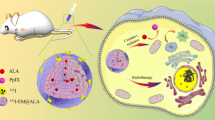
Abstract
Background
Tumor-derived exosomes (TEX) have shown great potential for drug delivery and tumor targeting. Here, we developed a novel multi-drug loaded exosomes nanoprobe for combined antitumor chemotherapy and photodynamic therapy, and monitoring the drug delivery capabilities with pre-targeting technique.
Methods
TEX of human colorectal cancer HCT116 was prepared, and Doxorubicin and the photodynamic therapy agent 5-aminolevulinic acid (ALA) were loaded and named as TEX@DOX@ALA. Tumor uptake was first examined using fluorescence imaging of the fluorescent dye Cy5 (TEX@DOX@ALA@Cy5). Visualization of exosome aggregation in tumor were realized by positron-emission tomography/computed tomography (PET/CT) with pre-targeting technique. Tumor-bearing mice were first injected with TEX@DOX@ALA labeled with azide (N3) (TEX@DOX@ALA@N3), and then 68Ga-(2,2′-((6-amino-1-(4,7-bis (carboxymethyl)-1,4,7-triazonan-1-yl) hexan-2-yl) azanediyl) diacetic acid-dibenzocyclooctyne (68Ga-L-NETA-DBCO) was injected after 24 h for PET/CT imaging via in vivo click chemistry. For the antitumor therapy with photodynamic and/or chemotherapy, seven groups of tumor-bearing mice with different therapy were monitored, and the tumor size, animal weight and the survival time were recorded. Furthermore, the samples of blood and interested tissues (heart, lung, liver, kidney, and spleen) were harvested for hematological analysis and H&E staining.
Results
The drug loading process did not influence the structure or the function of the HCT116 TEX membranes. In a fluorescence imaging experiment, higher fluorescence could be seen in tumor after TEX@DOX@ALA@Cy5 injected, and reached the highest signal at 24 h. From PET/CT images with subcutaneous and orthotopic colon tumor-bearing mice, clear radioactivity could be seen in tumors, which suggested the successes of TEX accumulation in tumors. TEX@DOX@ALA group with photodynamic therapy and chemotherapy had the best tumor inhibition effect compared with the other groups, with the longest survival time (36 days, 37.5%). No significant damage was found on histological observation and the blood biochemical analysis, which suggested the safety of the multi-drug loaded exosomes.
Conclusions
We successfully engineered an exosome-based nanoprobe integrating PET imaging components and therapeutic drugs. This drug-loaded exosome system may effectively target tumors and enable synergistic chemotherapeutic and photodynamic antitumor effects.







Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortalityworldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Peng P-C, Hong R-L, Tsai T, Chen C-T. Co-Encapsulation of Chlorin e6 and Chemotherapeutic Drugs in a PEGylated Liposome Enhance the Efficacy of Tumor Treatment: Pharmacokinetics and Therapeutic Efficacy. Pharmaceutics. 2019;11(11).
Legha SS, Benjamin RS, Mackay B, Ewer M, Wallace S, Valdivieso M, et al. Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion. p. 133-9.
Wang M, Zhai Y, Ye H, Lv Q, Sun B, Luo C, et al. High co-loading capacity and stimuli-responsive release based on Cascade reaction of self-destructive polymer for improved chemo-photodynamic therapy. ACS Nano. 2019;13(6):7010–23.
Dolmans DEJG, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3(5):380–7.
Chen W, Ouyang J, Liu H, Chen M, Zeng K, Sheng J, et al. Black Phosphorus Nanosheet-Based Drug Delivery System for Synergistic Photodynamic/Photothermal/Chemotherapy of Cancer. Advanced materials (Deerfield Beach, Fla). 2017;29(5).
Wang Y, Yang M, Qian J, Xu W, Wang J, Hou G, et al. Sequentially self-assembled polysaccharide-based nanocomplexes for combined chemotherapy and photodynamic therapy of breast cancer. Carbohydr Polym. 2019;203:203–13.
Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38(6):754–63.
Syn NL, Wang L, Chow EK-H, Lim CT, Goh B-C. Exosomes in Cancer nanomedicine and immunotherapy: prospects and challenges. Trends Biotechnol. 2017;35(7):665–76.
Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–35.
Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Advanced drug delivery reviews. 2016;106(Pt A):148-56.
Liu D, Yang F, Xiong F, Gu N. The smart drug delivery system and its clinical potential. Theranostics. 2016;6(9):1306–23.
Yang B, Chen Y, Shi J. Exosome Biochemistry and Advanced Nanotechnology for Next-Generation Theranostic Platforms. Advanced materials (Deerfield Beach, Fla). 2019;31(2):e1802896.
Qi H, Liu C, Long L, Ren Y, Zhang S, Chang X, et al. Blood exosomes endowed with magnetic and targeting properties for Cancer therapy. ACS Nano. 2016;10(3):3323–33.
Bellavia D, Raimondo S, Calabrese G, Forte S, Cristaldi M, Patinella A, et al. Interleukin 3- receptor targeted exosomes inhibit in vitro and in vivo chronic myelogenous leukemia cell growth. Theranostics. 2017;7(5):1333–45.
Jing B, Qian R, Jiang D, Gai Y, Liu Z, Guo F, et al. Extracellular vesicles-based pre-targeting strategy enables multi-modal imaging of. Journal of nanobiotechnology. 2021;19(1):151.
Jing B, Gai Y, Qian R, Liu Z, Zhu Z, Gao Y, et al. Hydrophobic insertion-based engineering of tumor cell-derived exosomes for SPECT/NIRF imaging of colon cancer. Journal of nanobiotechnology. 2021;19(1):7.
Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T, et al. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol. 2013;165(2):77–84.
Lim E-K, Kim T, Paik S, Haam S, Huh Y-M, Lee K. Nanomaterials for theranostics: recent advances and future challenges. Chem Rev. 2015;115(1):327–94.
Luo D, Goel S, Liu H-J, Carter KA, Jiang D, Geng J, et al. Intrabilayer (64) cu labeling of photoactivatable Doxorubicin-Loaded Stealth Liposomes. ACS nano. 2017;11(12):12482–91.
Kneepkens E, Fernandes A, Nicolay K, Grüll H. Iron (III)-based magnetic resonance-Imageable liposomal T1 contrast agent for monitoring temperature-induced image-guided drug delivery. Investig Radiol. 2016;51(11):735–45.
Ektate K, Kapoor A, Maples D, Tuysuzoglu A, VanOsdol J, Ramasami S, et al. Motion compensated ultrasound imaging allows thermometry and image guided drug delivery monitoring from echogenic liposomes. Theranostics. 2016;6(11):1963–74.
Stapleton S, Dunne M, Milosevic M, Tran CW, Gold MJ, Vedadi A, et al. Radiation and heat improve the delivery and efficacy of Nanotherapeutics by modulating Intratumoral fluid dynamics. ACS Nano. 2018;12(8):7583–600.
He S, Tourkakis G, Berezin O, Gerasimchuk N, Zhang H, Zhou H, et al. Temperature-dependent shape-responsive fluorescent nanospheres for image-guided drug delivery. J Mater Chem C. 2016;4(14):3028–35.
Gao N, Bozeman EN, Qian W, Wang L, Chen H, Lipowska M, et al. Tumor penetrating Theranostic nanoparticles for enhancement of targeted and image-guided drug delivery into peritoneal tumors following intraperitoneal delivery. Theranostics. 2017;7(6):1689–704.
Velikyan I. Continued rapid growth in (68) Ga applications: update 2013 to June 2014. Journal of labelled compounds & radiopharmaceuticals. 2015;58(3):99–121.
Zhang X, Ding B, Qu C, Li H, Sun Y, Gai Y, et al. A thiopyrylium salt for PET/NIR-II tumor imaging and image-guided surgery. Mol Oncol. 2020;14(5):1089–100.
Li M, Fang H, Liu Q, Gai Y, Yuan L, Wang S, et al. Red blood cell membrane-coated upconversion nanoparticles for pretargeted multimodality imaging of triple-negative breast cancer. Biomaterials science. 2020;8(7):1802–14.
Ding N, Zou Z, Sha H, Su S, Qian H, Meng F, et al. iRGD synergizes with PD-1 knockout immunotherapy by enhancing lymphocyte infiltration in gastric cancer. Nat Commun. 2019;10(1):1336.
Gregson AL, Hoji A, Injean P, Poynter ST, Briones C, Palchevskiy V, et al. Altered Exosomal RNA profiles in Bronchoalveolar lavage from lung transplants with acute rejection. Am J Respir Crit Care Med. 2015;192(12):1490–503.
Fu W, Lei C, Liu S, Cui Y, Wang C, Qian K, et al. CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat Commun. 2019;10(1):4355.
Cheng Z, Liu S, Wu X, Raza F, Li Y, Yuan W, et al. Autologous erythrocytes delivery of berberine hydrochloride with long-acting effect for hypolipidemia treatment. Drug delivery. 2020;27(1):283–91.
Liu H, Hu Y, Sun Y, Wan C, Zhang Z, Dai X, et al. Co-delivery of bee venom Melittin and a photosensitizer with an organic-inorganic hybrid Nanocarrier for photodynamic therapy and immunotherapy. ACS Nano. 2019;13(11):12638–52.
Milman N, Ginini L, Gil Z. Exosomes and their role in tumorigenesis and anticancer drug resistance. Drug Resist Updat. 2019;45.
He C, Zheng S, Luo Y, Wang B. Exosome Theranostics: biology and translational medicine. Theranostics. 2018;8(1):237–55.
Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev. 2004;56(11):1649–59.
Yi YW, Lee JH, Kim S-Y, Pack C-G, Ha DH, Park SR, et al. Advances in Analysis of Biodistribution of Exosomes by Molecular Imaging. International journal of molecular sciences. 2020;21(2).
Jung KO, Jo H, Yu JH, Gambhir SS, Pratx G. Development and MPI tracking of novel hypoxia-targeted theranostic exosomes. Biomaterials. 2018;177:139–48.
Kooijmans SAA, Stremersch S, Braeckmans K, de Smedt SC, Hendrix A, Wood MJA, et al. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. Journal of controlled release : official journal of the Controlled Release Society. 2013;172(1):229–38.
Toffoli G, Hadla M, Corona G, Caligiuri I, Palazzolo S, Semeraro S, et al. Exosomal doxorubicin reduces the cardiac toxicity of doxorubicin. Nanomedicine (Lond). 2015;10(19):2963–71.
Betzer O, Barnoy E, Sadan T, Elbaz I, Braverman C, Liu Z, et al. Advances in imaging strategies for in vivo tracking of exosomes. Wiley interdisciplinary reviews Nanomedicine and nanobiotechnology. 2020;12(2):e1594.
Molavipordanjani S, Khodashenas S, Abedi SM, Moghadam MF, Mardanshahi A, Hosseinimehr SJ. (99m)Tc-radiolabeled HER2 targeted exosome for tumor imaging. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2020;148:105312.
Abello J, Nguyen TDT, Marasini R, Aryal S, Weiss ML. Biodistribution of gadolinium- and near infrared-labeled human umbilical cord mesenchymal stromal cell-derived exosomes in tumor bearing mice. 2019(1838-7640 (Electronic)).
Jing B, Qian R, Jiang D, Gai Y, Liu Z, Guo F, et al. Extracellular vesicles-based pre-targeting strategy enables multi-modal imaging of orthotopic colon cancer and image-guided surgery. J Nanobiotechnology. 2021;19(1):151.
Li M, Fang H, Liu Q, Gai Y, Yuan L, Wang S, et al. Red blood cell membrane-coated upconversion nanoparticles for pretargeted multimodality imaging of triple-negative breast cancer. Biomater Sci. 2020;8(7):1802–14.
Fang H, Li M, Liu Q, Gai Y, Yuan L, Wang S, et al. Ultra-sensitive Nanoprobe modified with tumor cell membrane for UCL/MRI/PET multimodality precise imaging of triple-negative breast Cancer. Nanomicro Lett. 2020;12(1):62.
Hameed S, Bhattarai P, Liang X, Zhang N, Xu Y, Chen M, et al. Self-assembly of porphyrin-grafted lipid into nanoparticles encapsulating doxorubicin for synergistic chemo-photodynamic therapy and fluorescence imaging. Theranostics. 2018;8(19):5501–18.
Su J, Sun H, Meng Q, Zhang P, Yin Q, Li Y. Enhanced blood Suspensibility and laser-activated tumor-specific drug release of Theranostic mesoporous silica nanoparticles by functionalizing with erythrocyte membranes. Theranostics. 2017;7(3):523–37.
Ma L, Kohli M, Smith A. Nanoparticles for combination drug therapy. ACS Nano. 2013;7(11):9518–25.
Liao B, Liang H, Chen J, Liu Q, Zhang B, Chen X. Suberoylanilide hydroxamic acid enhances chemosensitivity to 5-fluorouracil in hepatocellular carcinoma via inhibition of thymidylate synthase. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2015;36(12):9347–56.
Luo D, Goel S, Liu HJ, Carter KA, Jiang D, Geng J, et al. Intrabilayer (64)cu labeling of photoactivatable. Doxorubicin-Loaded Stealth Liposomes ACS Nano. 2017;11(12):12482–91.
Zhang J, Shen L, Li X, Song W, Liu Y, Huang L. Nanoformulated Codelivery of quercetin and Alantolactone promotes an antitumor response through synergistic immunogenic cell death for microsatellite-stable colorectal Cancer. ACS Nano. 2019;13(11):12511–24.
Nam G-H, Choi Y, Kim GB, Kim S, Kim SA, Kim I-S. Emerging Prospects of Exosomes for Cancer Treatment: From Conventional Therapy to Immunotherapy. Advanced materials (Deerfield Beach, Fla). 2020;32(51):e2002440.
Acknowledgements
We would like to thank Ms. Guang-Xin Wang and Ms. Yan Wang at The Analysis and Testing Center of Institute of Hydrobiology, Chinese Academy of Sciences for discussion and comments on the manuscript. We would also like to acknowledge the service provided by Beijing novel medical equipment Ltd. for image acquisition.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81873904 and 82071966).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All experimental schemes were performed under the guidance and approved by the Institutional Animal Care and Use Committee of Tongji Medical College of Huazhong University of Science and Technology. BALB/c nude mice (female, 5-6 weeks old) purchased from Weitong Lihua Laboratory Animal Center (Beijing, China), and maintained in a pathogen-free environment. Extensive efforts were made to ensure minimal suffering of the animals used during the study.
Competing interests
The authors have declared that no competing interest exists.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Translational research.
Supplementary Information
ESM 1
(DOCX 2278 kb)
Rights and permissions
About this article
Cite this article
Qian, R., Jing, B., Jiang, D. et al. Multi-antitumor therapy and synchronous imaging monitoring based on exosome. Eur J Nucl Med Mol Imaging 49, 2668–2681 (2022). https://doi.org/10.1007/s00259-022-05696-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05696-x