocardial hypertrophy in mice. Proc Natl Acad Sci USA 92(11):4862–4866
Milano CA, Dolber PC, Rockman HA, Bond RA, Venable ME, Allen LF, Lefkowitz RJ (1994) Myocardial expression of a constitutively active alpha 1B-adrenergic receptor in transgenic mice induces cardiac hypertrophy. Proc Natl Acad Sci USA 91(21):10109–10113
Graham BH, Waymire KG, Cottrell B, Trounce IA, MacGregor GR, Wallace DC (1997) A mouse model for mitochondrial myopathy and cardiomyopathy resulting from a deficiency in the heart/muscle isoform of the adenine nucleotide translocator. Nat Genet 16(3):226–234
Blankenburg R, Hackert K, Wurster S, Deenen R, Seidman JG, Lohse MJ, Schmitt JP (2014) β-Myosin heavy chain variant Met606Val causes very mild hypertrophic cardiomyopathy in mice, but exacerbates HCM phenotypes in mice carrying other HCM mutations. Circ Res 115(2):227–237
Schulz EM, Wilder T, Chowdhury SA, Sheikh HN, Wolska BM, Solaro RJ, Wieczorek DF (2013) Decreasing tropomyosin phosphorylation rescues tropomyosin-induced familial hypertrophic cardiomyopathy. J Biol Chem 288(40):28925–28935
Zhao W, Zhao T, Chen Y, Zhao F, Gu Q, Williams RW, Bhattacharya SK, Lu L, Sun Y (2015) A murine hypertrophic cardiomyopathy model: the DBA/2J strain. PLoS ONE 10(8):e0133132
Davis J, Davis LC, Correll RN, Makarewich CA, Schwanekamp JA, Moussavi-Harami F, Wang D, York AJ, Wu H, Houser SR, Seidman CE, Seidman JG, Regnier M, Metzger JM, Wu JC, Molkentin JD (2016) A tension-based model distinguishes hypertrophic versus dilated cardiomyopathy. Cell 165(5):1147–1159
Dai S, Yuan F, Mu J, Li C, Chen N, Guo S, Kingery J, Prabhu SD, Bolli R, Rokosh G (2010) Chronic AMD3100 antagonism of SDF-1alpha-CXCR4 exacerbates cardiac dysfunction and remodeling after myocardial infarction. J Mol Cell Cardiol 49(4):587–597
Wang G, Hamid T, Keith RJ, Zhou G, Partridge CR, Xiang X, Kingery JR, Lewis RK, Li Q, Rokosh DG, Ford R, Spinale FG, Riggs DW, Srivastava S, Bhatnagar A, Bolli R, Prabhu SD (2010) Cardioprotective and antiapoptotic effects of heme oxygenase-1 in the failing heart. Circulation 121(17):1912–1925
Liu X, Simpson JA, Brunt KR, Ward CA, Hall SR, Kinobe RT, Barrette V, Tse MY, Pang SC, Pachori AS, Dzau VJ, Ogunyankin KO, Melo LG (2007) Preemptive heme oxygenase-1 gene delivery reveals reduced mortality and preservation of left ventricular function 1 year after acute myocardial infarction. Am J Physiol Heart Circ Physiol 293(1):H48–H59
van den Bos EJ, Mees BM, de Waard MC, de Crom R, Duncker DJ (2005) A novel model of cryoinjury-induced myocardial infarction in the mouse: a comparison with coronary artery ligation. Am J Physiol Heart Circ Physiol 289(3):H1291–H1300
Polizzotti BD, Ganapathy B, Haubner BJ, Penninger JM, Kuhn B (2016) A cryoinjury model in neonatal mice for cardiac translational and regeneration research. Nat Protoc 11(3):542–552
Weinstein DM, Mihm MJ, Bauer JA (2000) Cardiac peroxynitrite formation and left ventricular dysfunction following doxorubicin treatment in mice. J Pharmacol Exp Ther 294(1):396–401
Robert J (2007) Long-term and short-term models for studying anthracycline cardiotoxicity and protectors. Cardiovasc Toxicol 7(2):135–139
Oudit GY, Crackower MA, Eriksson U, Sarao R, Kozieradzki I, Sasaki T, Irie-Sasaki J, Gidrewicz D, Rybin VO, Wada T, Steinberg SF, Backx PH, Penninger JM (2003) Phosphoinositide 3-kinase gamma-deficient mice are protected from isoproterenol-induced heart failure. Circulation 108(17):2147–2152
Shan J, Kushnir A, Betzenhauser MJ, Reiken S, Li J, Lehnart SE, Lindegger N, Mongillo M, Mohler PJ, Marks AR (2010) Phosphorylation of the ryanodine receptor mediates the cardiac fight or flight response in mice. J Clin Invest 120(12):4388–4398
Wang QD, Bohlooly YM, Sjoquist PO (2004) Murine models for the study of congestive heart failure: implications for understanding molecular mechanisms and for drug discovery. J Pharmacol Toxicol Methods 50(3):163–174
Martino TA, Liu P, Sole MJ (1994) Viral infection and the pathogenesis of dilated cardiomyopathy. Circ Res 74(4):182–188
Matsumori A, Kawai C (1982) An experimental model for congestive heart failure after encephalomyocarditis virus myocarditis in mice. Circulation 65(6):1230–1235
Liu P, Penninger J, Aitken K, Sole M, Mak T (1995) The role of transgenic knockout models in defining the pathogenesis of viral heart disease. Eur Heart J 16(Suppl O):25–27
Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S (1993) Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA 90(2):770–774
Sharaf AR, Narula J, Nicol PD, Southern JF, Khaw BA (1994) Cardiac sarcoplasmic reticulum calcium ATPase, an autoimmune antigen in experimental cardiomyopathy. Circulation 89(3):1217–1228
Jane-wit D, Yu M, Edling AE, Kataoka S, Johnson JM, Stull LB, Moravec CS, Tuohy VK (2002) A novel class II-binding motif selects peptides that mediate organ-specific autoimmune disease in SWXJ, SJL/J, and SWR/J mice. J Immunol 169(11):6507–6514
Hirasawa M, Ito Y, Shibata MA, Otsuki Y (2007) Mechanism of inflammation in murine eosinophilic myocarditis produced by adoptive transfer with ovalbumin challenge. Int Arch Allergy Immunol 142(1):28–39
Jean-Charles PY, Li YJ, Nan CL, Huang XP (2011) Insights into restrictive cardiomyopathy from clinical and animal studies. J Geriatr Cardiol 8(3):168–183
Boerma M, Hauer-Jensen M (2010) Preclinical research into basic mechanisms of radiation-induced heart disease. Cardiol Res Pract. doi:10.4061/2011/858262
Lemaire R, Farina G, Kissin E, Shipley JM, Bona C, Korn JH, Lafyatis R (2004) Mutant fibrillin 1 from tight skin mice increases extracellular matrix incorporation of microfibril-associated glycoprotein 2 and type I collagen. Arthritis Rheum 50(3):915–926
Teng MH, Yin JY, Vidal R, Ghiso J, Kumar A, Rabenou R, Shah A, Jacobson DR, Tagoe C, Gallo G, Buxbaum J (2001) Amyloid and nonfibrillar deposits in mice transgenic for wild-type human transthyretin: a possible model for senile systemic amyloidosis. Lab Invest 81(3):385–396
Ruberg FL, Berk JL (2012) Transthyretin (TTR) cardiac amyloidosis. Circulation 126(10):1286–1300
Dewald O, Frangogiannis NG, Zoerlein MP, Duerr GD, Taffet G, Michael LH, Welz A, Entman ML (2004) A murine model of ischemic cardiomyopathy induced by repetitive ischemia and reperfusion. Thorac Cardiovasc Surg 52(5):305–311
Li Y, Takemura G, Kosai K, Takahashi T, Okada H, Miyata S, Yuge K, Nagano S, Esaki M, Khai NC, Goto K, Mikami A, Maruyama R, Minatoguchi S, Fujiwara T, Fujiwara H (2004) Critical roles for the Fas/Fas ligand system in postinfarction ventricular remodeling and heart failure. Circ Res 95(6):627–636
Lim DS, Lutucuta S, Bachireddy P, Youker K, Evans A, Entman M, Roberts R, Marian AJ (2001) Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation 103(6):789–791
Teekakirikul P, Eminaga S, Toka O, Alcalai R, Wang L, Wakimoto H, Nayor M, Konno T, Gorham JM, Wolf CM, Kim JB, Schmitt JP, Molkentin JD, Norris RA, Tager AM, Hoffman SR, Markwald RR, Seidman CE, Seidman JG (2010) Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-β. J Clin Invest 120(10):3520–3529
Haudek SB, Trial J, Xia Y, Gupta D, Pilling D, Entman ML (2008) Fc receptor engagement mediates differentiation of cardiac fibroblast precursor cells. Proc Natl Acad Sci USA 105(29):10179–10184
Metzler B, Jehle J, Theurl I, Ludwiczek S, Obrist P, Pachinger O, Weiss G (2007) Short term protective effects of iron in a murine model of ischemia/reperfusion. Biometals 20(2):205–215
Stansfield WE, Moss NC, Willis MS, Tang R, Selzman CH (2007) Proteasome inhibition attenuates infarct size and preserves cardiac function in a murine model of myocardial ischemia–reperfusion injury. Ann Thorac Surg 84(1):120–125
Hataishi R, Rodrigues AC, Neilan TG, Morgan JG, Buys E, Shiva S, Tambouret R, Jassal DS, Raher MJ, Furutani E, Ichinose F, Gladwin MT, Rosenzweig A, Zapol WM, Picard MH, Bloch KD, Scherrer-Crosbie M (2006) Inhaled nitric oxide decreases infarction size and improves left ventricular function in a murine model of myocardial ischemia–reperfusion injury. Am J Physiol Heart Circ Physiol 291(1):H379–H384
Yada M, Shimamoto A, Hampton CR, Chong AJ, Takayama H, Rothnie CL, Spring DJ, Shimpo H, Yada I, Pohlman TH, Verrier ED (2004) FR167653 diminishes infarct size in a murine model of myocardial ischemia–reperfusion injury. J Thorac Cardiovasc Surg 128(4):588–594
Stansfield WE, Tang RH, Moss NC, Baldwin AS, Willis MS, Selzman CH (2008) Proteasome inhibition promotes regression of left ventricular hypertrophy. Am J Physiol Heart Circ Physiol 294(2):H645–H650
Moss NC, Stansfield WE, Willis MS, Tang RH, Selzman CH (2007) IKKbeta inhibition attenuates myocardial injury and dysfunction following acute ischemia–reperfusion injury. Am J Physiol Heart Circ Physiol 293(4):H2248–H2253
Passariello CL, Gayanilo M, Kritzer MD, Thakur H, Cozacov Z, Rusconi F, Wieczorek D, Sanders M, Li J, Kapiloff MS (2013) p90 ribosomal S6 kinase 3 contributes to cardiac insufficiency in alpha-tropomyosin Glu180Gly transgenic mice. Am J Physiol Heart Circ Physiol 305(7):H1010–H1019
Anderson ME (2005) Calmodulin kinase signaling in heart: an intriguing candidate target for therapy of myocardial dysfunction and arrhythmias. Pharmacol Ther 106(1):39–55
Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J Jr, Bers DM, Brown JH (2003) The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res 92(8):912–919
Zong J, Salim M, Zhou H, Bian ZY, Dai J, Yuan Y, Deng W, Zhang JY, Zhang R, Wu QQ, Tang QZ (2013) NOD2 deletion promotes cardiac hypertrophy and fibrosis induced by pressure overload. Lab Invest 93(10):1128–1136
Woo YJ, Panlilio CM, Cheng RK, Liao GP, Atluri P, Hsu VM, Cohen JE, Chaudhry HW (2006) Therapeutic delivery of cyclin A2 induces myocardial regeneration and enhances cardiac function in ischemic heart failure. Circulation 114(1 Suppl):I206–I213
Kolossov E, Bostani T, Roell W, Breitbach M, Pillekamp F, Nygren JM, Sasse P, Rubenchik O, Fries JW, Wenzel D, Geisen C, Xia Y, Lu Z, Duan Y, Kettenhofen R, Jovinge S, Bloch W, Bohlen H, Welz A, Hescheler J, Jacobsen SE, Fleischmann BK (2006) Engraftment of engineered ES cell-derived cardiomyocytes but not BM cells restores contractile function to the infarcted myocardium. J Exp Med 203(10):2315–2327
Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P (2001) Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA 98(18):10344–10349
Harada M, Qin Y, Takano H, Minamino T, Zou Y, Toko H, Ohtsuka M, Matsuura K, Sano M, Nishi J, Iwanaga K, Akazawa H, Kunieda T, Zhu W, Hasegawa H, Kunisada K, Nagai T, Nakaya H, Yamauchi-Takihara K, Komuro I (2005) G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med 11(3):305–311
Pankajakshan D, Kansal V, Agrawal DK (2013) In vitro differentiation of bone marrow derived porcine mesenchymal stem cells to endothelial cells. J Tissue Eng Regen Med. 7(11):911–920
Ou L, Li W, Liu Y, Zhang Y, Jie S, Kong D, Steinhoff G, Ma N (2010) Animal models of cardiac disease and stem cell therapy. Open Cardiovasc Med J 4:231–239
Aminzadeh MA, Tseliou E, Sun B, Cheng K, Malliaras K, Makkar RR, Marbán E (2015) Therapeutic efficacy of cardiosphere-derived cells in a transgenic mouse model of non-ischaemic dilated cardiomyopathy. Eur Heart J 36(12):751–762
Ieronimakis N, Hays AL, Janebodin K, Mahoney WM Jr, Duffield JS, Majesky MW, Reyes M (2013) Coronary adventitial cells are linked to perivascular cardiac fibrosis via TGFbeta1 signaling in the mdx mouse model of Duchenne muscular dystrophy. J Mol Cell Cardiol 63:122–134
van Putten M, van der Pijl EM, Hulsker M, Verhaart IE, Nadarajah VD, van der Weerd L, Aartsma-Rus A (2014) Low dystrophin levels in heart can delay heart failure in mdx mice. J Mol Cell Cardiol 69:17–23
Seok HY, Chen J, Kataoka M, Huang ZP, Ding J, Yan J, Hu X, Wang DZ (2014) Loss of MicroRNA-155 protects the heart from pathological cardiac hypertrophy. Circ Res 114(10):1585–1595
Quattrocelli M, Crippa S, Montecchiani C, Camps J, Cornaglia AI, Boldrin L, Morgan J, Calligaro A, Casasco A, Orlacchio A, Gijsbers R, D’Hooge J, Toelen J, Janssens S, Sampaolesi M (2013) Long-term miR-669a therapy alleviates chronic dilated cardiomyopathy in dystrophic mice. J Am Heart Assoc 2(4):e000284
Bernardo BC, Nguyen SS, Gao XM, Tham YK, Ooi JY, Patterson NL, Kiriazis H, Su Y, Thomas CJ, Lin RC, Du XJ, McMullen JR (2016) Inhibition of miR-154 protects against cardiac dysfunction and fibrosis in a mouse model of pressure overload. Sci Rep 6:22442
Napoli C, Grimaldi V, De Pascale MR, Sommese L, Infante T, Soricelli A (2016) Novel epigenetic-based therapies useful in cardiovascular medicine. World J Cardiol 8(2):211–219
Saxena A, Dobaczewski M, Rai V, Haque Z, Chen W, Li N, Frangogiannis NG (2014) Regulatory T cells are recruited in the infarcted mouse myocardium and may modulate fibroblast phenotype and function. Am J Physiol Heart Circ Physiol 307(8):H1233–H1242
Acknowledgments
This work was supported by research Grants R01 HL112597, R01 HL116042, and R01 HL120659 to DK Agrawal from the National Heart, Lung and Blood Institute, National Institutes of Health, USA. The content of this review article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial and competing interests’ disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with financial interest or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Rights and permissions
About this article
Cite this article
Rai, V., Sharma, P., Agrawal, S. et al. Relevance of mouse models of cardiac fibrosis and hypertrophy in cardiac research. Mol Cell Biochem 424, 123–145 (2017). https://doi.org/10.1007/s11010-016-2849-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2849-0