Abstract
Purpose
Approximately 40–70% of biochemically persistent or recurrent prostate cancer (PCa) patients after radical prostatectomy (RPE) are oligo-metastatic in 68gallium-prostate-specific membrane antigen positron emission tomography (68Ga-PSMA PET). Those lesions are frequently located outside the prostate bed, and therefore not cured by the current standards of care like external-beam radiotherapy (EBRT) of the prostatic fossa. This retrospective study analyzes the influence of oligo-metastases’ site on outcome after metastasis-directed radiotherapy (MDR).
Methods
Retrospectively, 359 patients with PET-positive PCa recurrences after RPE were analyzed. Biochemical recurrence-free survival (BRFS) (prostate-specific antigen (PSA) < post-radiotherapy nadir + 0.2 ng/mL) was assessed using Kaplan-Meier survival and Cox regression analysis.
Results
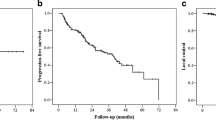
All patients were initially clinically without distant metastases (cM0). Seventy-five patients had local recurrence within the prostatic fossa, 32 patients had pelvic nodal plus local recurrence, 117 patients had pelvic nodal recurrence, 51 patients had paraaortic lymph node metastases with/without locoregional recurrence, and 84 patients had bone or visceral metastases with/without locoregional recurrence. Median PSA before MDR was 1.2 ng/mL (range, 0.04–47.5). Additive androgen deprivation therapy (ADT) was given in 35% (125/359) of patients. Median PSA nadir after MDR was 0.23 ng/mL (range, < 0.03–18.30). After a median follow-up of 16 months (1–57), 239/351 (68%) patients had no biochemical recurrence. Patients with distant lymph node and/or distant metastases, the so-called oligo-body cohort, had an overall in-field control of 90/98 (91%) but at the same time, an ex-field progress of 44/96 (46%). In comparison, an ex-field progress was detected in 28/154 (18%) patients with local and/or pelvic nodal recurrence (oligo-pelvis group). Compared with the oligo-pelvis group, there was a significantly lower BRFS in oligo-body patients at the last follow-up.
Conclusion
Overall, BRFS was dependent on patterns of metastatic disease. Thus, MDR of PSMA PET-positive oligo-metastases can be offered considering that about one-third of the patients progressed within a median follow-up of 16 months.


Similar content being viewed by others
References
Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8–10.
Afshar-Oromieh A, Holland-Letz T, Giesel FL, Kratochwil C, Mier W, Haufe S, et al. Diagnostic performance of (68)Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44(8):1258–68.
Pollack A, Karrison T, Balogh A, Low D, Bruner D, Wefel J. Short term androgen deprivation therapy without or with pelvic lymph node treatment added to prostate bed only salvage radiotherapy: the NRG oncology/RTOG 0534 SPPORT trial. Int J Radiat Oncol Biol Phys. 2018;102:1605.
Caroli P, Sandler I, Matteucci F, De Giorgi U, Uccelli L, Celli M, et al. 68Ga-PSMA PET/CT in patients with recurrent prostate cancer after radical treatment: prospective results in 314 patients. Eur J Nucl Med Mol Imaging. 2018;45(12):2035–44.
Sachpekidis C, Bäumer P, Kopka K, Hadaschik BA, Hohenfellner M, Kopp-Schneider A, et al. 68Ga-PSMA PET/CT in the evaluation of bone metastases in prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45(6):904–12.
Grubmüller B, Baltzer P, D’Andrea D, Korn S, Haug AR, Hacker M, et al. 68Ga-PSMA 11 ligand PET imaging in patients with biochemical recurrence after radical prostatectomy – diagnostic performance and impact on therapeutic decision-making. Eur J Nucl Med Mol Imaging. 2018;45(2):235–42.
Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, et al. Diagnostic efficacy of (68)gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol. 2016;195(5):1436–43.
Sawicki LM, Kirchner J, Buddensieck C, Antke C, Ullrich T, Schimmöller L, et al. Prospective comparison of whole-body MRI and 68Ga-PSMA PET/CT for the detection of biochemical recurrence of prostate cancer after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2019;46(7):1542–50.
Lawton CA, Michalski J, El-Naqa I, Buyyounouski MK, Lee WR, Menard C. RTOG GU radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74(2):383–7.
https://www.rtog.org/CoreLab/ContouringAtlases/ProstatePostOp.aspx. Accessed 01.29.2018, 2018.
Gillessen S, Attard G, Beer TM, Beltran H, Bossi A, Bristow R, et al. Management of patients with advanced prostate cancer: the report of the Advanced Prostate cancer Consensus Conference APCCC 2017. Eur Urol. 2018;73(2):178–211.
Foster CC, Weichselbaum RR, Pitroda SP. Oligometastatic prostate cancer: reality or figment of imagination? Cancer. 2019;125(3):340–52.
Emmett L, Van Leeuwen P, Nandurkar R, Scheltema MJ, Cusick T, Hruby G, et al. Treatment outcomes from (68)GaPSMA PET CT informed salvage radiation treatment in men with rising PSA following radical prostatectomy: prognostic value of a negative PSMA PET. J Nucl Med. 2017;58:1972–6.
Henkenberens C, Bengel F, Wester HJ, Christiansen H, Derlin T. Early efficacy of 68 ga-PSMA ligand positron emission tomography/computed tomography–based radiation treatment in locally recurrent and oligometastatic prostate cancer after primary therapy. Int J Radiat Oncol Biol Phys. 2016;96:267.
Schmidt-Hegemann N-S, Stief C, Kim T-H, Eze C, Kirste S, Strouthos I, et al. Outcome after PSMA PET/CT–based salvage radiotherapy in patients with biochemical recurrence after radical prostatectomy: a 2-institution retrospective analysis. J Nucl Med. 2019;60(2):227–33.
Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393:2051–8.
Baumann R, Koncz M, Luetzen U, Krause F, Dunst J. Oligometastases in prostate cancer. Strahlenther Onkol. 2018;194(4):318–24.
Guler OC, Engels B, Onal C, Everaert H, Van den Begin R, Gevaert T, et al. The feasibility of prostate-specific membrane antigen positron emission tomography (PSMA PET/CT)-guided radiotherapy in oligometastatic prostate cancer patients. Clin Transl Oncol. 2018;20(4):484–90.
Henkenberens C, von Klot CA, Ross TL, Bengel FM, Wester HJ, Merseburger AS, et al. (68)Ga-PSMA ligand PET/CT-based radiotherapy in locally recurrent and recurrent oligometastatic prostate cancer : early efficacy after primary therapy. Strahlenther Onkol. 2016;192(7):431–9.
Kneebone A, Hruby G, Ainsworth H, Byrne K, Brown C, Guo L, et al. Stereotactic body radiotherapy for oligometastatic prostate cancer detected via prostate-specific membrane antigen positron emission tomography. Eur Urol Oncol. 2018;1(6):531–7.
Soldatov A, von Klot CAJ, Walacides D, Derlin T, Bengel FM, Ross TL, et al. Patterns of progression after 68Ga-PSMA-ligand PET/CT-guided radiation therapy for recurrent prostate cancer. Int J Radiat Oncol Biol Phys. 2019;103(1):95–104.
Artigas C, Flamen P, Charlier F, Levillain H, Wimana Z, Diamand R, et al. 68Ga-PSMA PET/CT-based metastasis-directed radiotherapy for oligometastatic prostate cancer recurrence after radical prostatectomy. World J Urol. 2019;37(8):1535–42.
Ong WL, Koh TL, Lim Joon D, Chao M, Farrugia B, Lau E, et al. Prostate-specific membrane antigen-positron emission tomography/computed tomography (PSMA-PET/CT)-guided stereotactic ablative body radiotherapy for oligometastatic prostate cancer: a single-institution experience and review of the published literature. BJU Int. 2019;124(S1):19–30.
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686–700.
Kyriakopoulos CE, Chen Y-H, Carducci MA, Liu G, Jarrard DF, Hahn NM, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36(11):1080–7.
Siva S, Bressel M, Murphy DG, Shaw M, Chander S, Violet J, et al. Stereotactic ablative body radiotherapy (SABR) for oligometastatic prostate cancer: a prospective clinical trial. Eur Urol. 2018;74(4):455–62.
Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2017;36(5):446–53.
Jereczek-Fossa BA, Fanetti G, Fodor C, Ciardo D, Santoro L, Francia CM, et al. Salvage stereotactic body radiotherapy for isolated lymph node recurrent prostate cancer: single institution series of 94 consecutive patients and 124 lymph nodes. Clin Genitourin Cancer. 2017;15(4):e623–e32.
Kroeze SGC, Henkenberens C, Schmidt-Hegemann NS, Vogel MME, Kirste S, Becker J, et al. Prostate-specific membrane antigen positron emission tomography-detected oligorecurrent prostate cancer treated with metastases-directed radiotherapy: role of addition and duration of androgen deprivation. Eur Urol Focus. 2019;S2405-4569(19):30270–6. https://doi.org/10.1016/j.euf.2019.08.012.
Nini A, Gandaglia G, Fossati N, Suardi N, Cucchiara V, Dell’Oglio P, et al. Patterns of clinical recurrence of node-positive prostate cancer and impact on long-term survival. Eur Urol. 2015;68(5):777–84.
Weineisen M, Simecek J, Schottelius M, Schwaiger M, Wester H-J. Synthesis and preclinical evaluation of DOTAGA-conjugated PSMA ligands for functional imaging and endoradiotherapy of prostate cancer. Eur J Nucl Med Mol Imaging Res. 2014;4(1):1–15.
Eder M, Schäfer M, Bauder-Wüst U, Hull W-E, Wängler C, Mier W, et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem. 2012;23(4):688–97.
Schäfer M, Bauder-Wüst U, Leotta K, Zoller F, Mier W, Haberkorn U, et al. A dimerized urea-based inhibitor of the prostate-specific membrane antigen for 68Ga-PET imaging of prostate cancer. Eur J Nucl Med Mol Imaging Res. 2012;2(1):23.
Fendler WP, Eiber M, Beheshti M, Bomanji J, Ceci F, Cho S, et al. 68Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2017;44(6):1014–24.
Sheikhbahaei S, Afshar-Oromieh A, Eiber M, Solnes LB, Javadi MS, Ross AE, et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur J Nucl Med Mol Imaging. 2017;44(12):2117–36.
Afshar-Oromieh A, Sattler LP, Steiger K, Holland-Letz T, da Cunha ML, Mier W, et al. Tracer uptake in mediastinal and paraaortal thoracic lymph nodes as a potential pitfall in image interpretation of PSMA ligand PET/CT. Eur J Nucl Med Mol Imaging. 2018;45(7):1179–87.
https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Accessed 06.05.2018, 2018.
D’Amico AV, Whittington R, Malkowicz SB, Weinstein M, Tomaszewski JE, Schultz D, et al. Predicting prostate specific antigen outcome preoperatively in the prostate specific antigen era. J Urol. 2001;166:2185–8.
Farolfi A, Ceci F, Castellucci P, Graziani T, Siepe G, Lambertini A, et al. 68Ga-PSMA-11 PET/CT in prostate cancer patients with biochemical recurrence after radical prostatectomy and PSA < 0.5 ng/ml. Efficacy and impact on treatment strategy. Eur J Nucl Med Mol Imaging. 2019;46(1):11–9.
Ceci F, Castellucci P, Graziani T, Farolfi A, Fonti C, Lodi F, et al. 68Ga-PSMA-11 PET/CT in recurrent prostate cancer: efficacy in different clinical stages of PSA failure after radical therapy. Eur J Nucl Med Mol Imaging. 2019;46(1):31–9.
Müller J, Ferraro DA, Muehlematter UJ, Garcia Schüler HI, Kedzia S, Eberli D, et al. Clinical impact of 68Ga-PSMA-11 PET on patient management and outcome, including all patients referred for an increase in PSA level during the first year after its clinical introduction. Eur J Nucl Med Mol Imaging. 2019;46(4):889–900.
Lecouvet FE, Oprea-Lager DE, Liu Y, Ost P, Bidaut L, Collette L, et al. Use of modern imaging methods to facilitate trials of metastasis-directed therapy for oligometastatic disease in prostate cancer: a consensus recommendation from the EORTC Imaging Group. Lancet Oncol. 2018;19:534–45.
Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JMC, Papaemmanuil E, et al. The evolutionary history of lethal metastatic prostate cancer. Nature. 2015;520:353–7.
Budäus L, Leyh-Bannurah SR, Salomon G, Michl U, Heinzer H, Huland H, et al. Initial experience of (68)Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. Eur Urol. 2016;69(3):393–6.
Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. New Engl J Med. 2019;381(2):121–31.
Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–46.
Nagarajah J. Pilot study: Lutetium-177-PSMA-617 in low volume metastatic prostate cancer [accessed September, 22 2019]. Available from: https://clinicaltrials.gov/ct2/show/NCT03828838.
De Bari B, Mazzola R, Aiello D, Aloi D, Gatta R, Corradini S, et al. (68Ga)-PSMA-PET/CT for the detection of postoperative prostate cancer recurrence: possible implications on treatment volumes for radiation therapy. Cancer Radiother. 2019;23(3):194–200.
Tran P, Radwan N, Phillips R, Ross A, Rowe S, Gorin M, et al. OC-0505: interim results of a randomized trial of observation versus SABR for oligometastatic prostate cancer. Radiother Oncol. 2018;127:261.
Heidenreich A, Moul JW, Shariat S, Karnes RJ. Role of salvage lymph node dissection in prostate cancer. Curr Opin Urol. 2016;26(6):581–9.
Abdollah F, Briganti A, Montorsi F. Contemporary role of salvage lymphadenectomy in patients with recurrence following radical prostatectomy. Eur Urol. 2015;67(5):839–49.
Ploussard G, Gandaglia G, Borgmann H, de Visschere P, Heidegger I, Kretschmer A, et al. Salvage lymph node dissection for nodal recurrent prostate cancer: a systematic review. Eur Urol. 2019;76(4):493–504.
James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377(4):338–51.
Parikh NR, Huiza C, Patel JS, Tsai S, Kalpage N, Thein M, et al. Systemic and tumor-directed therapy for oligometastatic prostate cancer: study protocol for a phase II trial for veterans with de novo oligometastatic disease. BMC Cancer. 2019;19(1):291.
Jadvar H. Oligometastatic prostate cancer: molecular imaging and clinical management implications in the era of precision oncology. J Nucl Med. 2018;59(9):1338–9.
Habl G, Sauter K, Schiller K, Dewes S, Maurer T, Eiber M, et al. 68Ga-PSMA-PET for radiation treatment planning in prostate cancer recurrences after surgery: individualized medicine or new standard in salvage treatment. Prostate. 2017;77(8):920–7.
Steuber T, Jilg C, Tennstedt P, De Bruycker A, Tilki D, Decaestecker K, et al. Standard of care versus metastases-directed therapy for PET-detected nodal oligorecurrent prostate cancer following multimodality treatment: a multi-institutional case-control study. Eur Urol Focus. 2019;5(6):1007–13.
Triggiani L, Alongi F, Buglione M, Detti B, Santoni R, Bruni A, et al. Efficacy of stereotactic body radiotherapy in oligorecurrent and in oligoprogressive prostate cancer: new evidence from a multicentric study. Br J Cancer. 2017;116(12):1520–5.
Ost P, Jereczek-Fossa BA, As NV. Progression-free survival following stereotactic body radiotherapy for oligometastatic prostate cancer treatment-naive recurrence: a multi-institutional analysis. Eur Urol. 2016;69(1):9–12.
Decaestecker K, Meerleer G, Lambert B, Delrue L, Fonteyne V, Claeys T. Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat Oncol. 2014;9.
Pasqualetti F, Panichi M, Sainato A, Matteucci F, Galli L, Cocuzza P, et al. [18F]Choline PET/CT and stereotactic body radiotherapy on treatment decision making of oligometastatic prostate cancer patients: preliminary results. Radiat Oncol. 2016;11(1):9.
Franzese C, Zucali PA, Di Brina L, D’Agostino G, Navarria P, Franceschini D, et al. The efficacy of stereotactic body radiation therapy and the impact of systemic treatments in oligometastatic patients from prostate cancer. Cancer Med. 2018;7:4379–86.
Cysouw M, Bouman-Wammes E, Hoekstra O, van den Eertwegh A, Piet M, van Moorselaar J, et al. Prognostic value of [18F]-fluoromethylcholine positron emission tomography/computed tomography before stereotactic body radiation therapy for oligometastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2018;101(2):406–10.
Tran S, Jorcano S, Falco T, Lamanna G, Miralbell R, Zilli T. Oligorecurrent nodal prostate cancer: long-term results of an elective nodal irradiation approach. Am J Clin Oncol. 2018;41:960–2.
Bouman-Wammes EW, van Dodewaard-De Jong JM, Dahele M, Cysouw MCF, Hoekstra OS, van Moorselaar RJA, et al. Benefits of using stereotactic body radiotherapy in patients with metachronous oligometastases of hormone-sensitive prostate cancer detected by [18F]fluoromethylcholine PET/CT. Clin Genitourin Cancer. 2017;15:773–82.
Ingrosso G, Trippa F, Maranzano E, Carosi A, Ponti E, Arcidiacono F, et al. Stereotactic body radiotherapy in oligometastatic prostate cancer patients with isolated lymph nodes involvement: a two-institution experience. World J Urol. 2017;35:45–9.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by N.-S. Schmidt-Hegemann, S.G.C. Kroeze, C. Henkenberens, M.M.E. Vogel, S. Kirste, and J. Becker. The first draft of the manuscript was written by N.-S. Schmidt-Hegemann, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committees of the respective Medical Faculties (Business Administration System for Ethics Committees Number (BASEC-Nr. 2017–01499)) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
This retrospective multicenter study was undertaken in six university hospitals in Switzerland and Germany, was approved by the local Ethics Committee of the respective Medical Faculties (BASEC-Nr. 2017–01499), and the need for written informed consent was waived.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology
Electronic supplementary material
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Schmidt-Hegemann, NS., Kroeze, S., Henkenberens, C. et al. Influence of localization of PSMA-positive oligo-metastases on efficacy of metastasis-directed external-beam radiotherapy—a multicenter retrospective study. Eur J Nucl Med Mol Imaging 47, 1852–1863 (2020). https://doi.org/10.1007/s00259-020-04708-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04708-y