Abstract
Purpose
To evaluate the pathological complete response (pCR) rate of locally advanced rectal cancer (LARC) after adaptive high-dose neoadjuvant chemoradiation (CRT) based on 18 F-fluorodeoxyglucose positron emission tomography/computed tomography (18 F-FDG-PET/CT).
Methods
The primary endpoint was the pCR rate. Secondary endpoints were the predictive value of 18 F-FDG-PET/CT on pathological response and acute and late toxicity. All patients performed 18 F-FDG-PET/CT at baseline (PET0) and after 2 weeks during CRT (PET1). The metabolic PET parameters were calculated both at the PET0 and PET1. The total CRT dose was 45 Gy to the pelvic lymph nodes and 50 Gy to the primary tumor, corresponding mesorectum, and to metastatic lymph nodes. Furthermore, a sequential boost was delivered to a biological target volume defined by PET1 with an additional dose of 5 Gy in 2 fractions. Capecitabine (825 mg/m2 twice daily orally) was prescribed for the entire treatment duration.
Results
Eighteen patients (13 males, 5 females; median age 55 years [range, 41–77 years]) were enrolled in the trial. Patients underwent surgical resection at 8–9 weeks after the end of neoadjuvant CRT. No patient showed grade > 1 acute radiation-induced toxicity. Seven patients (38.8%) had TRG = 0 (complete regression), 5 (27.0%) showed TRG = 2, and 6 (33.0%) had TRG = 3. Based on the TRG results, patients were classified in two groups: TRG = 0 (pCR) and TRG = 1, 2, 3 (non pCR). Accepting p < 0.05 as the level of significance, at the Kruskal–Wallis test, the medians of baseline-MTV, interim-SUVmax, interim-SUVmean, interim-MTV, interim-TLG, and the MTV reduction were significantly different between the two groups. 18 F-FDG-PET/CT was able to predict the pCR in 77.8% of cases through compared evaluation of both baseline PET/CT and interim PET/CT.
Conclusions
Our results showed that a dose escalation on a reduced target in the final phase of CRT is well tolerated and able to provide a high pCR rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer is still one of the most prevalent cancers in industrialized countries and about one third of tumors are in the rectum [1]. Preoperative chemoradiation (CRT) is currently considered the standard treatment of locally advanced rectal cancer (LARC) due to the positive impact on loco-regional control and on probability of sphincter-sparing resection [2, 3].
Traditionally, preoperative CRT is based on the combination of 45 to 50.4 Gy delivered with conventional fractionation and concurrent fluoropirimidine-based chemotherapy. This approach achieved a complete pathological response (pCR) in up to 10–20% of patients [4, 5].
Intensity-modulated radiation therapy with simultaneous integrated boost (IMRT-SIB) technique is able to deliver the same doses to the prophylactic volumes plus a boost on the macroscopic disease in the same treatment session, with optimal sparing of the surrounding normal tissues. Therefore, IMRT-SIB technique is potentially associated with lower acute toxicity rates and thus to improved feasibility of dose-escalated CRT [6, 7].
It is worth noting that LARCs significantly shrink, in most cases, during CRT. Therefore, escalating the dose only to the residual gross target volume (GTV), at the end of CRT, could improve treatment tolerability and pCR rates and provide better chances of conservative surgery [8,9,10].
Adaptive radiotherapy is a technique based on the progressive conformation of the irradiated volumes during treatment. This approach was mainly tested in patients with head and neck cancers [11, 12], while reliable data on LARC are still lacking. Moreover, the possibility to achieve the same pCR rate as standard treatments, despite the reduction of treated volumes during CRT, was never proven. Furthermore, no data is available on the imaging technique of choice for early evaluation of tumor response.
18 F-FDG-PET/CT is not routinely used in the staging or tumor response assessment of LARCs [13]. However, some studies showed a high reliability of 18 F-FDG-PET/CT in predicting the pathological response after CRT [14,15,16].
Therefore, based on this background, aims of this study were to (i) evaluate pCR rates of LARC after adaptive high-dose neoadjuvant CRT with concomitant and sequential boost based on 18 F-FDG-PET/CT and (ii) confirm the value of 18 F-FDG-PET/CT in predicting the pCR rate.
Material and methods
Study design and aims
This was a prospective phase II study approved by the local ethics committee and registered in an international public registry (NCT03479814). All patients enrolled in the study signed an informed consent.
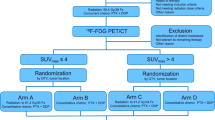
The primary aim was to assess the pCR rate after 18 F-FDG-PET/CT-based neoadjuvant CRT. Secondary aims were as follows: (a) treatment-related acute and late toxicity, (b) GTV-boost and PTV-boost reduction through 18 F-FDG-PET/CT re-evaluation 2 weeks after starting CRT, (c) predictive value of 18 F-FDG-PET/CT on pathological response, (d) progression-free survival (PFS), (e) overall survival (OS), and (f) treatment impact on quality of life (QoL). A flow chart of the trial is summarized in Fig. 1.
Eligibility criteria
Inclusion criteria were biopsy proven LARC with cT3-4N0-2M0 (any tumor site) or T2N1-2 M0 (only lower rectum) stage; age ≥ 18 years; Eastern Cooperative Oncology Group performance status ≤ 2; adequate hematological count and liver/renal function. Exclusion criteria were as follows: patients unfit for chemotherapy or surgery, metastatic disease not amenable for local radical treatments, pregnant or breast-feeding, severe cardiovascular disease, prior pelvic radiotherapy, patients with other primary neoplasms (except non-melanoma skin cancer or in situ cervical carcinoma), and patients not able to provide informed consent.
Outcome measures
At baseline, a clinical evaluation based on rectal examination and complete clinical history was performed. Subsequent assessments included colonoscopy with biopsy, routine blood tests with carcino-embryonic antigen (CEA), liver and renal function, trans-rectal ultrasound (TRUS), contrast-enhanced thorax-abdomen-pelvis computed tomography (CT) scan, and pelvic magnetic resonance imaging (MRI).18 F-FDG-PET/CT was performed before the start (PET0) and 2 weeks after starting CRT (PET1) to plan the sequential boost.
Clinical response was assessed with clinical examination, contrast-enhanced thorax-abdomen-pelvis CT scan, and pelvic MRI. Surgery was planned about 8 weeks after CRT completion.
Pathological tumor response was scored according to the College of American Pathologists [17] as follows: tumor regression grade (TRG)-0: non-viable cancer cells (complete response); TRG-1: single cells or rare small groups of cancer cells (near complete response); TRG-2: residual cancer with evident tumor regression but more than single cells or rare small groups of cancer cells (partial response); TRG-3: extensive residual cancer with no evident tumor regression (poor or no response). Based on the TRG results, patients were classified in two groups: TRG = 0 (complete pathological response) and TRG = 1, 2, 3 (non-complete pathological response).
Radiotherapy planning
All patients were immobilised with full bladder in supine position using the Combifix™ frame and underwent a baseline 18 F-FDG-PET/CT scan. Whole-body18 F-FDG-PET/CT was performed using a standard procedure. Briefly, 3.0 MBq/kg of 18F-FDG was intravenously injected. All patients were required to fast for 6 h and the uptake time was 60 min. Images were acquired on a 3-D tomograph (Discovery STE; GE) for 2 min per bed position. A low-dose CT scan (120 kV, 80 mA) was performed both for attenuation correction and to provide an anatomical map. 18 F-FDG-PET/CT images were reconstructed using an iterative 3-D ordered subsets expectation maximization method with two iterations and 20 subsets, followed by smoothing (with a 6-mm 3-D Gaussian kernel) with CT-based attenuation, scatter, and random coincidence event correction [18].
PET-CT analysis and target volumes definition
All 18 F-FDG-PET/CT scans were reviewed by two experienced nuclear medicine physicians who defined the PET positive regions. Sites of primary tumor and metastatic lymph nodes were defined as the GTV-PET0. A radiation oncologist with over 10-year experience in LARC treatment defined two clinical target volumes (CTV1 and CTV2). The CTV1 included the GTV-PET0 and the corresponding mesorectum (same cranio-caudal level) plus 2 cm cranio-caudally. The CTV2 included the CTV1 plus the entire mesorectum, the pre-sacral space, the internal iliac nodes, and the obturator nodes. Inguinal nodes were included in case of positive inguinal nodes or in patients with infiltration of the anal canal and/or external anal sphincter, and/or lower third of the vagina. The planning target volumes (PTV1 and PTV2) were generated by adding an isotropic expansion of 0.8 cm from CTV1 and CTV2, respectively.
Two weeks after CRT start, an interim 18F-FDG-PET/CT (PET1) was performed to plan the second phase of the treatment (sequential boost). Two nuclear medicine physicians examined the interim 18F-FDG-PET/CT (PET1), compared it with the PET0, and finally defined the PET1 positive region sites at primary tumor and metastatic lymph nodes as the GTV-PET1. Then, the radiation oncologist defined the CTV of the sequential boost (CTV3) as the GTV-PET1 plus an isotropic expansion of 0.5 cm. The PTV3 was generated by isotropically adding 0.8 cm to the CTV3. Figure 2 depicts the CTV delineation.
Image analysis and interpretation criteria
The following metabolic 18F-FDG-PET/CT parameters of LARCs were measured both at baseline and at the interim PET: SUVmax (maximum standardized uptake value), SUVmean (mean standardized uptake value), MTV (metabolic tumor volume), and TLG (total lesion glycolysis). MTV measurement was calculated on 18F-FDG-PET/CT images using a semi-quantitative (40% threshold) analysis. When necessary a visual evaluation was added to the semi quantitative analysis to avoid missing the tumor at the boundaries, SUVmax and SUVmean, normalized to body weight, were calculated within the MTV defined as above. TLG values were calculated as the product of MTV and SUVmean [19]. When the bladder (filled with radioactive urine) was very close to the primary lesion, a visual correction of the adjacent region of interest margin was necessary. Moreover, we calculated the SUVmax, SUVmean, MTV, and TLG percentage reduction between the baseline and interim scan using the following formula:
Organs at risk (OARs)
The following OARs were considered: bowel (defined as the “bowel bag”), bladder, and femoral heads. The acceptability of dose distribution to the OaRs was evaluated based on the dose/volume constraints suggested by the quantitative analysis of normal tissue effects in the clinic (QUANTEC) guidelines [20].
Intensity-modulated RT (IMRT)
IMRT was delivered using an Elekta Sinergy Linac (Elekta, Crowley, United Kingdom), equipped with standard multi leaf collimators, with 6–15 MV photon energy. During the first phase of CRT (IMRT-SIB on PTV1 and PTV2), a daily online check of the set-up was performed using an electronic portal imaging device, as previously described [21]. During the sequential boost (PTV3), before each daily session, patients underwent KV-Cone-Beam-CT to check and eventually correct organ motion and set-up inaccuracies. The RT dose delivered to the PTV2 was 45 Gy (1.8 Gy/fraction) with 50 Gy (2 Gy/fraction) SIB dose to the PTV1 in five consecutive days per week. The dose delivered to the PTV3 was 5.0 Gy (2.5 Gy/die) on two consecutive days (PTV3 total dose: 55 Gy). Planning and delivery processes underwent a systematic independent-check procedures, as previously described [22]. In patients with grade ≥ 3 acute toxicity, CRT was stopped until toxicity was decreased to at least grade 2 toxicity.
Chemotherapy
Concurrent chemotherapy was based on capecitabine (825 mg/m2 twice daily orally) and was prescribed for the entire CRT duration. The choice of adjuvant chemotherapy was left at the medical oncologist’s discretion based on initial stage and pathological examination.
Surgery
Surgery was scheduled about eight weeks after the end of the CRT. The total mesorectal excision with pelvic autonomic nerve preservation was performed when technically feasible. However, the choice among surgical approaches (abdomino-perineal resection or low anterior resection) was based on physical examination and the results of pelvic MRI-based restaging.
Follow-up
The first follow-up visit included physical examination and full blood count and was performed 4 weeks after surgery. Chest CT scan and abdominal-pelvic CT scan or MRI were performed every 6 months in the first 5 years and yearly thereafter. Local control was calculated from the date of diagnosis to the time of local–regional failure or last follow-up. Disease-free survival was defined as the time from diagnosis to local or distant recurrence or last follow-up. Overall survival was defined as the time from diagnosis until death from any cause or last follow-up. Acute and late toxicity data were scored according to the Common Terminology Criteria for Adverse Events (CTCAE v4.03). Quality of Life was evaluated using the EORTC QLQ-C30 questionnaire at the beginning and at the end of radiotherapy.
Sample size and statistical analysis
According to the Simon’s optimal two-stage design [23], this study required the enrolment of nine to 17 patients to prove or exclude a significant improvement of pCR rates. We planned the closure of the trial in case of no pCR in the first nine patients, while, in case of at least one pCR, the study was continued by including eight additional patients. However, considering a possible 5% drop-out rate, we increased the sample size of the second step to nine patients. Due to the small sample size, we used both the non-parametric Kruskal–Wallis test to compare the medians of 18F-FDG-PET/CT parameters and the Student’s t-test to compare the means of the same variables between the two groups (TRG = 0 versus TRG = 1, 2, 3) [24]. The univariate logistic regression was used to investigate whether metabolic PET parameters may predict the TRG [25]. Statistical analyses were performed using SPSS 20 (IBM Corp., Armonk, NY).
Results
Between September 2017 and October 2018, eighteen patients were enrolled in the trial (13 males, 5 female; median age: 55 years, range: 41–77 years). Tumor site was the inferior, middle, and superior rectum in 10 (55%), six (33%), and two (11%) patients, respectively. According to the American Joint Committee on Cancer (AJCC 2010), the clinical stages were T2N1M0 (2 patients), T3N1M0 (11 patients), T3N2M0 (2 patients), and T4N1M0 (3 patients). The mean GTV-PET0 and GTV-PET1 values were 21.97 cc (SD: ± 24.32) and 9.76 cc (SD: ± 15.26), respectively (p = 0.002). The mean PTV2 and PTV3 values were 175.6 cc (SD: ± 81.4) and 41.1 cc (SD: ± 36.5), respectively (p < 0.001).
Nineteen episodes of G1 acute toxicity were recorded in 17 patients (gastrointestinal: 7, genitourinary: 7, hematological: 2, skin: 3) while no patient showed acute G 2 toxicity. One patient with dihydro-pyrimidine dehydrogenase deficiency had acute G3 toxicity (hematological and gastrointestinal) while no patient showed G > 3 acute toxicity. Based on the EORTC QLQ-C30 questionnaire, no patient had relevant (> 20) changes in terms of quality of life. No patient showed late toxicity during the follow-up.
Surgical resection was performed eight-nine weeks after the end of CRT. Low anterior resection was performed in 14 patients (77%), abdomino-perineal resection in three patients (16%), and local excision in one patient (5.5%) who refused radical surgery after achieving a clinical complete response. According to the College of American Pathologists, seven patients (38.8%) had TRG = 0; five patients (27%) TRG = 2 and six patients (33%) had TRG 3.
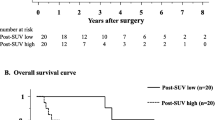
The median follow-up was 41.5 months (range: 13.0–50.0 months). Three patients showed hematogenous metastases, while two had distant metastases and synchronous local recurrence. The latter were both males, with low rectal cancer, and clinical stage T4N1. One-, 2-, 3-, and 4-year PFS rates were 100%, 76.7%, 76.7%, and 65.8%, respectively (Fig. 3). Finally, 1-, 2-, 3-, and 4-year OS rates were 100%, 94.1%, 88.2%, and 88.2%, respectively (Fig. 4).
At Kruskal–Wallis test, the median values of baseline-MTV, interim-SUVmax, interim-SUVmean, interim-MTV, interim-TLG, and the MTV reduction were significantly different between the group with TRG = 0 and the group with TRG = 1, 2, 3 (p = 0.016, p = 0.013, p = 0.030, p = 0.006, p = 0.006, and p = 0.033, respectively). Moreover, at the Student’s t-test, the means of the same variable reported above were significantly different between the two groups (p = 0.007, p = 0.025, p = 0.027, p = 0.007, p = 0.008, and p = 0.021, respectively). The results of the Kruskal–Wallis test and the Student’s t-test are presented in Tables 1 and 2, respectively.
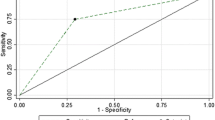
All variables were tested with a univariate logistic regression analysis to evaluate their role as TRG predictors. The only independent predictor of TRG was the MTV reduction between baseline- and interim-PET (odds ratio = 1.048, 95% CI = 1.001–1.097, Nagelkerke R2 = 0.378; p = 0.045). The logistic model built with this regressor correctly predicts the TRG after CRT in 77.8% of patients. Independent variables are reported in terms of odds ratios (OR) and their 95% confidence intervals (CI) in Table 3.
Discussion
The achievement of pCR after CRT is an important predictor of improved long-term outcome in LARC patients [26]. Standard CRT delivered with conventional doses (45–50 Gy) induces up to 20% pCR rates. RT dose intensification based on the delivery of SIB [27] as well as adaptive strategies [28] was investigated in order to improve these figures and clinical outcome in LARC.
To the best of our knowledge, this is the first report on 18 F-FDG-PET/CT -based adaptive dose-escalated CRT in LARC. Main aim of the current study was to evaluate the pathological response of LARC after adaptive high-dose neoadjuvant CRT with both simultaneous and sequential boost planned based on 18 F-FDG-PET/CT. We recorded 38.8% pCR rate with a low incidence of severe toxicity. In fact, only one case of grade 3 diarrhea was registered, yielding a severe toxicity rate of 5.5%.
The findings of our study are noteworthy for at least three reasons. First of all, we showed that 18 F-FDG-PET/CT was able to predict pCR in more than 75% of cases. Secondly, the implementation of adaptive CRT by 18 F-FDG-PET/CT allowed the delivery of dose-escalated treatment without worsening acute and late toxicity. Arguably, the dramatic reduction in PTV (by 76.6%), made possible by the interim 18 F-FDG-PET/CT, contributed to this result. Finally, this approach increased the pCR rate up to about 40% of cases.
18 F-FDG-PET/CT, as well as MRI, can be theoretically used for adaptive dose-escalated CRT in LARC [29–, 30,31,32]. Moreover, some studies reported a significant correlation between early 18 F-FDG-PET/CT and pathological tumor response [33,34,35]. Furthermore, the use of 18 F-FDG-PET/CT has been studied to optimize the initial target volume in preoperative CRT of LARC [34]. In the literature, the values of SUVmax have been found to correlate with response to CRT in rectal cancer [36]; interestingly, in our experience, the strongest prognosticator is the metabolic tumor volume reduction from the baseline to the interim 18 F-FDG-PET/CT.
In fact, in the prospective study by Alongi et al. [34], SIB-based dose intensification was tested in patients with LARC using 18 F-FDG-PET/CT. The latter was performed before CRT and was merged with the planning-CT scan to define a high-dose volumes including the hyper-metabolic areas of the primary tumor and metastatic nodes. Sixty and 54 Gy were delivered in 30 fractions to the hyper-metabolic areas and to the prophylactic volume, respectively. Tumor downstaging was reported in 62.5% of cases but the pCR rate was only 17.5%. Furthermore, 18 F-FDG-PET/CT was not able to predict pCR and no correlation was found between pre-treatment SUV-max values and pCR. However, unlike in our study, 18 F-FDG-PET/CT was carried out only before and not during CRT. This could explain the different results about pCR rate and 18 F-FDG-PET/CT predictive value.
Furthermore, as mentioned above, an interim 18 F-FDG-PET/CT, showing a rapid reduction of LARCs during CRT [37], has the further advantage of reducing the OaR-irradiated volume in the final phase of the treatment, with improved feasibility of intensified CRT regimens. Obviously, the greatest concern regarding dose-escalated CRT in LARC patients is the increased risk of toxicity, particularly in terms of gastrointestinal adverse effects. Some prospective studies based on IMRT (+ / − SIB), but without an adaptive strategy, have shown 23–35% pCR and 5–27% grade ≥ 3 toxicity rates [26, 27]. Our combined approach allowed to further improved pCR but without worsening of toxicity. This has important clinical implication based on the emerging data on the possibility to avoid major surgery in LARC patients with complete clinical response after preoperative CRT [30, 38].
The main limitations of our study are both small sample size and lack of a control group. Nevertheless, this trial should be considered as an exploratory study since it is the first prospective test of early 18 F-FDG-PET/CT to allow CRT dose escalation in LARC. Based on the results of our study, we can speculate that higher doses may be tested through this combined ad interim 18 F-FDG-PET/CT-based approach.
In conclusion, the results of our trial showed that adaptive individualized high-dose neoadjuvant CRT delivered with simultaneous and sequential boosts planned with 18 F-FDG-PET/CT is feasible and effective. In fact, our study showed that dose-escalation in the final phase of CRT is well tolerated and able to provide high pCR rate with a favorable toxicity profile.
Abbreviations
- CRT:
-
Chemoradiation
- LARC:
-
Locally advanced rectal cancer
- pCR:
-
Pathological complete response
- IMRT-SIB:
-
Intensity modulated radiation therapy with simultaneous integrated boost
- GTV:
-
Gross target volume
- 18 F-FDG-PET/CT:
-
18 F-fluorodeoxyglucose positron emission tomography/computed tomography
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- QoL:
-
Quality of life
- CEA:
-
Carcino-embryonic antigen
- TRUS:
-
Trans-rectal ultrasound
- CT:
-
Contrast-enhanced thorax-abdomen-pelvis computed tomography
- MRI:
-
Pelvic magnetic resonance imaging
- PET0 :
-
18 F-FDG-PET/CT at baseline
- PET1 :
-
18 F-FDG-PET/CT after 2 weeks during CRT
- TRG:
-
Tumor regression grade
- CTV:
-
Clinical target volume
- PTV:
-
Planning target volume
- SUVmax:
-
Maximum standardized uptake value
- SUVmean:
-
Mean standardized uptake value
- MTV:
-
Metabolic tumor volume
- TLG:
-
Total lesion glycolysis
- OARs:
-
Organs at risk
- IMRT:
-
Intensity-modulated RT
- QUANTEC:
-
Quantitative analysis of normal tissue effects in the clinic
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- AJCC:
-
American Joint Committee on Cancer
References
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–53.
Valentini V, Aristei C, Glimelius B, Minsky BD, Beets-Tan R, Borras JM, et al. Multidisciplinary rectal cancer management: 2nd European Rectal Cancer Consensus Conference (EURECA-CC2). Radiother Oncol 2009;92:148e63.
Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Sphincter preservation following pre-operative radiotherapy for rectal cancer: report of randomized trial comparing short-term radiotherapy versus conventionally fractionated radiochemotherapy. Radiother Oncol. 2004;72:15–24.
Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg. 2012;99:918–28.
Hiotis SP, Weber SM, Cohen AM, et al. Assessing the predictive value of clinical complete response to neoadjuvant therapy for rectal cancer: an analysis of 488 patients. J Am Coll Surg. 2002;194:131–5.
Engels B, Platteaux N, Van den Begin R, Gevaert T, Sermeus A, Storme G, Verellen D, De Ridder M. Preoperative intensity-modulated and image-guided radiotherapy with a simultaneous integrated boost in locally advanced rectal cancer: report on late toxicity and outcome. Radiother Oncol. 2014;110:155–9. https://doi.org/10.1016/j.radonc.2013.10.026.
Picardi V, Deodato F, Guido A, Giaccherini L, Macchia G, Gambacorta MA, Arcelli A, Farioli A, Cellini F, Cuicchi D, DI Fabio F, Poggioli G, Ardizzoni A, Frezza G, Cilla S, Caravatta L, Valentini V, Fuccio L, Morganti AG. Concurrent chemoradiation with concomitant boost in locally advanced rectal cancer: a phase II study. Anticancer Res. 2016;36:4081-7.
Habr-Gama A, Perez RO, São Julião GP, Proscurshim I, Gama-Rodrigues J. Nonoperative approaches to rectal cancer: a critical evaluation. Semin Radiat Oncol. 2011;21:234–9.
Maas M, Beets-Tan RG, Lambregts DM, Lammering G, Nelemans PJ, Engelen SM, van Dam RM, Jansen RL, Sosef M, Leijtens JW, Hulsewé KW, Buijsen J, Beets GL. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29:4633–40.
Appelt AL, Pløen J, Harling H, Jensen FS, Jensen LH, Jørgensen JCR, Lindebjerg J, Rafaelsen SR, Jakobsen A. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol 2015; 16: 919–27].
Surucu M, Shah KK, Roeske JC, Choi M, Small W Jr, Emami B. Adaptive radiotherapy for head and neck cancer. Technol Cancer Res Treat. 2017;16:218–23.
Zhao L, Wan Q, Zhou Y, Deng X, Xie C, Wu S. The role of replanning in fractionated intensity modulated radiotherapy for nasopharyngeal carcinoma. Radiother Oncol. 2011;98:23–7.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Rectal cancer, version 2.2021. Available at https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Accessed on 30 December 2021.
Maffione AM, Ferretti A, Grassetto G, Bellan E, Capirci C, Chondrogiannis S, Gava M, Marzola MC, Rampin L, Bondesan C, Colletti PM, Rubello D. Fifteen different 18F-FDG PET/CT qualitative and quantitative parameters investigated as pathological response predictors of locally advanced rectal cancer treated by neoadjuvant chemoradiation therapy. Eur J Nucl Med Mol Imaging. 2013;40:853–64.
Janssen MH, Ollers MC, Riedl RG, et al. Accurate prediction of pathological rectal tumor response after two weeks of preoperative radiochemotherapy using (18)fluorodeoxyglucose-positron emission tomography computed tomography imaging. Int J Radiat Oncol Biol Phys. 2010;77:392–9.
Kristiansen C, Loft A, Berthelsen AK, et al. PET/CT and histopathologic response to preoperative chemoradiation therapy in locally advanced rectal cancer. Dis Colon Rectum. 2008;51:21–5.
Washington MK, Berlin J, Branton P, Burgart LJ, Carter DK, Fitzgibbons PL, Halling K, Frankel W, Jessup J, Kakar S, Minsky B, Nakhleh R, Compton CC. Members of the Cancer Committee, College of American Pathologists. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Arch Pathol Lab Med. 2009;133:1539–51.
Lima GM, Matti A, Vara G, et al. Prognostic value of posttreatment 18 F-FDG PET/CT and predictors of metabolic response to therapy in patients with locally advanced cervical cancer treated with concomitant chemoradiation therapy: an analysis of intensity- and volume-based PET parameters. Eur J Nucl Med Mol Imaging. 2018;45:2139–46.
Lee JA. Segmentation of positron emission tomography images: some recommendations for target delineation in radiation oncology. Radiother Oncol. 2010;96:302–7.
Jackson A, Marks LB, Bentzen SM, Eisbruch A, Yorke ED, Ten Haken RK, Constine LS, Deasy JO. The lessons of QUANTEC: recommendations for reporting and gathering data on dose-volume dependencies of treatment outcome. Int J Radiat Oncol Biol Phys. 2010;76:S155–60.
Deodato F, Cilla S, Massaccesi M, Macchia G, Ippolito E, Caravatta L, Picardi V, Romanella M, Di Falco C, Bartollino A, Valentini V, Cellini N, De Spirito M, Piermattei A, Morganti AG. Daily on-line set-up correction in 3D-conformal radiotherapy: is it feasible? Tumori. 2012;98:441–4. https://doi.org/10.1700/1146.12637.
Morganti AG, Deodato F, Zizzari S, Cilla S, Digesu’ C, Macchia G, Panunzi S, De Gaetano A, Piermattei A, Cellini N, Valentini V. Complexity index (COMIX) and not type of treatment predicts undetected errors in radiotherapy planning and delivery. Radiother Oncol. 2008;89:320–9.
Simon R. Optimal two- stage design for phase II clinical trials. Control Clin Trials. 1989;10:1–10.
Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952;47:583–621.
Bagley SC, White H, Golomb BA. Logistic regression in the medical literature: standards for use and reporting, with particular attention to one medical domain. J Clin Epidemiol. 2001;54:979–85.
Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–44.
Picardi V, Macchia G, Guido A, Giaccherini L, Deodato F, Farioli A, Cilla S, Compagnone G, Ardizzoni A, Cuicchi D, Gambacorta MA, Cellini F, Frezza G, Poggioli G, Valetini V, Fuccio F, Morganti AG. Preoperative chemoradiation with VMAT-SIB in rectal cancer: a phase II study. Clin Colorectal Cancer. 2017;16:16–22.
Passoni P, Fiorino C, Slim N, Ronzoni M, Ricci V, Di Palo S, De Nardi P, Orsenigo Tamburini A, De Cobelli F, Losio C, Iacovelli NA, Broggi S, Staudacher C, Calandrino R, Di Muzio N. Feasibility of an adaptive strategy in preoperative radiochemotherapy for rectal cancer with image-guided tomotherapy: boosting the dose to the shrinking tumor. Int J Radiat Oncol Biol Phys. 2013;87:67–72.
Van Wickle JD, Paulson ES, Landry JC, Erickson BA, Hall WA. Adaptive radiation dose escalation in rectal adenocarcinoma: a review. J Gastrointest Oncol. 2017;8:902–14.
Bulens P, Thomas M, M. Deroose C, Haustermans K PET imaging in adaptive radiotherapy of gastrointestinal tumors. The Quarterly Journal of Nuclear Medicine and Molecular Imaging 2018;62:385–403.
Del Vescovo R, Trodella LE, Sansoni I, Cazzato RL, Battisti S, Giurazza F, Ramella S, Cellini F, Grasso RF, Trodella L, Beomonte ZB. MR imaging of rectal cancer before and after chemoradiation therapy. Radiol Med. 2012 Oct;117(7):1125–38. https://doi.org/10.1007/s11547-012-0804-2.
Valentini V, Gambacorta MA, Cellini F, Aristei C, Coco C, Barbaro B, Alfieri S, D’Ugo D, Persiani R, Deodato F, Crucitti A, Lupattelli M, Mantello G, Navarria F, Belluco C, Buonadonna A, Boso C, Lonardi S, Caravatta L, Barba MC, Vecchio FM, Maranzano E, Genovesi D, Doglietto GB, Morganti AG, La Torre G, Pucciarelli S, De Paoli A. The INTERACT Trial: Long-term results of a randomised trial on preoperative capecitabine-based radiochemotherapy intensified by concomitant boost or oxaliplatin, for cT2 (distal)-cT3 rectal cancer. Radiother Oncol. 2019 May;134:110–8. https://doi.org/10.1016/j.radonc.2018.11.023.
Cascini GL, Avallone A, Delrio P, Guida C, Tatangelo F, Marone P, Aloj L, De Martinis F, Comella P, Parisi V and Lastoria S 18F-FDG PET is an early predictor of pathologic tumor response to preoperative radiochemotherapy in locally advanced rectal cancer .J Nucl Med. 2006;47:1241–8
Alongi F, Fersino S, Mazzola R, Fiorentino A, Giaj-Levra N, Ricchetti F, Ruggieri R, Di Paola G, Cirillo M, Gori S, Salgarello M, Zamboni G, Ruffo G. Radiation dose intensification in pre-operative chemo-radiotherapy for locally advanced rectal cancer. Clin Transl Oncol. 2017;19:189–96.
Wu KC, Chen SW, Hsieh TC, Yen KY, Law KM, Kuo YC, Chang RF, Kao CH. Prediction of Neoadjuvant chemoradiotherapy response in rectal cancer with metric learning using pretreatment 18F-fluorodeoxyglucose positron emission tomography. Cancers. 2021;13:6350.
Memon S, Lynch AC, Akhurst T, Ngan SY, Warrier SK, Michael M, Heriot AG. Systematic review of FDG-PET prediction of complete pathological response and survival in rectal cancer. Ann Surg Oncol. 2014 Oct;21(11):3598–607. https://doi.org/10.1245/s10434-014-3753-z.
Anderson C, Koshy M, Staley C, Esiashvili N, Ghavidel S, Fowler Z, et al. PET/CT fusion in radiation management of patients with anorectal tumors. Int J Radiat Oncol Biol Phys. 2007;69:155–62.
Whelan S, Burneikis D, Kalady MF. Rectal cancer: maximizing local control and minimizing toxicity. J Surg Oncol. 2022;125:46–54.
Funding
Open access funding provided by Università Cattolica del Sacro Cuore within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology - Digestive tract
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guido, A., Cuicchi, D., Castellucci, P. et al. Adaptive Individualized high-dose preoperAtive (AIDA) chemoradiation in high-risk rectal cancer: a phase II trial. Eur J Nucl Med Mol Imaging 50, 572–580 (2023). https://doi.org/10.1007/s00259-022-05944-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05944-0