Abstract
Purpose
99mTc-Annexin A5 has been used as a molecular imaging probe for the visualization, characterization and measurement of apoptosis. In an effort to define the quantitative 99mTc-annexin A5 uptake criteria that best predict tumor response to treatment, we performed a systematic review and meta-analysis of the results of all clinical imaging trials found in the literature or publicly available databases.
Methods
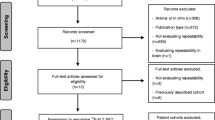
Included in this review were 17 clinical trials investigating quantitative 99mTc-annexin A5 (qAnx5) imaging using different parameters in cancer patients before and after the first course of chemotherapy and/or radiation therapy. Qualitative assessment of the clinical studies for diagnostic accuracy was performed using the QUADAS-2 criteria. Of these studies, five prospective single-center clinical trials (92 patients in total) were included in the meta-analysis after exclusion of one multicenter clinical trial due to heterogeneity. Pooled positive predictive values (PPV) and pooled negative predictive values (NPV) (with 95 % CI) were calculated using Meta-Disc software version 1.4.
Results
Absolute quantification and/or relative quantification of 99mTc-annexin A5 uptake were performed at baseline and after the start of treatment. Various quantitative parameters have been used for the calculation of 99mTc-annexin A5 tumor uptake and delta (Δ) tumor changes post-treatment compared to baseline including: tumor-to-background ratio (TBR), ΔTBR, tumor-to-noise ratio, relative tumor ratio (TR), ΔTR, standardized tumor uptake ratio (STU), ΔSTU, maximum count per pixel within the tumor volume (Cmax), Cmax%, absolute ΔU and percentage (ΔU%), maximum ΔU counts, semiquantitative visual scoring, percent injected dose (%ID) and %ID/cm3. Clinical trials investigating qAnx5 imaging have included patients with lung cancer, lymphoma, breast cancer, head and neck cancer and other less common tumor types. In two phase I/II single-center clinical trials, an increase of ≥25 % in uptake following treatment was considered a significant threshold for an apoptotic tumor response (partial response, complete response). In three other phase I/II clinical trials, increases of ≥28 %, ≥42 % and ≥47 % in uptake following treatment were found to be the mean cut-off levels in responders. In a phase II/III multicenter clinical trial, an increase of ≥23 % in uptake following treatment was found to be the minimum cut-off level for a tumor response. In one clinical trial, no significant difference in 99mTc-annexin A5 uptake in terms of %ID was found in healthy tissues after chemotherapy compared to baseline. In two other clinical trials, intraobserver and interobserver measurements of 99mTc-annexin A5 tumor uptake were found to be reproducible (mean difference <5 %, kappa = 0.90 and 0.82, respectively) and to be highly correlated with treatment outcome (Spearman r = 0.99, p < 0.0001). The meta-analysis demonstrated a pooled positive PPV of 100 % (95 % CI 92 – 100 %) and a pooled NPV of 70 % (95 % CI 55 – 82 %) for prediction of a tumor response after the first course of chemotherapy and/or radiotherapy in terms of ΔU%. In a symmetric sROC analysis, the AUC was 0.919 and the Q* index was 85.21 %.
Conclusion
Quantitative 99mTc-annexin A5 imaging has been investigated in clinical trials for the assessment of apoptotic tumor responses. This meta-analysis showed a high pooled PPV and a moderate pooled NPV with ΔU cut-off values ranging between 20 % and 30 %. Standardization of quantification and harmonization of results are required for high-quality clinical research. A standardized uptake value score (SUV, ΔSUV) using quantitative SPECT/CT imaging may be a promising approach to the simple, reproducible and semiquantitative assessment of apoptotic tumor changes.



Similar content being viewed by others
References
Lockshin RA, Osborne B, Zakeri Z. Cell death in the third millennium. Cell Death Differ. 2000;7(1):2–7. Editorial
Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73(8):2013–26.
Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, et al. Molecular definitions of cell death subroutines: recommendations of the nomenclature committee on cell death 2012. Cell Death Differ. 2012;19(1):107–20. Review.
Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp Cell Res. 2000;256(1):42–9. Review.
Staunton MJ, Gaffney EF. Tumor type is a determinant of susceptibility to apoptosis. Am J Clin Pathol. 1995;103(3):300–7.
van Genderen HO, Kenis H, Hofstra L, Narula J, Reutelingsperger CP. Extracellular annexin A5: functions of phosphatidylserine-binding and two-dimensional crystallization. Biochim Biophys Acta. 2008;1783(6):953–63.
Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, Laface DM, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl 2 and Abl. J Exp Med. 1995;182(5):1545–56.
Boersma HH, Kietselaer BL, Stolk LM, Bennaghmouch A, Hofstra L, Narula J, et al. Past, present, and future of annexin A5: from protein discovery to clinical applications. J Nucl Med. 2005;46(12):2035–50. Review.
Schaper FL, Reutelingsperger CP. 99mTc-HYNIC-annexin A5 in oncology: evaluating efficacy of anti-cancer therapies. Cancers (Basel). 2013;5(2):550–68.
Mankoff DA, Pryma DA, Clark AS. Molecular imaging biomarkers for oncology clinical trials. J Nucl Med. 2014;55(4):525–8.
Blankenberg FG, Tait JF, Strauss HW. Apoptotic cell death: its implications for imaging in the next millennium. Eur J Nucl Med. 2000;27(3):359–67. Review.
Green AM, Steinmetz ND. Monitoring apoptosis in real time. Cancer J. 2002;8(2):82–92. Review.
Maffione AM, Grassetto G, Chondrogiannis S, Colletti PM, Rubello D. Quantitative PET factors predictive of the response to therapy in solid tumors: which is the best? Clin Nucl Med. 2014;39(2):160–3.
Bailey DL, Willowson KP. An evidence-based review of quantitative SPECT imaging and potential clinical applications. J Nucl Med. 2013;54(1):83–9.
Rigo P, Paulus P, Kaschten BJ, Hustinx R, Bury T, Jerusalem G, et al. Oncological applications of positron emission tomography with fluorine-18 fluorodeoxyglucose. Eur J Nucl Med. 1996;23(12):1641–74. Review.
Jerusalem G, Belhocine TZ. Metabolic monitoring of chemosensitivity with 18FDG PET. Methods Mol Med. 2005;111:417–40.
Marsden PK. Quantification in PET: what is it? Can we do it? Do we need it? Nucl Med Commun. 2004;25(7):635–6.
Ritt P, Vija H, Hornegger J, Kuwert T. Absolute quantification in SPECT. Eur J Nucl Med Mol Imaging. 2011;38 Suppl 1:S69–77.
Atuegwu NC, Gore JC, Yankeelov TE. The integration of quantitative multi-modality imaging data into mathematical models of tumors. Phys Med Biol. 2010;55(9):2429–49.
Takei T, Kuge Y, Zhao S, Sato M, Strauss HW, Blankenberg FG, et al. Time course of apoptotic tumor response after a single dose of chemotherapy: comparison with 99mTc-annexin V uptake and histologic findings in an experimental model. J Nucl Med. 2004;45(12):2083–7.
King M, Member S, Boening G, Baker S, Steinmetz N. Study of relative quantitation of Tc-99m annexin localization in pulmonary nodules using an anthropomorphic phantom. IEEE Trans Nucl Sci. 2004;51(5):2606–11.
Belhocine T, Steinmetz N, Hustinx R, Bartsch P, Jerusalem G, Seidel L, et al. Increased uptake of the apoptosis-imaging agent (99m)Tc recombinant human Annexin V in human tumors after one course of chemotherapy as a predictor of tumor response and patient prognosis. Clin Cancer Res. 2002;8(9):2766–74.
Belhocine T.-Z. Nouvelles technologies en oncologie nucleaire. Doctoral Thesis. Dissertation. University of Liège (Ulg), Liège, Belgium. 2002. 130 p.
Steinmetz N. NAS 2020 European Multicenter Trials. North American Scientific Release October 2003.
Steinmetz N. North American Scientific Releases updated observations from European clinical trials of Tc-99m Hynic-annexin. Theseus Imaging Corporation Inc. Presented at the 51st Annual Meeting of the Society of Nuclear Medicine, 19–23 June 2004, Philadelphia.
Vermeersch H, Loose D, Lahorte C, Mervillie K, Dierckx R, Steinmetz N, et al. 99mTc-HYNIC annexin-V imaging of primary head and neck carcinoma. Nucl Med Commun. 2004;25(3):259–63.
Rottey S, Slegers G, Van Belle S, Goethals I, Van de Wiele C. Sequential 99mTc-hydrazinonicotinamide-annexin V imaging for predicting response to chemotherapy. J Nucl Med. 2006;47(11):1813–8.
Rottey S, Loose D, Vakaet L, Lahorte C, Vermeersch H, Van Belle S, et al. 99mTc-HYNIC annexin-V imaging of tumors and its relationship to response to radiotherapy and/or chemotherapy. Q J Nucl Med Mol Imaging. 2007;51(2):182–8.
Rottey S. 99mTc-HYNIC Annexin-V for monitoring response to chemotherapy: methodology and feasibility studies. Doctoral Thesis. Dissertation. University of Gent (AZ UGent). Gent, Belgium. 2007. 139 p.
Rottey S, Van den Bossche B, Slegers G, Van Belle S, van de Wiele C. Influence of chemotherapy on the biodistribution of [99mTc]hydrazinonicotinamide annexin V in cancer patients. Q J Nucl Med Mol Imaging. 2009;53(2):127–32.
Kartachova M, Haas RL, Olmos RA, Hoebers FJ, van Zandwijk N, Verheij M. In vivo imaging of apoptosis by 99mTc-annexin V scintigraphy: visual analysis in relation to treatment response. Radiother Oncol. 2004;72(3):333–9.
Kartachova M, van Zandwijk N, Burgers S, van Tinteren H, Verheij M, Valdés Olmos RA. Prognostic significance of 99mTc Hynic-rh-annexin V scintigraphy during platinum-based chemotherapy in advanced lung cancer. J Clin Oncol. 2007;25(18):2534–9.
Kartachova MS. Imaging apoptosis in malignancies. Doctoral Thesis. Dissertation. University of Amsterdam (AMC-UvA). Amsterdam, The Netherlands. 2007. 151 p.
Kartachova MS, Valdés Olmos RA, Haas RL, Hoebers FJ, van Herk M, Verheij M. 99mTc-HYNIC-rh-annexin-V scintigraphy: visual and quantitative evaluation of early treatment-induced apoptosis to predict treatment outcome. Nucl Med Commun. 2008;29(1):39–44.
Haas RL, de Jong D, Valdés Olmos RA, Hoefnagel CA, van den Heuvel I, Zerp SF, et al. In vivo imaging of radiation-induced apoptosis in follicular lymphoma patients. Int J Radiat Oncol Biol Phys. 2004;59(3):782–7.
Hoebers FJP. Prognostic factors and predictive tests in the treatment of head and neck squamous cell carcinoma. Doctoral Thesis. Dissertation. University of Amsterdam (AMC-UvA). Amsterdam, The Netherlands. 2007. 160 p.
Hoebers FJ, Kartachova M, de Bois J, van den Brekel MW, van Tinteren H, van Herk M, et al. 99mTc Hynic-rh-annexin V scintigraphy for in vivo imaging of apoptosis in patients with head and neck cancer treated with chemoradiotherapy. Eur J Nucl Med Mol Imaging. 2008;35(3):509–18.
Loose D, Vermeersch H, De Vos F, Deron P, Slegers G, Van de Wiele C. Prognostic value of 99mTc-HYNIC annexin-V imaging in squamous cell carcinoma of the head and neck. Eur J Nucl Med Mol Imaging. 2008;35(1):47–52.
Vermeersch H, Ham H, Rottey S, Lahorte C, Corsetti F, Dierckx R, et al. Intraobserver, interobserver, and day-to-day reproducibility of quantitative 99mTc-HYNIC annexin-V imaging in head and neck carcinoma. Cancer Biother Radiopharm. 2004;19(2):205–10.
Steinmetz N. Theseus Imaging Corporation Inc. Clinical study protocol NAS-2021-21-23-01. Phase II/III study of technetium 99mTc-HYNIC-rh-Annexin V for imaging of apoptosis in patients with non-small-cell lung cancer. Version 1.2, May 2, 2002. Released 28 May 2002.
Green AM, Steinmetz ND. Theseus Imaging Corporation Inc. NSCLC Investigator Group. Correlation of biodistribution changes of 99mTc-Hynic-rh-annexin V before and after the initial dose of chemotherapy for non-small cell lung cancer with subsequent patient response. Presented at the Annual Congress of the EANM, 5–8 September 2004, Helsinki. Poster 250.
Yang TJ, Haimovitz-Friedman A, Verheij M. Anticancer therapy and apoptosis imaging. Exp Oncol. 2012;34(3):269–76. Review.
Van de Wiele C, Lahorte C, Vermeersch H, Loose D, Mervillie K, Steinmetz ND, et al. Quantitative tumor apoptosis imaging using technetium-99m-HYNIC annexin V single photon emission computed tomography. J Clin Oncol. 2003;21(18):3483–7.
Vermeersch H, Mervillie K, Lahorte C, Loose D, Dierck RA, Steinmetz N, et al. Relationship of 99mTc-HYNIC annexin V uptake to microvessel density, FasL and MMP-9 expression, and the number of tumour-infiltrating lymphocytes in head and neck carcinoma. Eur J Nucl Med Mol Imaging. 2004;31(7):1016–21.
Kurihara H, Yang DJ, Cristofanilli M, Erwin WD, Yu DF, Kohanim S, et al. Imaging and dosimetry of 99mTc EC annexin V: preliminary clinical study targeting apoptosis in breast tumors. Appl Radiat Isot. 2008;66(9):1175–82.
Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. Review.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Whiting P, Rutjes A, Westwood M, Mallett S, Leeflang M, Reitsma H, et al. Updating QUADAS: evidence to inform the development of QUADAS-2. 2010. http://www.bristol.ac.uk/media-library/sites/quadas/migrated/documents/quadas2reportv4.pdf. Accessed 9 August 2015.
Sterne JAC, Egger M, Moher D, editors. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. www.cochrane-handbook.org. Accessed 9 August 2015.
Weber WA. Assessing tumor response to therapy. J Nucl Med. 2009;50 Suppl 1:1S–10S.
Costelloe CM, Chuang HH, Madewell JE, Ueno NT. Cancer response criteria and bone metastases: RECIST 1.1, MDA and PERCIST. J Cancer. 2010;1:80–92.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35(13):1773–82.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1:122S–150S.
Belhocine TZ, Blankenberg FG. The imaging of apoptosis with the radiolabelled annexin A5: a new tool in translational research. Curr Clin Pharmacol. 2006;1(2):129–37. Review.
Tait JF, Smith C, Levashova Z, Patel B, Blankenberg FG, Vanderheyden JL. Improved detection of cell death in vivo with annexin V radiolabeled by site-specific methods. J Nucl Med. 2006;47(9):1546–53.
Weber WA. Use of PET for monitoring cancer therapy and for predicting outcome. J Nucl Med. 2005;46(6):983–95. Review.
de Langen AJ, Vincent A, Velasquez LM, van Tinteren H, Boellaard R, Shankar LK, et al. Repeatability of 18F-FDG uptake measurements in tumors: a metaanalysis. J Nucl Med. 2012;53(5):701–8.
Takei T, Kuge Y, Zhao S, Sato M, Strauss HW, Blankenberg FG, et al. Enhanced apoptotic reaction correlates with suppressed tumor glucose utilization after cytotoxic chemotherapy: use of 99mTc-Annexin V, 18F-FDG, and histologic evaluation. J Nucl Med. 2005;46(5):794–9.
Li D, Yao Q, Li L, Wang L, Chen J. Correlation between hybrid 18F-FDG PET/CT and apoptosis induced by neoadjuvant chemotherapy in breast cancer. Cancer Biol Ther. 2007;6(9):1442–8.
Celik B, Yalcin AD, Bisgin A, Dimitrakopoulou-Strauss A, Kargi A, Strauss LG. Level of TNF-related apoptosis-inducing-ligand and CXCL8 correlated with 2-[18F]fluoro-2-deoxy-D-glucose uptake in anti-VEGF treated colon cancers. Med Sci Monit. 2013;19:875–82.
Deron P, Vangestel C, Goethals I, De Potter A, Peeters M, Vermeersch H, et al. FDG uptake in primary squamous cell carcinoma of the head and neck. The relationship between overexpression of glucose transporters and hexokinases, tumour proliferation and apoptosis. Nuklearmedizin. 2011;50(1):15–21.
Yeatman TJ, Mule J, Dalton WS, Sullivan D. On the eve of personalized medicine in oncology. Cancer Res. 2008;68(18):7250–2.
Basu S. Personalized versus evidence-based medicine with PET-based imaging. Nat Rev Clin Oncol. 2010;7(11):665–8.
Dimitrakopoulou-Strauss A. PET-based molecular imaging in personalized oncology: potential of the assessment of therapeutic outcome. Future Oncol. 2015;11(7):1083–91.
De Saint-Hubert M, Bauwens M, Mottaghy FM. Molecular imaging of apoptosis for early prediction of therapy efficiency. Curr Pharm Des. 2014;20(14):2319–28. Review.
Mariani G, Strauss HW. Positron emission and single-photon emission imaging: synergy rather than competition. Eur J Nucl Med Mol Imaging. 2011;38(7):1189–90.
Blankenberg FG. Imaging the molecular signatures of apoptosis and injury with radiolabeled annexin V. Proc Am Thorac Soc. 2009;6(5):469–76.
Belhocine T, Steinmetz N, Li C, Green A, Blankenberg FG. The imaging of apoptosis with the radiolabeled annexin V: optimal timing for clinical feasibility. Technol Cancer Res Treat. 2004;3(1):23–32. Review.
Beekman CA, Buckle T, van Leeuwen AC, Valdés Olmos RA, Verheij M, Rottenberg S, et al. Questioning the value of (99m)Tc-HYNIC-annexin V based response monitoring after docetaxel treatment in a mouse model for hereditary breast cancer. Appl Radiat Isot. 2011;69(4):656–62.
Erba PA, Manfredi C, Lazzeri E, Minichilli F, Pauwels EK, Sbrana A, et al. Time course of paclitaxel-induced apoptosis in an experimental, model of virus-induced breast cancer. J Nucl Med. 2010;51(5):775–81.
Kamal A, Faazil S, Malik MS. Apoptosis-inducing agents: a patent review (2010–2013). Expert Opin Ther Pat. 2014;24(3):339–54.
Meyn RE, Milas L, Ang KK. The role of apoptosis in radiation oncology. Int J Radiat Biol. 2009;85(2):107–15.
Van de Wiele C, Vermeersch H, Loose D, Signore A, Mertens N, Dierckx R. Radiolabeled annexin-V for monitoring treatment response in oncology. Cancer Biother Radiopharm. 2004;19(2):189–94. Review.
Verheij M. Clinical biomarkers and imaging for radiotherapy-induced cell death. Cancer Metastasis Rev. 2008;27(3):471–80.
Belhocine TZ. Imaging apoptosis. In: Pomper MG, Gelovani JG, editors. Molecular imaging in oncology. New York: Informa Healthcare; 2008. p. 493–502.
Kartachova M, Verheij MM, Eck BV, Hoefnagel K, Valdés Olmos R. Methodological aspects and applications of in vivo imaging of apoptosis in oncology: an illustrative review. Curr Med Imaging Rev. 2005;1(3):1–8.
Shcherbinin S, Celler A, Belhocine T, Vanderwerf R, Driedger A. Accuracy of quantitative reconstructions in SPECT/CT imaging. Phys Med Biol. 2008;53(17):4595–604.
Zeintl J, Vija AH, Yahil A, Hornegger J, Kuwert T. Quantitative accuracy of clinical 99mTc SPECT/CT using ordered-subset expectation maximization with 3-dimensional resolution recovery, attenuation, and scatter correction. J Nucl Med. 2010;51(6):921–8.
Seret A, Nguyen D, Bernard C. Quantitative capabilities of four state-of-the-art SPECT-CT cameras. EJNMMI Res. 2012;2(1):45.
HERMES Medical Solutions. New quantitative SPECT-CT reconstruction SUV SPECT™ now available. Presented at the SNMMI Annual Meeting, 7–11 June 2014, St. Louis, MO.
Haidich AB. Meta-analysis in medical research. Hippokratia. 2010;14 Suppl 1:29–37.
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Chichester: Wiley; 2009.
Belhocine TZ, Vanderheyden JL. Annexin A5 imaging: an academic research – clinical trials and theses. Curr Mol Imaging. 2014;3(1):52–63.
Begg CB. The role of meta-analysis in monitoring clinical trials. Stat Med. 1996;15(12):1299–306. Discussion 1307–11. Review.
Byrd D, Linden H, Kinahan P. Efforts addressing SUV accuracy for PET quantitation and standardization. PET Center Excellence Newsl. 2013;10(4):1–3.
Graham MM. The Clinical Trials Network of the Society of Nuclear Medicine. Semin Nucl Med. 2010;40(5):327–31.
Tatsch K. Standardisation and harmonisation boost the credibility of nuclear medicine procedures. Eur J Nucl Med Mol Imaging. 2012;39(1):186–7.
Acknowledgments
The authors acknowledge the invaluable scientific contribution of Dr. Allan Green as a previous President and CEO of Theseus Imaging Corporation Inc. (Boston, MA, USA) for the single-center and multicenter clinical trials on 99mTc-annexin A5 imaging of apoptosis in cancer patients. The authors thank Dr. Neil Steinmetz as a previous Vice-President and Medical Monitor of Theseus Imaging Corporation Inc. for providing his unique data on the quantitative results from the European multicenter phase II/III 99mTc-Hynic-rh-annexin A5 clinical trial (NAS-2020). The authors also thank Dr. Rick L.M. Haas of The Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Department of Radiotherapy (Amsterdam, The Netherlands) for providing detailed data on the 99mTc-annexin A5 clinical trial he conducted.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
None.
Ethical approval
All procedures performed in studies involving human participants in the single-center and multicenter clinical trials included in this systematic review and meta-analysis of quantitative 99mTc-annexin A5 imaging of apoptosis were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This article does not describe any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the studies described.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Belhocine, T.Z., Blankenberg, F.G., Kartachova, M.S. et al. 99mTc-Annexin A5 quantification of apoptotic tumor response: a systematic review and meta-analysis of clinical imaging trials. Eur J Nucl Med Mol Imaging 42, 2083–2097 (2015). https://doi.org/10.1007/s00259-015-3152-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-3152-0