Abstract
Purpose
The aim of the current study was to evaluate the feasibility of phase analysis on gated myocardial perfusion SPECT (GMPS) for the assessment of left ventricular (LV) diastolic dyssynchrony in a head-to-head comparison with tissue Doppler imaging (TDI).
Methods
The population consisted of patients with end-stage heart failure of New York Heart Association functional class III or IV with a reduced LV ejection fraction of ≤35%. LV diastolic dyssynchrony was calculated using TDI as the maximal time delay between early peak diastolic velocities of two opposing left ventricle walls (diastolic mechanical delay). Significant LV diastolic dyssynchrony was defined as a diastolic mechanical delay of >55 ms on TDI. Furthermore, phase analysis on GMPS was performed to evaluate LV diastolic dyssynchrony; diastolic phase standard deviation (SD) and histogram bandwidth (HBW) were used as markers of LV diastolic dyssynchrony.
Results
A total of 150 patients (114 men, mean age 66.0 ± 10.4 years) with end-stage heart failure were enrolled. Both diastolic phase SD (r = 0.81, p < 0.01) and diastolic HBW (r = 0.75, p < 0.01) showed good correlations with LV diastolic dyssynchrony on TDI. Additionally, patients with LV diastolic dyssynchrony on TDI (>55 ms) showed significantly larger diastolic phase SD (68.1 ± 13.4° vs. 40.7 ± 14.0°, p < 0.01) and diastolic HBW (230.6 ± 54.3° vs. 129.0 ± 55.6°, p < 0.01) as compared to patients without LV diastolic dyssynchrony on TDI (≤55 ms). Finally, phase analysis on GMPS showed a good intra- and interobserver reproducibility for the determination of diastolic phase SD (ICC 0.97 and 0.88) and diastolic HBW (ICC 0.98 and 0.93).
Conclusion
Phase analysis on GMPS showed good correlations with TDI for the assessment of LV diastolic dyssynchrony.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Left ventricular (LV) systolic dyssynchrony is considered an important pathophysiological condition in patients with heart failure (HF) [1, 2]. In addition, it has been shown that a dyssynchronous relaxation pattern of the left ventricle, which is commonly referred to as LV diastolic dyssynchrony, plays an important role in patients with HF [3–5]. In these patients, LV diastolic dyssynchrony has been related to abnormalities in LV diastolic filling and LV filling rate, which may contribute to a further impairment of the haemodynamics of the failing heart [5]. Importantly, it has been recognized that LV diastolic dyssynchrony is a common pathophysiological condition in patients with HF with an estimated prevalence of more than 50% [3, 6].
At present, the majority of available studies used echocardiography for the assessment of LV diastolic dyssynchrony in patients with HF [3, 4, 6–8]. Among the echocardiographic techniques, tissue Doppler imaging (TDI) has been used predominantly for the evaluation of diastolic relaxation patterns of the left ventricle [6–8]. Although TDI is an accurate approach for the assessment of LV diastolic dyssynchrony, the post-processing of Doppler images requires special expertise and standardized protocols to optimize the interobserver variability.
Phase on gated myocardial perfusion single photon emission computed tomography (SPECT) (GMPS) has emerged as a practical technique for the assessment of LV mechanical dyssynchrony as it automatically provides robust and reproducible indices of mechanical dyssynchrony [9, 10]. Moreover, phase analysis can be performed on conventional GMPS studies without the use of additional image acquisitions. Although studies have shown that phase analysis on GMPS can be used for the assessment of LV systolic dyssynchrony, its feasibility to assess LV diastolic dyssynchrony has not been demonstrated. Accordingly, the aim of the current study was to evaluate the feasibility of phase analysis on GMPS for the assessment of LV diastolic dyssynchrony in a head-to-head comparison with TDI.
Material and methods
Patient population and protocol
The patient population consisted of patients with end-stage HF (New York Heart Association functional class III or IV) with a depressed LV ejection fraction (LVEF) of ≤35%. Patient data were prospectively collected in the departmental Cardiology Information System (EPD-Vision, Leiden University Medical Center (LUMC), Leiden, The Netherlands) and retrospectively analysed. All patients were treated according to the MISSION! heart failure care track operational at Leiden University Medical Center. All patients were stable on maximum tolerated doses of HF medication. Patients with recent myocardial infarction (within 3 months prior to GMPS imaging), atrial fibrillation, decompensated HF or acute coronary syndrome were excluded. Patients were diagnosed with ischaemic cardiomyopathy in the presence of ≥50% stenosis in one or more of the major epicardial coronary arteries, previous myocardial infarction, percutaneous coronary intervention or coronary artery bypass grafting.
Resting GMPS imaging and 2-D echocardiography with TDI were performed as part of the clinical evaluation of the patients with HF to determine therapeutic options. A standard 12-lead electrocardiogram was obtained prior to GMPS imaging.
GMPS imaging and TDI were compared for the assessment of LV diastolic dyssynchrony. TDI was considered the standard of reference for evaluation of LV diastolic dyssynchrony, which was calculated as the maximal time delay between early peak diastolic velocities of two opposing left ventricle walls. As previously reported, a cut-off value of 55 ms of diastolic mechanical delay on TDI was used as a marker of significant LV diastolic dyssynchrony [6]. Phase analysis on GMPS imaging was used to evaluate LV diastolic dyssynchrony and systolic dyssynchrony. Both diastolic phase histogram bandwidth (HBW) and diastolic phase standard deviation (SD) as derived from the onset of mechanical relaxation (OMR) distribution were used as markers of LV diastolic dyssynchrony, whereas systolic HBW and systolic phase SD were used as markers of LV systolic dyssynchrony, as previously described [11].
TDI and phase analysis on GMPS were then compared for the assessment of LV diastolic dyssynchrony. Additionally, the extent of LV diastolic dyssynchrony on GMPS imaging was evaluated in patients with (>55 ms) and without (≤55 ms) significant LV diastolic dyssynchrony on TDI. Finally, the relationship between LV diastolic and systolic dyssynchrony as assessed by GMPS was evaluated.
Gated myocardial perfusion SPECT
Acquisition
Resting GMPS imaging with 99mTc-tetrofosmin was performed on a triple-head SPECT camera (GCA 9300/HG; Toshiba, Tokyo, Japan) equipped with low-energy high-resolution collimators. At rest, an average dose of 500 MBq of 99mTc-tetrofosmin (Myoview; GE Healthcare, UK) was administered intravenously. A 20% window was centred around the 140-KeV energy peak of 99mTc-tetrofosmin. A total of 90 projections (step and shoot method, 35 s per projection, 64 × 64 matrix, total imaging time 23 min) were obtained over a 360° circular orbit. Electrocardiogram gating was applied on the cardiac cycle with 16 frames per cardiac cycle using a tolerance window of 50%.
The raw data were uploaded to the Emory Cardiac Toolbox (Emory University, Atlanta, GA) for data reconstruction, reorientation and phase analysis processing. All patient studies were reconstructed by ordered subsets expectation maximization with three iterations and ten subsets. A Butterworth filter with a cut-off frequency of 0.35 cycles per centimetre and a power of 10 was used to filter the gated images. The reconstructed images were thereafter reoriented manually with the assistance of the automatic reorientation tool in the Emory Reconstruction Toolbox to generate gated short-axis images. The gated short-axis images were sampled for regional maximum counts using the Emory Cardiac Toolbox and then submitted to the multiharmonic phase analysis tool. Finally, phase analysis processing was performed blinded at Emory University.
Phase analysis on GMPS
Phase analysis of GMPS studies was performed to derive LV diastolic and systolic dyssynchrony. LV diastolic dyssynchrony was assessed using phase analysis which consisted of several consecutive processing steps, as illustrated in Fig. 1. Initially, regional maximal count detection was performed in 3-D for each temporal frame using the standard gated short-axis SPECT image. Phase analysis processing was based on the partial volume effect wherein alterations in regional maximal counts are proportional to changes in regional myocardial wall thickening over the cardiac cycle [12, 13]. This linear relationship was demonstrated in a phantom study [13]. The third Fourier harmonic function was then used to approximate the discrete sample points into a continuous wall-thickening curve. For each region, the wall-thickening curve provided a phase angle that represented the OMR of the particular region. The OMR phase is the time point when the curves cross the middle line from positive to negative. Once the OMR phase angles of all regions (>600 regions over the entire left ventricle) were obtained, a phase distribution was generated that provided information on the degree of diastolic dyssynchrony for the entire left ventricle. The phase distribution was displayed in a polar map and histogram as shown in Fig. 1. The quantitative parameters for LV diastolic dyssynchrony were derived from the phase histogram, and included the diastolic phase SD (SD of the OMR phase distribution) and diastolic HBW (the width of the band that included 95% of the OMR phase angles), similar to the LV systolic dyssynchrony indices [11].
Schematic illustration of the processing steps involved in the assessment of diastolic dyssynchrony using phase analysis of GMPS studies. For each temporal frame, a regional maximal count detection was performed in 3-D using the standard gated short-axis SPECT images. Consecutively, the third Fourier harmonic function was used to approximate the discrete sample points into a continuous wall-thickening curve. The wall-thickening curve provided a phase angle that represented the OMR of the region (>600 regions for the entire left ventricle). The OMR phase angles of all left ventricle regions were used to generate a phase distribution, which was displayed in a polar map and histogram. The phase histogram was used to obtain the LV diastolic dyssynchrony indices including the diastolic phase SD (SD of the OMR phase distribution) and the diastolic HBW (the width of the band that includes 95% of the OMR phase angles)
Additionally, LV systolic dyssynchrony was evaluated measuring the systolic phase SD and systolic HBW [11]. As previously described [11], the onset of mechanical contraction per cardiac region was measured using count-based wall-thickening curves which were derived from alternations in myocardial counts during the cardiac cycle. Each myocardial segment yielded a phase angle representing the onset of mechanical contraction for that particular segment of the myocardium. Finally, the phase angles of the entire left ventricle (exceeding 600 per left ventricle) were plotted in a polar map and histogram, wherein the phase histogram was used to calculate systolic phase SD and systolic HBW as markers of LV systolic dyssynchrony [11].
Furthermore, the intra- and interobserver reproducibility of phase analysis for the assessment of diastolic phase SD and diastolic HBW were determined in a subset of 25 patients.
Transthoracic echocardiography with tissue Doppler imaging
Transthoracic echocardiography was performed in patients at rest lying in the left lateral decubitus position using a commercially available ultrasound device (M3S probe, Vivid 7; GE-Vingmed, Horten, Norway). Data were acquired with a 3.5 MHz transducer in the parasternal and apical views (standard long- and short-axis images, and two- and four-chamber and apical long-axis images). Conventional 2-D images were obtained during breath-hold and saved in cine-loop format from three consecutive beats for offline analysis (EchoPac 108.1.5; GE-Vingmed, Horten, Norway). From the apical two- and four-chamber views, LV end-systolic volume and LV end-diastolic volume along with the LVEF were measured using the biplane Simpson approach [14]. Colour-coded TDI was then performed to assess diastolic dyssynchrony of the left ventricle, as shown in Fig. 2. In each patient, colour-coded TDI of the left ventricle was obtained in the apical two- and four-chamber views during end-expiration. The sector size and depth were optimized for the highest possible frame rate (>115 frames per second). A sample volume (12 × 6 mm) was then positioned in the middle of the basal portion of the four left ventricle wall segments (anterior, lateral, inferior and septal) to obtain regional colour-coded TDI velocity curves. For each region, the maximal diastolic delay was obtained measuring the time between the onset QRS complex and the peak early diastolic myocardial velocity (E′) [7, 15]. LV dyssynchrony was calculated as the time delay between peak early diastolic velocities of two opposing walls (diastolic mechanical delay). Significant LV diastolic dyssynchrony was defined as a diastolic mechanical delay of >55 ms [6]. As previously reported [6], the cut-off value of 55 ms was derived from the mean ±2×SD of the maximal diastolic delay in 38 control patients who had no history of cardiovascular disease. The TDI recordings were post-processed by two independent observers who were blinded to the other clinical and phase analysis data.
LV diastolic dyssynchrony was assessed with colour-coded TDI. The times between the onset of the QRS complex and the peak early diastolic myocardial velocities (E′) of four left ventricle wall segments (anterior, lateral, inferior and septal) were obtained using colour-coded TDI. LV dyssynchrony was calculated as the maximal time delay between peak early diastolic velocities of two opposing walls (diastolic mechanical delay). In this example, the diastolic mechanical delay was 70 ms between the septal and lateral left ventricle walls (four-chamber view; upper panel) and 95 ms between the anterior and inferior left ventricle walls (two-chamber view; lower panel)
Statistical analysis
Continuous variables are expressed as means ± SD as they were normally distributed when evaluated by Kolmogorov-Smirnov tests. Categorical data are presented as numbers or percentages. Phase analysis on GMPS and TDI were compared using Pearson’s linear regression analysis. Furthermore, patients were stratified into those with and those without LV diastolic dyssynchrony using a cut-off value of 55 ms of diastolic mechanical delay on TDI [6]. Phase SD and HBW were compared between patients with LV diastolic dyssynchrony (>55 ms) and those without LV diastolic dyssynchrony (≤55 ms) on TDI using Student’s t test. LV diastolic dyssynchrony and systolic dyssynchrony indices were compared using Pearson’s linear regression analysis. Intra- and interobserver reproducibility were evaluated by calculating the intraclass correlation coefficients (ICC). Excellent agreement was defined as an ICC of >0.8.Statistical analyses were performed with the SPSS software package, version 16.0 (SPSS, Chicago, IL).
Results
Patient population
A total of 150 patients with end-stage HF (114, 76%, men; mean age 66.0 ± 10.4 years) were included. The baseline characteristics of the patient population are shown in Table 1. Of the 150 patients, 101 (67%) had ischaemic cardiomyopathy and 49 (33%) had non-ischaemic cardiomyopathy. Patients showed a severely reduced LVEF (27 ± 8%) on 2-D echocardiography. Medication consisted of diuretics (87% of patients), angiotensin-converting enzyme inhibitors (ACE-I) or angiotensin (AT) II antagonists (91% of patients) and beta-blockers (73% of patients).
LV diastolic dyssynchrony
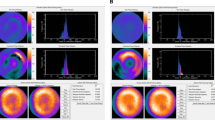
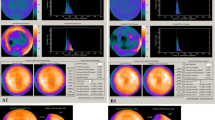
The mean values of LV diastolic dyssynchrony indices are shown in Table 2. The patient population showed a mean diastolic mechanical delay of 53.4 ± 21.4 ms on TDI. Phase analysis on GMPS showed a mean diastolic phase SD of 53.3 ± 19.4° and diastolic HBW of 175.7 ± 74.7°. Example findings in a patient with and in a patient without extensive LV diastolic dyssynchrony are provided in Fig. 3.
LV diastolic dyssynchrony as assessed by phase analysis on GMPS and 2-D echocardiography with TDI. Left Patient with extensive LV diastolic dyssynchrony on GMPS (a) and TDI (b). Extensive LV diastolic dyssynchrony is reflected in a heterogeneous colour-coded phase polar map and a broad phase histogram (a). Diastolic phase SD and diastolic HBW are 90.6° and 312.0°, respectively. Similarly, TDI shows extensive LV dyssynchrony with a diastolic mechanical delay of 60 ms (b). Right Patient without LV diastolic dyssynchrony on GMPS (c) and TDI (d). Phase analysis on GMPS shows a homogeneous colour-coded phase polar map and a narrow phase histogram (c). Diastolic phase SD and diastolic HBW are 14.2° and 46.0°, respectively. Diastolic mechanical delay on TDI is 11 ms (d)
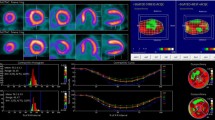
Furthermore, phase analysis on GMPS showed a good correlation with TDI for the assessment of LV diastolic dyssynchrony. Diastolic phase SD (r = 0.81, p < 0.01) and diastolic HBW (r = 0.75, p < 0.01) were well-correlated with LV diastolic dyssynchrony on TDI, as illustrated in Fig. 4.
Additionally, phase analysis on GMPS showed a good correlation with TDI for the assessment of LV diastolic dyssynchrony in patients with non-ischaemic cardiomyopathy as compared to patients with ischaemic cardiomyopathy (diastolic phase SD, r = 0.86 vs. r = 0.78; diastolic HBW, r = 0.78 vs. r = 0.73; p < 0.01 for all analyses). Patients with ischaemic cardiomyopathy showed more extensive LV diastolic dyssynchrony, as reflected by diastolic phase SD (55.9 ± 18.5° vs. 47.9 ± 20.1°, p = 0.2) and diastolic HBW (185.0 ± 72.5° vs. 156.6 ± 76.3°, p = 0.4) than patients with non-ischaemic cardiomyopathy.
In total, 69 (46%) patients showed LV diastolic dyssynchrony (diastolic mechanical delay >55 ms) on TDI, whereas 81 (54%) patients showed no LV diastolic dyssynchrony (diastolic mechanical delay ≤55 ms). Patients with LV diastolic dyssynchrony showed a significantly larger diastolic phase SD (68.1 ± 13.4° vs. 40.7 ± 14.0°, p < 0.01) and diastolic HBW (230.6 ± 54.3° vs. 129.0 ± 55.6°, p < 0.01) than patients without LV diastolic dyssynchrony on TDI (Fig. 5).
The patient population was divided into those with (white bars) and those without (black bars) significant LV diastolic dyssynchrony on TDI using a cut-off value of 55 ms of diastolic mechanical delay [6]. GMPS with phase analysis was used to calculate diastolic phase SD and diastolic HBW, which were used as markers of LV diastolic dyssynchrony. Patients with significant LV diastolic dyssynchrony (>55 ms) on TDI (white bars) showed significantly higher values of diastolic phase SD (68.1 ± 13.4° vs. 40.7 ± 14.0°, p < 0.01) and diastolic HBW (230.6 ± 54.3° vs. 129.0 ± 55.6°, p < 0.01) than patients without significant LV diastolic dyssynchrony (≤55 ms) on TDI (black bars)
Finally, phase analysis on GMPS showed good intra- and interobserver reproducibilities for diastolic phase SD (ICC 0.97 and 0.88, respectively) and diastolic HBW (ICC 0.98 and 0.93, respectively) in a subset of 25 randomly selected patients.
LV diastolic dyssynchrony and QRS duration
QRS duration was not correlated with LV diastolic dyssynchrony as assessed by phase analysis on GMPS or TDI. Diastolic phase SD (r = 0.11, p = NS) and diastolic HBW (r = 0.16, p = NS) showed no significant correlation with QRS duration. Similarly, LV diastolic dyssynchrony on TDI (r = 0.12, p = NS) showed no significant correlation with QRS duration.
LV diastolic dyssynchrony and systolic dyssynchrony on GMPS
LV diastolic phase SD (r = 0.91, p < 0.01) and diastolic HBW (r = 0.94, p < 0.01) were well-correlated with LV systolic SD and systolic HBW, respectively. In addition, LV diastolic phase SD showed a good correlation with LV systolic HBW (r = 0.90, p < 0.01). Finally, LV diastolic HBW and systolic phase SD (r = 0.92, p < 0.01) were well-correlated.
Discussion
The current study showed that phase analysis on GMPS was significantly correlated with TDI for the assessment of LV diastolic dyssynchrony. Diastolic phase SD and diastolic HBW as derived from GMPS showed good correlations with diastolic mechanical delay on TDI. Accordingly, phase analysis on GMPS represents a feasible technique for evaluation of LV diastolic dyssynchrony in patients with HF.
Dyssynchrony of the left ventricle contraction pattern (LV systolic dyssynchrony) is considered an important condition in patients with HF [1, 2]. Previous studies have shown that patients with extensive LV systolic dyssynchrony are at risk of adverse cardiac events [1, 2]. In addition, it has been suggested that LV diastolic dyssynchrony, which is a dyssynchronous relaxation pattern of the left ventricle, plays an important role in patients with HF [3–5]. In patients with HF, it is known that left bundle branch block can cause LV diastolic dyssynchrony and marked diastolic LV filling impairment by inducing a reduced diastolic filling rate, and prolonged isovolumetric contraction and relaxation times. As a consequence, LV diastolic dyssynchrony is related to abnormalities in the diastolic filling pattern of the left ventricle, which may further compromise the haemodynamic function of the failing heart [3, 4].
At present, TDI echocardiography is a feasible method for evaluation of diastolic relaxation patterns of the left ventricle [6–8]. By measuring the time delays in peak diastolic velocities of left ventricle segments diastolic dyssynchrony can be derived [6–8]. Even though a detailed evaluation of LV diastolic dyssynchrony can be made with TDI, the post-processing of Doppler images remains observer-dependent and requires substantial expertise.
Phase analysis on GMPS is another approach to the evaluation of mechanical dyssynchrony [16, 17]. In recent years, phase analysis on GMPS has received increasing attention as it provides robust and reproducible indices of mechanical dyssynchrony using an automated approach [9, 10]. Moreover, GMPS with phase analysis provides integrated information on mechanical dyssynchrony, regional mechanical activation pattern and myocardial perfusion using a single SPECT study. Several studies have shown its feasibility for the assessment of LV systolic dyssynchrony in patients with HF [16, 17]. Henneman et al. [17] showed that phase analysis on GMPS is well-correlated with 2-D echocardiography with TDI for the assessment of LV systolic dyssynchrony. Good correlations were found between systolic phase SD (r = 0.80, p < 0.05) or systolic HBW (r = 0.89, p < 0.01) and LV systolic dyssynchrony on TDI in 75 patients with moderate-to-severe HF. Additionally, phase analysis on GMPS has been compared with real-time 3-D echocardiography (RT3DE) for the assessment of LV systolic dyssynchrony in 40 patients with severe HF [18]. Both techniques showed good agreement for LV systolic dyssynchrony: systolic phase SD (r = 0.80, p < 0.05) and systolic HBW (r = 0.76, p < 0.05) as derived from GMPS were well-correlated with LV systolic dyssynchrony on RT3DE. Although these studies have shown that phase analysis on GMPS can be used for the assessment of LV systolic dyssynchrony, no study has evaluated its feasibility for the assessment of LV diastolic dyssynchrony in patients with HF. The present study showed the feasibility of phase analysis on GMPS for the evaluation of LV diastolic dyssynchrony, as reflected by a good correlations between LV diastolic dyssynchrony indices on GMPS (phase SD and HBW) and LV diastolic dyssynchrony on TDI. Moreover, patients with LV diastolic dyssynchrony on TDI (diastolic mechanical delay >55 ms) showed significantly larger diastolic phase SD and diastolic HBW than patients without LV diastolic dyssynchrony on TDI (diastolic mechanical delay ≤55 ms). Accordingly, phase analysis on GMPS represents a feasible technique for the assessment of both LV diastolic and LV systolic dyssynchrony in patients with HF.
Furthermore, a subanalysis was performed to evaluate whether phase analysis on GMPS could be used for the assessment of LV diastolic dyssynchrony in patients with ischaemic cardiomyopathy and in those with non-ischaemic cardiomyopathy. In the current study, the agreement between phase analysis on GMPS and TDI for measuring LV diastolic dyssynchrony was slightly reduced in patients with ischaemic cardiomyopathy as compared to those with non-ischaemic cardiomyopathy. An explanation for this finding could be that the presence of myocardial scar tissue may have influenced the comparison between the two techniques. In patients with ischaemic cardiomyopathy, the presence of extensive scar tissue may have reduced the accuracy of TDI in assessing LV diastolic dyssynchrony. Although the agreement between the two techniques was slightly reduced in patients with ischaemic HF, the study demonstrated that phase analysis on GMPS can also be used for the assessment of LV diastolic dyssynchrony in patients with ischaemic cardiomyopathy. Finally, the current study showed that LV diastolic dyssynchrony was a common phenomenon in patients with HF. In the present study, 69 patients (46%) showed LV diastolic dyssynchrony defined as a diastolic mechanical delay of >55 ms on TDI. Similarly, Schuster et al. [3] have reported that diastolic dyssynchrony of the left ventricle was prevalent in patients with HF: 58% of patients showed LV diastolic dyssynchrony on TDI. Furthermore, the current findings are in line with those of Shanks et al. [6] who showed that LV diastolic dyssynchrony as assessed with TDI was present in nearly 52% of patients with HF. Accordingly, phase analysis on GMPS allows detection of LV diastolic dyssynchrony.
LV diastolic dyssynchrony and QRS duration
Several studies have shown that electrical dyssynchrony, as reflected by a prolonged QRS interval, is not associated with either systolic dyssynchrony or diastolic dyssynchrony of the left ventricle [3, 19, 20]. Schuster et al. [3] showed that QRS duration was poorly correlated with LV systolic dyssynchrony and LV diastolic dyssynchrony in 108 patients with HF. Similar findings were reported by Wang et al. [7] who found that LV diastolic dyssynchrony and QRS interval (r = 0.36, p = 0.03) were poorly correlated in 60 patients with HF. In line with the findings of these studies, the present study showed that QRS duration was not correlated with LV diastolic dyssynchrony as assessed by phase analysis on GMPS or TDI.
Limitations
Although the study demonstrated that phase analysis on GMPS could be used to assess LV diastolic dyssynchrony, some limitations need to be addressed. First, the current findings were based on a relatively small subset of patients, whereas a large cohort of patients with HF would have been preferred to evaluate the feasibility of phase analysis on GMPS for the assessment of LV diastolic dyssynchrony. Secondly, even though the current evaluation showed that phase analysis on GMPS was well-correlated with TDI for the assessment of LV diastolic dyssynchrony, some variation was observed between the two imaging techniques. This may be explained by the fact that GMPS with phase analysis allows evaluation of the time dispersion of mechanical phenomena (both diastolic relaxation and systolic contraction) in 3-D, whereas TDI represents the time difference between two opposing walls. For this reason, 3-D echocardiography (rather than 2-D echocardiography with TDI) would be preferred to evaluate the feasibility of phase analysis on GMPS for the assessment of LV diastolic dyssynchrony. At present, however, TDI is the most commonly used technique for the assessment of LV diastolic dyssynchrony. In addition, the implications of GMPS-derived LV diastolic dyssynchrony on response to cardiac resynchronization therapy (CRT) or the effects of CRT on LV diastolic mechanical relaxation were not evaluated. Additional studies are needed in order to establish the accuracy of GMPS-derived LV diastolic dyssynchrony to predict response to CRT or long-term outcome.
Conclusion
Phase analysis on GMPS showed a good correlation with TDI for the assessment of LV diastolic dyssynchrony. Accordingly, phase analysis represents a feasible technique for evaluation of LV diastolic dyssynchrony in patients with HF.
References
Bader H, Garrigue S, Lafitte S, Reuter S, Jais P, Haissaguerre M, et al. Intra-left ventricular electromechanical asynchrony. A new independent predictor of severe cardiac events in heart failure patients. J Am Coll Cardiol. 2004;43:248–56.
Cho GY, Song JK, Park WJ, Han SW, Choi SH, Doo YC, et al. Mechanical dyssynchrony assessed by tissue Doppler imaging is a powerful predictor of mortality in congestive heart failure with normal QRS duration. J Am Coll Cardiol. 2005;46:2237–43.
Schuster I, Habib G, Jego C, Thuny F, Avierinos JF, Derumeaux G, et al. Diastolic asynchrony is more frequent than systolic asynchrony in dilated cardiomyopathy and is less improved by cardiac resynchronization therapy. J Am Coll Cardiol. 2005;46:2250–7.
Xiao HB, Lee CH, Gibson DG. Effect of left bundle branch block on diastolic function in dilated cardiomyopathy. Br Heart J. 1991;66:443–7.
Waggoner AD, Rovner A, de las Fuentes L, Faddis MN, Gleva MJ, Sawhney N, et al. Clinical outcomes after cardiac resynchronization therapy: importance of left ventricular diastolic function and origin of heart failure. J Am Soc Echocardiogr. 2006;19:307–13.
Shanks M, Bertini M, Delgado V, Ng ACT, Nucifora G, Van Bommel RJ, et al. Effect of biventricular pacing on diastolic dyssynchrony. J Am Coll Cardiol. 2010;56:1567–75.
Wang J, Kurrelmeyer KM, Torre-Amione G, Nagueh SF. Systolic and diastolic dyssynchrony in patients with diastolic heart failure and the effect of medical therapy. J Am Coll Cardiol. 2007;49:88–96.
Yu CM, Zhang Q, Yip GW, Lee PW, Kum LC, Lam YY, et al. Diastolic and systolic asynchrony in patients with diastolic heart failure: a common but ignored condition. J Am Coll Cardiol. 2007;49:97–105.
Trimble MA, Velazquez EJ, Adams GL, Honeycutt EF, Pagnanelli RA, Barnhart HX, et al. Repeatability and reproducibility of phase analysis of gated single-photon emission computed tomography myocardial perfusion imaging used to quantify cardiac dyssynchrony. Nucl Med Commun. 2008;29:374–81.
Lin X, Xu H, Zhao X, Folks RD, Garcia EV, Soman P, et al. Repeatability of left ventricular dyssynchrony and function parameters in serial gated myocardial perfusion SPECT studies. J Nucl Cardiol. 2010;17:811–6.
Chen J, Garcia EV, Folks RD, Cooke CD, Faber TL, Tauxe EL, et al. Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. J Nucl Cardiol. 2005;12:687–95.
Hoffman EJ, Huang SC, Phelps ME. Quantitation in positron emission computed tomography: 1. Effect of object size. J Comput Assist Tomogr. 1979;3:299–308.
Galt JR, Garcia EV, Robbins WL. Effects of myocardial wall thickness on SPECT quantification. IEEE Trans Med Imaging. 1990;9:144–50.
Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67.
Chang SA, Kim HK, Kim DH, Kim YJ, Sohn DW, Oh BH, et al. Left ventricular systolic and diastolic dyssynchrony in asymptomatic hypertensive patients. J Am Soc Echocardiogr. 2009;22:337–42.
Henneman MM, Chen J, Dibbets-Schneider P, Stokkel MP, Bleeker GB, Ypenburg C, et al. Can LV dyssynchrony as assessed with phase analysis on gated myocardial perfusion SPECT predict response to CRT? J Nucl Med. 2007;48:1104–11.
Henneman MM, Chen J, Ypenburg C, Dibbets-Schneider P, Bleeker GB, Boersma E, et al. Phase analysis of gated myocardial perfusion single-photon emission computed tomography compared with tissue Doppler imaging for the assessment of left ventricular dyssynchrony. J Am Coll Cardiol. 2007;49:1708–14.
Marsan NA, Henneman MM, Chen J, Ypenburg C, Dibbets-Schneider P, Ghio S, et al. Real-time 3-dimensional echocardiography as a novel approach to quantify left ventricular dyssynchrony: a comparison study with phase analysis of gated myocardial perfusion single photon emission computed tomography. J Am Soc Echocardiogr. 2008;21:801–7.
Bleeker GB, Schalij MJ, Molhoek SG, Holman ER, Verwey HF, Steendijk P, et al. Frequency of left ventricular dyssynchrony in patients with heart failure and a narrow QRS complex. Am J Cardiol. 2005;95:140–2.
Yu CM, Chan YS, Zhang Q, Yip GW, Chan CK, Kum LC, et al. Benefits of cardiac resynchronization therapy for heart failure patients with narrow QRS complexes and coexisting systolic asynchrony by echocardiography. J Am Coll Cardiol. 2006;48:2251–7.
Acknowledgments
Mark J. Boogers is supported by the Dutch Heart Foundation (grant number 2006 T102). This study was also supported in part by a NIH-funded research project (1R01HL094438, PI: Ji Chen).
Conflicts of Interest
Martin J. Schalij received research grants from Boston Scientific, Medtronic and Biotronik. Jeroen J. Bax received research grants from Medtronic, Boston Scientific, Biotronik, Edwards Lifesciences, BMS Medical Imaging, St. Jude Medical and GE Healthcare. Ji Chen and Ernest V. Garcia receive royalties from the sale of the Emory Cardiac Toolbox. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict-of-interest practice. The other authors have indicated they have no financial or other conflicts of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Boogers, M.J., Chen, J., Veltman, C.E. et al. Left ventricular diastolic dyssynchrony assessed with phase analysis of gated myocardial perfusion SPECT: a comparison with tissue Doppler imaging. Eur J Nucl Med Mol Imaging 38, 2031–2039 (2011). https://doi.org/10.1007/s00259-011-1870-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-011-1870-5