Abstract
Purpose
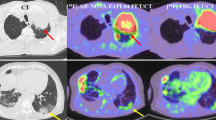
[18F]FAPI-42 is a new fibroblast activation protein (FAP)-specific tracer used for cancer imaging. Here, we describe the optimal acquisition time and in vivo evaluation of [18F]FAPI-42 and compared intra-individual biodistribution, tumor uptake, and detection ability to [68Ga]Ga-FAPI-04.
Methods
A total of 22 patients with various types of cancer received [18F]FAPI-42 whole-body positron emission tomography/computed tomography (PET/CT). Among them, 4 patients underwent PET/CT scans, including an early dynamic 20-min, static 1-h, and static 2-h scans. The in vivo biodistribution in normal organs and tumor uptake were semiquantitatively evaluated using the standardized uptake value (SUV) and tumor-to-background ratio (TBR). Furthermore, both [18F]FAPI-42 and [68Ga]Ga-FAPI-04 PET/CT were performed in 12 patients to compare biodistribution, tumor uptake, and tumor detection ability.
Results
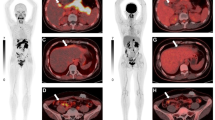
[18F]FAPI-42 uptake in the tumors was rapid and reached a high level with an average SUVmax of 15.8 at 18 min, which stayed at a similarly high level to 2 h. The optimal image acquisition time for [18F]FAPI-42 was determined to be 1 h postinjection. For tumor detection, [18F]FAPI-42 had a high uptake and could be clearly visualized in the lesions. Compared to [68Ga]Ga-FAPI-04, [18F]FAPI-42 had the same detectability for 144 positive lesions. In addition, [18F]FAPI-42 showed a higher SUVmax in liver and bone lesions (P < 0.05) and higher TBRs in liver, bone, lymph node, pleura, and peritoneal lesions (all P < 0.05).
Conclusion
The present study demonstrates that the optimal image acquisition time of [18F]FAPI-42 is 1 h postinjection and that [18F]FAPI-42 exhibits comparable lesion detectability to [68Ga]Ga-FAPI-04.
Trial registration
Chinese Clinical Trial Registry (ChiCTR2100045757).







Similar content being viewed by others
References
Bušek P, Mal R, Šedo A. Dipeptidyl peptidase IV activity and / or structure homologues (DASH) and their substrates in cancer. Int J Biochem Cell Biol. 2004;36:408–21.
Liu F, Qi L, Liu B, Liu J, Zhang H, Che DH, et al. Fibroblast activation protein overexpression and clinical implications in solid tumors: a meta-analysis. PLoS ONE. 2015;10:1–18.
Yang L, Ma L, Lai D. Over-expression of fibroblast activation protein alpha increases tumor growth in xenografts of ovarian cancer cells. Acta Biochim Biophys Sin. 2013 928–37.
Busek P, Mateu R, Zubal M, Kotackova L, Sedo A. Targeting fibroblast activation protein in cancer–prospects and caveats. Front Biosci. 2018 1933–68.
van der Geest T, Roeleveld DM, Walgreen B, Helsen MM, Nayak TK, Klein C, et al. Imaging fibroblast activation protein to monitor therapeutic effects of neutralizing interleukin-22 in collagen-induced arthritis. Rheumatol. 2018;57:737–47.
Brokopp CE, Schoenauer R, Richards P, Bauer S, Lohmann C, Emmert MY, et al. Fibroblast activation protein is induced by inflammation and degrades type i collagen in thin-cap fibroatheromata. Eur Heart J. 2011;32:2713–22.
Fan MH, Zhu Q, Li HH, Ra HJ, Majumdar S, Gulick DL, et al. Fibroblast activation protein (FAP) accelerates collagen degradation and clearance from lungs in mice. J Biol Chem. 2016;291:8070–89.
Lay AJ, Zhang HE, Mccaughan GW, Gorrell MD, Fap S, Fap C, et al. Fibroblast activation protein in liver fibrosis. Front Biosci. 2019 1–17.
Rettig WJ, Garin-Chesa P, Beresford HR, Oettgen HF, Melamed MR, Old LJ. Cell-surface glycoproteins of human sarcomas: differential expression in normal and malignant tissues and cultured cells. Proc Natl Acad Sci U S A. 1988;85:3110–4.
Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, Debus J, et al. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med. 2018;59:1415–22.
Chen H, Pang Y, Wu J, Zhao L, Hao B, Wu J, et al. Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F]FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur J Nucl Med Mol Imaging. 2020. https://doi.org/10.1007/s00259-020-04769-z.
Loktev A, Lindner T, Mier W, Debus J, Altmann A, Jäger D, et al. A tumor-imaging method targeting cancer-associated fibroblasts. J Nucl Med. 2018;59:1423–9.
Kuten J, Levine C, Shamni O, Pelles S, Wolf I, Lahat G, et al. Head-to-head comparison of [68Ga]Ga-FAPI-04 and [18F]FDG PET/CT in evaluating the extent of disease in gastric adenocarcinoma. Eur J Nucl Med Mol Imaging. 2021. https://doi.org/10.1007/s00259-021-05494-x.
Shi X, Xing H, Yang X, Li F, Yao S, Zhang H, et al. Fibroblast imaging of hepatic carcinoma with 68Ga-FAPI-04 PET/CT : a pilot study in patients with suspected hepatic nodules. Eur J Nucl Med Mol Imaging. 2021;48(1):196–203.
Kesch C, Yirga L, Dendl K, Handke A, Darr C, Krafft U, et al. High fibroblast‑activation‑protein expression in castration‑resistant prostate cancer supports the use of FAPI‑molecular theranostics. Eur J Nucl Med Mol Imaging. 2021 1–5.
Windisch P, Röhrich M, Regnery S, Tonndorf-martini E, Held T, Lang K, et al. Fibroblast activation protein (FAP) specific PET for advanced target volume delineation in glioblastoma. Radiother Oncol. 2020;150:159–63.
Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. 2019;60:801–5.
Wang H, Zhu W, Ren S, Kong Y, Huang Q, Zhao J. Ga-FAPI-04 versus 18F-FDG PET/CT in the detection of hepatocellular carcinoma. Front Oncol. 2021;11:693640.
Miller PW, Long NJ, Vilar R, Gee AD. Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew Chemie-Int Ed. 2008;47:8998–9033.
Hu K, Li J, Wang L, Huang Y, Li L, Ye S, et al. Preclinical evaluation and pilot clinical study of [18F]AlF-labeled FAPI-tracer for PET imaging of cancer associated fibroblasts. Acta Pharm. Sin. B. 2021 In press. https://doi.org/10.1016/j.apsb.2021.09.032
Wang S, Zhou X, Xu X, Ding J, Liu S, Hou X, et al. Clinical translational evaluation of Al18F-NOTA-FAPI for fibroblast activation protein-targeted tumour imaging. Eur J Nucl Med Mol Imaging. 2021. https://doi.org/10.1007/s00259-021-05470-5.
Jiang X, Wang X, Shen T, Yao Y, Chen M, Li Z, et al. FAPI-04 PET/CT Using [18F]AlF labeling strategy: automatic synthesis, quality control, and in vivo assessment in patient. Front Oncol. 2021;11:1–9.
Huang Y, Li H, Ye S, Tang G, Liang Y, Hu K. Synthesis and preclinical evaluation of an Al18F radiofluorinated bivalent PSMA ligand. Eur J Med Chem. 2021;221:113502.
Loktev A, Lindner T, Burger EM, Altmann A, Giesel F, Kratochwil C, et al. Development of fibroblast activation protein-targeted radiotracers with improved tumor retention. J Nucl Med. 2019;60:1421–9.
Tan H, Sui X, Yin H, Yu H, Gu Y, Chen S, et al. Total-body PET/CT using half-dose FDG and compared with conventional PET/CT using full-dose FDG in lung cancer. Eur J Nucl Med Mol Imaging. 2021;48:1966–75.
Spencer BA, Berg E, Schmall JP, Omidvari N, Leung EK, Abdelhafez YG, et al. Performance evaluation of the uEXPLORER total-body PET/CT scanner based on NEMA NU 2–2018 with additional tests to characterize PET scanners with a long axial field of view. J Nucl Med. 2021;62:861–70.
Altmann A, Haberkorn U, Siveke J. The latest developments in imaging of fibroblast activation protein. J Nucl Med. 2021;62:160–7.
Giesel FL, Adeberg S, Syed M, Lindner T, Jiménez-Franco LD, Mavriopoulou E, et al. FAPI-74 PET/CT using either 18F-AlF or cold-kit 68Ga labeling: biodistribution, radiation dosimetry, and tumor delineation in lung cancer patients. J Nucl Med. 2021;62:201–7.
Meyer C, Dahlbom M, Lindner T, Vauclin S, Mona C, Slavik R, et al. Radiation dosimetry and biodistribution of 68Ga-FAPI-46 PET imaging in cancer patients. J Nucl Med. 2020;61:1171–7.
Toms J, Kogler J, Maschauer S, Daniel C, Schmidkonz C, Kuwert T, et al. Targeting fibroblast activation protein: radiosynthesis and preclinical evaluation of an 18F-labeled FAP inhibitor. J Nucl Med. 2020;61:1806–13.
Wang S, Zhou X, Xu X, Ding J, Liu T, Jiang J, et al. Dynamic PET/CT imaging of 68Ga-FAPI-04 in chinese subjects. Front Oncol. 2021;11:1–11.
Rohrich M, Naumann P, Giesel FL, Choyke PL, Staudinger F, Wefers A, et al. Impact of 68Ga-FAPI PET/CT imaging on the therapeutic management of primary and recurrent pancreatic ductal adenocarcinomas. J Nucl Med. 2021;62:779–86.
Mankoff DA, Eary JF, Link JM, Muzi M, Rajendran JG, Spence AM, et al. Tumor-specific positron emission tomography imaging in patients: [18F]fluorodeoxyglucose and beyond. Clin Cancer Res. 2007;13:3460–9.
Acknowledgements
We would like to thank all the patients who participated in this study. We also thank the staff at the Department of Nuclear Medicine for their contribution to this work.
Funding
This work was supported in part by the National Natural Science Foundation of China (91949121), Guangdong Basic and Applied Basic Research Foundation (2021A1515011099), Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University (2017J010), and Nanfang Hospital Talent Introduction Foundation of Southern Medical University (123456).
Author information
Authors and Affiliations
Contributions
Conception and design, K. Hu and G. Tang; acquiring data, S. Huang, Y. Tian, Q. Wang, C. Xiao, L. Wang, and Y. Han; analyzing data, L. Wang, Y. Han, Y. Tian, K. Hu, and H. Wu; drafting the manuscript: K. Hu, L. Wang, and H. Wu; revising the manuscript, G. Tang and Y. Han; and enhancing the manuscript’s intellectual content, H. Wu. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
All procedures involving human participants were carried out in accordance with the Ethics Committee of Nanfang Hospital (No. NFEC-2020–205) and registered in the Chinese Clinical Trial Registry (ChiCTR2100045757).
Consent to participate
Written informed consent was obtained from all participants included in the study.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kongzhen Hu and Lijuan Wang contributed equally to this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hu, K., Wang, L., Wu, H. et al. [18F]FAPI-42 PET imaging in cancer patients: optimal acquisition time, biodistribution, and comparison with [68Ga]Ga-FAPI-04. Eur J Nucl Med Mol Imaging 49, 2833–2843 (2022). https://doi.org/10.1007/s00259-021-05646-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05646-z