Abstract
Purpose
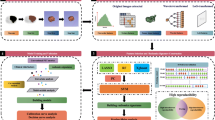
To develop and externally validate models incorporating a PET radiomics signature (R-signature) obtained by the cross-combination method for predicting the survival of patients with diffuse large B-cell lymphoma (DLBCL).
Methods
A total of 383 patients with DLBCL from two medical centres between 2011 and 2019 were included. The cross-combination method was used on three types of PET radiomics features from the training cohort to generate 49 feature selection-classification candidates based on 7 different machine learning models. The R-signature was then built by selecting the optimal candidates based on their progression-free survival (PFS) and overall survival (OS). Cox regression analysis was used to develop the survival prediction models. The calibration, discrimination, and clinical utility of the models were assessed and externally validated.
Results
The R-signatures determined by 12 and 31 radiomics features were significantly associated with PFS and OS, respectively (P<0.05). The combined models that incorporated R-signatures, metabolic metrics, and clinical risk factors exhibited significant prognostic superiority over the clinical models, PET-based models, and the National Comprehensive Cancer Network International Prognostic Index in terms of both PFS (C-index: 0.801 vs. 0.732 vs. 0.785 vs. 0.720, respectively) and OS (C-index: 0.807 vs. 0.740 vs. 0.773 vs. 0.726, respectively). For external validation, the C-indices were 0.758 vs. 0.621 vs. 0.732 vs. 0.673 and 0.794 vs. 0.696 vs. 0.781 vs. 0.708 in the PFS and OS analyses, respectively. The calibration curves showed good consistency, and the decision curve analysis supported the clinical utility of the combined model.
Conclusion
The R-signature could be used as a survival predictor for DLBCL, and its combination with clinical factors may allow for accurate risk stratification.






Similar content being viewed by others
Data availability
The datasets generated and analysed during the current study are available from the Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School, and the Jiangsu Province Hospital, the First Affiliated Hospital of Nanjing Medical University.
References
Liu Y, Barta SK. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2019;94(5):604–16.
Bakhshi TJ, Georgel PT. Genetic and epigenetic determinants of diffuse large B-cell lymphoma. Blood Cancer J. 2020;10(12):123.
Kwak JY. Treatment of diffuse large B cell lymphoma. Korean J Intern Med. 2012;27(4):369–77.
Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, Lefort S, Marit G, Macro M, Sebban C, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116(12):2040–2045.
Coiffier B, Sarkozy C. Diffuse large B-cell lymphoma: R-CHOP failure-what to do? Hematol Am Soc Hematol Educ Program. 2016;2016(1):366–78.
Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, Klasa R, Savage KJ, Shenkier T, Sutherland J, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109(5):1857–61.
International Non-Hodgkin's Lymphoma Prognostic Factors P. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329(14):987–994.
Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era[J]. Blood. 2014;123(6):837–42.
Gallicchio R, Mansueto G, Simeon V, Nardelli A, Guariglia R, Capacchione D, Soscia E, Pedicini P, Gattozzi D, Musto P, et al. F-18 FDG PET/CT quantization parameters as predictors of outcome in patients with diffuse large B-cell lymphoma. Eur J Haematol. 2014;92(5):382–9.
Jiang C, Teng Y, Chen J, Wang Z, Zhou Z, Ding C, Xu J. Value of (18)F-FDG PET/CT for prognostic stratification in patients with primary intestinal diffuse large B cell lymphoma treated with an R-CHOP-like regimen. Ann Nucl Med. 2020;34(12):911–9.
Xie M, Zhai W, Cheng S, Zhang H, Xie Y, He W. Predictive value of F-18 FDG PET/CT quantization parameters for progression-free survival in patients with diffuse large B-cell lymphoma. Hematology. 2016;21(2):99–105.
Xie M, Wu K, Liu Y, Jiang Q, Xie Y. Predictive value of F-18 FDG PET/CT quantization parameters in diffuse large B cell lymphoma: a meta-analysis with 702 participants. Med Oncol. 2015;32(1):446.
Vercellino L, Cottereau AS, Casasnovas O, Tilly H, Feugier P, Chartier L, Fruchart C, Roulin L, Oberic L, Pica GM, et al. High total metabolic tumor volume at baseline predicts survival independent of response to therapy. Blood. 2020;135(16):1396–405.
Stanta G, Bonin S. Overview on Clinical Relevance of Intra-Tumor Heterogeneity. Front Med (Lausanne). 2018;5:85.
McGranahan N, Swanton C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell. 2017;168(4):613–28.
Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, Sanduleanu S, Larue RTHM, Even AJG, Jochems A, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14(12):749–62.
Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, Zegers CM, Gillies R, Boellard R, Dekker A, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48(4):441–6.
Lue KH, Wu YF, Liu SH, Hsieh TC, Chuang KS, Lin HH, Chen YH. Prognostic Value of Pretreatment Radiomic Features of 18F-FDG PET in Patients With Hodgkin Lymphoma. Clin Nucl Med. 2019;44(10):e559–65.
Mayerhoefer ME, Riedl CC, Kumar A, Gibbs P, Weber M, Tal I, Schilksy J, Schöder H. Radiomic features of glucose metabolism enable prediction of outcome in mantle cell lymphoma. Eur J Nucl Med Mol Imaging. 2019;46(13):2760–9.
Wang H, Zhao S, Li L, Tian R. Development and validation of an (18)F-FDG PET radiomic model for prognosis prediction in patients with nasal-type extranodal natural killer/T cell lymphoma. Eur Radiol. 2020;30(10):5578–87.
Aide N, Fruchart C, Nganoa C, Gac AC, Lasnon C. Baseline (18)F-FDG PET radiomic features as predictors of 2-year event-free survival in diffuse large B cell lymphomas treated with immunochemotherapy. Eur Radiol. 2020;30(8):4623–32.
Lue KH, Wu YF, Lin HH, Hsieh TC, Liu SH, Chan SC, Chen YH. Prognostic Value of Baseline Radiomic Features of 18F-FDG PET in Patients with Diffuse Large B-Cell Lymphoma. Diagnostics (Basel). 2020;11(1):36.
Eertink JJ, van de Brug T, Wiegers SE, Zwezerijnen GJC, Pfaehler EAG, Lugtenburg PJ, van der Holt B, de Vet HCW, Hoekstra OS, Boellaard R, et al. 18F-FDG PET baseline radiomics features improve the prediction of treatment outcome in diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging. 2022;49(3):932–42.
Zhou Y, Ma XL, Zhang T, Wang J, Zhang T, Tian R. Use of radiomics based on (18)F-FDG PET/CT and machine learning methods to aid clinical decision-making in the classification of solitary pulmonary lesions: an innovative approach. Eur J Nucl Med Mol Imaging. 2021;48(9):2904–13.
Sun P, Wang D, Mok V C, Shi L. Comparison of feature selection methods and machine learning classifiers for radiomics analysis in glioma grading. IEEE Access. 2019;102010-102020.
Chang E, Joel MZ, Chang HY, Du J, Khanna O, Omuro A, et al. Comparison of radiomic feature aggregation methods for patients with multiple tumors. Sci Rep. 2021;11(1):9758.
Tixier F, Cheze-Le Rest C, Hatt M, Albarghach NM, Pradier O, Metges J-P, et al. Intratumor Heterogeneity Characterized by Textural Features on Baseline 18F-FDG PET Images Predicts Response to Concomitant Radiochemotherapy in Esophageal Cancer. J Nucl Med. 2011;52:369–78.
Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–354.
Huang S, Cai N, Pacheco PP, Narrandes S, Wang Y, Xu W. Applications of Support Vector Machine (SVM) Learning in Cancer Genomics. Cancer Genomics Proteomics. 2018;15(1):41–51.
Zhang Z, Jung C. GBDT-MO: Gradient-Boosted Decision Trees for Multiple Outputs. IEEE Trans Neural Netw Learn Syst. 2021;32(7):3156–67.
Hou N, Li M, He L, Xie B, Wang L, Zhang R, Yu Y, Sun X, Pan Z, Wang K. Predicting 30-days mortality for MIMIC-III patients with sepsis-3: a machine learning approach using XGboost. J Transl Med. 2020;18(1):462.
Zhang L, Liu M, Qin X, Liu G. Succinylation Site Prediction Based on Protein Sequences Using the IFS-LightGBM (BO) Model. Comput Math Methods Med. 2020;2020:8858489.
Sylvester EVA, Bentzen P, Bradbury IR, Clément M, Pearce J, Horne J, Beiko RG. Applications of random forest feature selection for fine-scale genetic population assignment. Evol Appl. 2018;11(2):153–65.
McEligot AJ, Poynor V, Sharma R, Panangadan A. Logistic LASSO Regression for Dietary Intakes and Breast Cancer. Nutrients. 2020;12(9):2652.
Nick TG, Campbell KM. Logistic regression. Methods Mol Biol. 2007;404:273–301.
Moon SH, Kim J, Joung JG, Cha H, Park WY, Ahn JS, Ahn MJ, Park K, Choi JY, Lee KH, et al. Correlations between metabolic texture features, genetic heterogeneity, and mutation burden in patients with lung cancer. Eur J Nucl Med Mol Imaging. 2019;46(2):446–54.
Choi ER, Lee HY, Jeong JY, Choi YL, Kim J, Bae J, Lee KS, Shim YM. Quantitative image variables reflect the intratumoral pathologic heterogeneity of lung adenocarcinoma. Oncotarget. 2016;7(41):67302–13.
Sala E, Mema E, Himoto Y, Veeraraghavan H, Brenton JD, Snyder A, Weigelt B, Vargas HA. Unravelling tumour heterogeneity using next-generation imaging: radiomics, radiogenomics, and habitat imaging. Clin Radiol. 2017;72(1):3–10.
Burrell RA, McGranahan N, Bartek J, Swanton C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. 2013;501(7467):338–45.
Liu J, Dang H, Wang XW. The significance of intertumor and intratumor heterogeneity in liver cancer. Exp Mol Med. 2018;50(1):e416.
Morris LG, Riaz N, Desrichard A, Şenbabaoğlu Y, Hakimi AA, Makarov V, Reis-Filho JS, Chan TA. Pan-cancer analysis of intratumor heterogeneity as a prognostic determinant of survival. Oncotarget. 2016;7(9):10051–63.
Sharma N, Gautam S K, Henry AA, Kumar A. Application of Big Data and Machine Learning. Machine Learning and Big Data: Concepts, Algorithms, Tools and Applications. 2020;305–333.
Gondaliyaa C P, Patel A M, Parikh S M. A Comparative Study on Machine Learning Based Algorithms. Proceedings of 3rd International Conference on Internet of Things and Connected Technologies (ICIoTCT). 2018;26–27.
Sasanelli M, Meignan M, Haioun C, Berriolo-Riedinger A, Casasnovas RO, Biggi A, Gallamini A, Siegel BA, Cashen AF, Véra P, et al. Pretherapy metabolic tumour volume is an independent predictor of outcome in patients with diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging. 2014;41(11):2017–22.
Mikhaeel NG, Smith D, Dunn JT, Phillips M, Møller H, Fields PA, Wrench D, Barrington SF. Combination of baseline metabolic tumour volume and early response on PET/CT improves progression-free survival prediction in DLBCL. Eur J Nucl Med Mol Imaging. 2016;43(7):1209–19.
Zucca E, Cascione L, Ruberto T, Facchinelli D, Schär S, Hayoz S, Dirnhofer S, Giovanella L, Bargetzi M, Mamot C, et al. Prognostic models integrating quantitative parameters from baseline and interim positron emission computed tomography in patients with diffuse large B-cell lymphoma: post-hoc analysis from the SAKK38/07 clinical trial. Hematol Oncol. 2020;38(5):715–25.
Miyazaki Y, Nawa Y, Miyagawa M, Kohashi S, Nakase K, Yasukawa M, Hara M. Maximum standard uptake value of 18F-fluorodeoxyglucose positron emission tomography is a prognostic factor for progression-free survival of newly diagnosed patients with diffuse large B cell lymphoma. Ann Hematol. 2013;92(2):239–44.
Shagera QA, Cheon GJ, Koh Y, Yoo MY, Kang KW, Lee DS, Kim EE, Yoon SS, Chung JK. Prognostic value of metabolic tumour volume on baseline (18)F-FDG PET/CT in addition to NCCN-IPI in patients with diffuse large B-cell lymphoma: further stratification of the group with a high-risk NCCN-IPI. Eur J Nucl Med Mol Imaging. 2019;46(7):1417–27.
Zhao P, Yu T, Pan Z. Prognostic value of the baseline 18F-FDG PET/CT metabolic tumour volume (MTV) and further stratification in low-intermediate (L-I) and high-intermediate (H-I) risk NCCNIPI subgroup by MTV in DLBCL MTV predict prognosis in DLBCL. Ann Nucl Med. 2021;35(1):24–30.
Funding
This work was partially supported by fundings for Clinical Trials from the Affiliated Drum Tower Hospital, Medical School of Nanjing University under Grant No. 2021-LCYJ-MS-04.
This work was also partially supported by fundings for the Key Project of Medical Science and Technology of Nanjing under Grant No.ZKX21011.
Author information
Authors and Affiliations
Contributions
Chong Jiang, Ang Li, Yue Teng, Xiangjun Huang, and Chongyang Ding collected data and analysed data; Jingyan Xu, Jianxin Chen, and Zhengyang Zhou participated in the research design; Chong Jiang and Ang Li contributed to the writing of the manuscript, discussed data, and supervised the study, and all authors performed data analysis and interpretation and read and approved the final version of the article.
Corresponding authors
Ethics declarations
Conflicts of interest/Competing interests
The authors declare that they have no conflicts of interest
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chong Jiang and Ang Li are co-first authors. They contributed equally to the work.
This article is part of the Topical Collection on Hematology
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiang, C., Li, A., Teng, Y. et al. Optimal PET-based radiomic signature construction based on the cross-combination method for predicting the survival of patients with diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging 49, 2902–2916 (2022). https://doi.org/10.1007/s00259-022-05717-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05717-9