Abstract
Atherosclerosis is the major cause of cardiovascular disease, which still has the leading position in morbidity and mortality in the Western world. Many risk factors and pathobiological processes are acting together in the development of atherosclerosis. This leads to different remodelling stages (positive and negative) which are both associated with plaque physiology and clinical presentation. The different remodelling stages of atherosclerosis are explained with their clinical relevance. Recent advances in basic science have established that atherosclerosis is not only a lipid storage disease, but that also inflammation has a fundamental role in all stages of the disease. The molecular events leading to atherosclerosis will be extensively reviewed and described. Further on in this review different modalities and their role in the different stages of atherosclerosis will be discussed. Non-nuclear invasive imaging techniques (intravascular ultrasound, intravascular MRI, intracoronary angioscopy and intravascular optical coherence tomography) and non-nuclear non-invasive imaging techniques (ultrasound with Doppler flow, electron-bean computed tomography, coronary computed tomography angiography, MRI and coronary artery MR angiography) will be reviewed. After that we focus on nuclear imaging techniques for detecting atherosclerotic plaques, divided into three groups: atherosclerotic lesion components, inflammation and thrombosis. This emerging area of nuclear imaging techniques can provide measures of biological activity of atherosclerotic plaques, thereby improving the prediction of clinical events. As we will see in the future perspectives, at present, there is no special tracer that can be called the diagnostic tool to diagnose prospective stroke or infarction in patients. Nevertheless, we expect such a tracer to be developed in the next few years and maybe, theoretically, it could even be used for targeted therapy (in the form of a beta-emitter) to combat cardiovascular disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in the Western world and is responsible for approximately 50% of all deaths in the USA, Europe and Japan [1]. CVD comprises three major areas, being coronary heart diseases (myocardial infarction, angina, heart failure and coronary death), cerebrovascular diseases (stroke, transient ischaemic attacks) and peripheral vascular diseases (claudicatio intermittens, gangrene). CVD is common in the general population, affecting the majority of adults past the age of 60 years. The lifetime risk of coronary heart disease (CHD) in individuals initially free of CHD is 49% in men and 32% in women at age 40. The lifetime risk is also appreciable in those free of CHD at age 70: 35% in men and 24% in women [2].
Atherosclerosis is the major cause of cardiovascular disease [3]. A variety of risk factors and pathobiological processes, often acting together, are associated with an increased risk for developing atherosclerosis in the coronary and peripheral vasculature. A major pathobiological process central to the pathogenesis of atherosclerosis is the deposition of cholesterol in the arterial wall [4]. Nearly all lipoproteins are involved in this process, including cholesterol carried by very low-density (VLDL), remnant lipoproteins [5] and low-density lipoproteins (LDL), particularly the small, dense form [6]. Conversely, cholesterol is carried away from the arterial wall by high-density lipoprotein (HDL) [7]. Therefore, it is well recognized that abnormalities in lipoprotein profile, both inherited or acquired, are the most important cause of premature atherosclerosis [8]. Consistently, several large-scale trials have demonstrated that the reduction of LDL by either diet or drugs is followed by a significant reduction of ischaemic cardiovascular events [9–11].
Other major, modifiable factors are diabetes mellitus, hypertension, cigarette smoking (active and passive), obesity and physical inactivity [12–14]. Family history of premature CHD (genetic predisposition), age and gender are not modifiable independent risk factors for atherosclerosis [12].
In addition to the above-mentioned major risk factors, clinical and epidemiological studies have indicated that once an atherosclerotic plaque reaches a certain stage of development, the plaque itself becomes a risk factor for future vascular events [15]. This is because existing plaques can undergo rupture or erosion, thus favouring the formation of an occluding thrombus [16]. This is clearly demonstrated by follow-up studies on patients undergoing coronary angiograms revealing that the probability of future coronary events relates to the extent of coronary atherosclerosis [17]. The more extensive the burden of atherosclerosis is, the greater is the frequency of plaque rupture [15]. As the severity of atherosclerosis rises with age, the usual way of estimating plaque burden in the clinical setting is to use age as a surrogate marker. This approach has been accepted for primary prevention [18]; however, once significant atherosclerosis has been definitely identified, the patient is often designated as having preclinical disease, without symptoms. As a result, plaque burden has to be integrated into risk assessment in asymptomatic patients [15].
In recent years several techniques for estimating the severity of atherosclerotic burden have been investigated. The aim of this article is to review advantages and disadvantages of these techniques, with special attention to the molecular imaging of atherosclerosis. In the last couple of years our knowledge of the molecular mechanisms of atherosclerosis has greatly expanded. This led to the recognition that the rupture of vulnerable plaques is critical in converting asymptomatic atherosclerosis to a clinical event. Therefore, in this article particular attention will be dedicated to those procedures designed to explore the “functional status” of the atherosclerotic plaque. The hypothesis is that measurement of plaque activity would improve the global risk assessment of patients with atherosclerosis, thus allowing more cost-effective risk reduction treatments.
Remodelling stages of atherosclerosis
The exact mechanisms by which atherogenic factors act are only partially known. However there are indications that they primarily alter the protective function of endothelium thus favouring the deposit of lipids and monocytes/macrophages into the vascular wall. Atherosclerosis develops over the course of 50 years, beginning in the early teenage years. Initial, fatty streaks in the arterial intima are seen in childhood and adolescence [19].
In the first steps, i.e. formation of stable plaque, an innate immune response plays a pivotal role in determining the evolution of the lesion. In particular monocytes/macrophages, recruited in the subintimal space by damaged endothelium, overload oxLDL via scavenger receptors and perpetuate the local inflammatory response secreting cytokines, degrading enzymes [matrix metalloproteinases (MMPs)] as well as growth factors that stimulate smooth muscle cell (SMC) migration and proliferation. The continuous influx of cells into the subintimal space converts the fatty streak into a more complex and advanced lesion in which inflammatory cells (monocytes/macrophages, lymphocytes), SMCs, necrotic debris mainly due to cell death, oxLDL elicit a chronic inflammatory response by the adaptive immune system. SMCs form a thick fibrous cap that cover the necrotic core and avoid the exposure of thrombogenic material to the bloodstream. The volume of lesion grows up and protrudes into the arterial lumen causing variable degrees of lumen stenosis. The degree of carotid lumen stenosis is a relevant marker for the risk of cerebrovascular disease [20, 21] even if the cause of most clinical events should rely on thromboembolism from a vulnerable carotid plaque [22].
The microenvironment of the plaque could elicit an adaptive immune response able to determine a selective recruitment of inflammatory cells. In this evolutive stage lymphocytes, instead of macrophages, orchestrate the immune response. The switch to a selective recruitment of T helper type 1 (Th1) T lymphocytes represents a key point toward plaque vulnerability/disruption. In particular Th1 T lymphocytes promote plaque destabilization triggering vascular inflammation and downregulating extracellular matrix production by SMCs.
Interferon (IFN)-γ, the cytokine that characterizes the Th1 pattern, strongly inhibits the proliferation of SMCs and the production of interstitial collagens by vascular SMCs, affecting the stability of the fibrous cap. In this context activated macrophages secrete proteases that can degrade collagen. In addition, ligation of CD40 expressed by macrophages increases the production of matrix-degrading proteases. Therefore the integrity of the fibrous cap is drastically regulated by inflammatory cells which cause the thinning of the collagen-rich fibrous cap overlying the atheroma. The physical disruption of the protective cap will cause the exposure of the procoagulant factors expressed within lesions to circulating clotting factors initiating the coagulation cascade responsible for thrombosis.
In the early phase of atherosclerosis development, luminal size is not affected by plaque growth because of expansion of external elastic membrane and enlargement of vessel size, representing “positive remodelling”, known as the “Glagov effect”. Functionally important lumen stenosis may be delayed until the lesion occupies 40% of the internal elastic lamina area. The preservation of a nearly normal lumen cross-sectional area despite the presence of a large plaque should be taken into account in evaluating atherosclerotic disease with use of coronary angiography [23]. With disease progression, repeated silent plaque ruptures are followed by wound healing, resulting in increased stenosis [24]. There is no further increase in vessel size, but rather the plaque encroaches on the lumen, which shrinks, representing “negative remodelling” [25].
Positive and negative arterial remodelling are both associated with plaque physiology and clinical presentation. Positive remodelling appears to be a feature of unstable vulnerable plaques, which have a higher lipid content and macrophage count, and is primarily determined by inflammation, calcification and medial thinning [26, 27]. This positive remodelling is mostly seen in patients presenting with acute coronary and cerebrovascular syndromes (unstable angina, myocardial infarction, sudden death and stroke) and is typically due to rupture of plaques with only modest luminal stenosis (less than 75% in angiography) [28, 29]. The risk of plaque disruption depends more on plaque composition and vulnerability (plaque type) than on degree of stenosis (plaque size) [30]. In contrast, negative remodelling is associated with smoother and stable plaques such as in patients with stable angina, but nevertheless eventually resulting in severe stenosis.
In a study performed by Schoenhagen et al. positive remodelling and larger plaque areas were associated with unstable clinical presentation, whereas negative remodelling was more common in patients with stable clinical presentation. This association between the extent of remodelling and clinical presentation may reflect a greater tendency of plaques with positive remodelling to cause unstable coronary syndromes [31].
The most vulnerable coronary plaques are relatively non-stenotic (<75% cross-sectional area stenosis, normal lumina on angiography), have a larger lipid core and an often inflamed thin fibrous cap, and are associated with positive remodelling [30, 32] In contrast, low-risk plaques tend to be fibrotic and severely stenotic after a while due to negative remodelling. In both remodelling stages, repeated neovascularization of the intima at sites of vulnerable lipid-rich plaques is associated with infiltration of inflammatory cells contributing to plaque destabilization [33, 34].
Exploring the functional status of the plaque in both remodelling stages would be therefore essential to predict the most probable clinical complication.
Molecular events in atherosclerosis
Atherosclerosis was always considered to be a lipid storage disease. However, recent advances in basic science have established a fundamental role for inflammation in all stages of this disease from initiation through progression and ultimately to the thrombotic complications of atherosclerosis [35]. Hypercholesterolaemia and other risk factors are important in approximately 90% of patients with cardiovascular disease [36], but atherosclerosis does not result simply from the accumulation of lipids. It is clearly an inflammatory disease [37]. An atherosclerotic lesion can represent different stages of an inflammatory process in the artery; if unabated and excessive, this process will eventually result in an advanced, complicated lesion with plaque rupture and thrombosis. This consideration has several practical clinical consequences in risk stratification, diagnosis and targeting of therapy.
Endothelial cells, which under normal circumstances prevent adhesion of leukocytes and platelets, now express cellular adhesion molecules, chemokines and vascular cell adhesion molecules such as vascular cell adhesion molecule 1 (VCAM-1) [35, 38–40]. In addition selectins, like P- and E-selectin, contribute to leukocyte recruitment in atherosclerosis [40, 41]. These processes are partially mediated by nuclear factor κB (NF-κB), one of the transcriptional controllers in vascular inflammation and an integrator in atherogenesis, and proinflammatory cytokines, such as interleukin 1β (IL-1β) and tumour necrosis factor α (TNF-α).
Sites predisposed to lesion formation are branch points of arteries, which experience flow disturbances rather than uniform laminar flow. In these regions, the mean shear stress is relatively low and the NF-κB pathway may be primed for activation. Systemic risk factors would therefore preferentially induce the expression of proatherogenic NF-κB-dependent genes at lesion-predisposed sites. On the other hand, a straight segment of the artery proximal to the bifurcation is associated with a uniformly laminar blood flow profile that may induce the expression of genes producing atheroprotective proteins [42]. For example, the antioxidant superoxide dismutase (SOD) combats oxidative stress, endothelial nitric oxide synthetase (eNOS) inhibits the activation of NF-κB and cyclooxygenase 2 (COX-2) has an anti-inflammatory effect [43].
Once adherent to the endothelial cell, via VCAM-1 binding, leukocytes and monocytes enter the intima by diapedesis between endothelial cells at their junctions. This phenomenon is mediated through chemokines, such as monocyte chemoattractant protein 1 (MCP-1) [44, 45] and IL-8 as leukocyte chemoattractant [45–47]. Angiotensin II promotes atherogenesis by direct activation of MCP-1 gene expression in vascular SMCs [48]. Chemokines are subdivided into four families based on the position of their cysteine residues (CC, CXC, C, CXC3). Lymphocyte recruitment occurs through expression of three CXC chemokines on endothelial cells as well as the expression of their receptor, CXCR3, by all T lymphocytes within atherosclerotic lesions. This attraction is induced by IFN-γ that binds to the CXCR3 receptor on T lymphocytes at the atherosclerotic lesion [49].
A recent paper by Schwarz et al. showed that wire-mediated arterial injury induced local and systemic expression of IFN-γ-inducible protein 10 (IP-10) and monokine induced by IFN-γ (MIG), resulting in enhanced recruitment of CXCR3+ leukocytes and haematopoietic progenitor cells. This was accompanied by profound activation of mammalian target of rapamycin complex 1 (mTORC1, playing a fundamental role in the regulation of cell viability, translation initiation and cell cycle progression), increased reactive oxygen species production, apoptosis and intimal hyperplasia. In vitro, stimulation of T cells with IP-10 directly activated mTORC1 and induced generation of reactive oxygen species and apoptosis in an mTORC1-dependent manner. These results strongly indicate that CXCR3-dependent activation of mTORC1 directly links stimulation of the Th1 lymphocytes’ immune system with the proliferative response of intimal cells in vascular remodelling: a process that is followed by expression of activation markers on T-cell surface such as the IL-2 receptor, CD25-CD122-CD132 heterotrimer [50].
In the intima, monocytes acquire the morphological characteristics of macrophages and express scavenger receptors that bind internalized lipoprotein particles. These lipid-laden cells, known as foam cells, characterize the early atherosclerotic lesion, the fatty streak, and secrete proinflammatory cytokines that amplify the local inflammatory response in the lesion, as well as growth factors, that stimulate the smooth muscle replication responsible for lesion growth. Macrophage colony-stimulating factor (M-CSF) acts as the main stimulator in this process, next to granulocyte-macrophage colony-stimulating factor (MG-CSF) and IL-2 for lymphocytes [51, 52]. Colony stimulating factor 1 (CSF-1) represents a link between inflammation and cardiovascular disease. Two distinct mechanisms were suggested by which CSF-1, which is known to be present in atherosclerotic lesions, may contribute to plaque progression. CSF-1 modulated the human monocyte-derived macrophages (HMDM) transcriptome to favour a proatherogenic environment. CSF-1 induced expression of the proatherogenic chemokines CXCL10/IP-10, CCL2 and CCL7 but repressed expression of the antiatherogenic chemokine receptor CXCR4 [53].
Human atherosclerotic plaques contain numerous T lymphocyte cells. In a plaque, ∼40% of the cells express macrophage markers, ∼10% are CD3+ T cells and most of the remainder have the characteristics of SMCs [54]. T lymphocyte cells can be activated by autoantigens, such as oxLDL and heat-shock proteins (HSPs, both of endogenous and microbial origin). Both autoantigens serve as the target for autoimmune responses in inflammatory diseases and produce cytokines that can influence the behaviour of other cells present in the atheroma and so stimulating inflammation and plaque formation [55].
Mononuclear phagocytes play a key role in the thrombotic complications of atherosclerosis producing degrading enzymes, i.e. MMPs able to degrade the extracellular matrix that gives strength to the fibrous cap [56–58]. Subsequent to cap thinning a disruption or tear in the cap causes blood to enter the lipid core of the plaque determining an initial phase of intraplaque haemorrhage.
Blood cells may make contact with another macrophage product, the potent procoagulant protein tissue factor, which may trigger thrombosis and thus promote sudden expansion of the atheromatous lesion [59]. This remodelling of the thrombus and the release of platelet-derived growth factor (PDGF) and the anti-inflammatory mediator transforming growth factor β (TGF-β) leads to fibrous tissue formation. Eventually the macrophages congregate in a central core in the typical atherosclerotic plaque. Macrophages can die in this location, some by apoptosis, so producing the “necrotic core” of the atherosclerotic lesion. All this happens in the beginning of intraplaque haemorrhage, thrombosis and necrosis that may or may not be followed by thrombosis within the lumen [35].
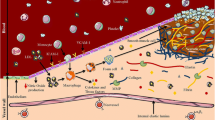
A brief overview of the molecular events leading to plaque formation (stable and unstable) is given in Figs. 1 and 2.
Plaque development is a complex cascade of events. Endothelial injury is initiated by a variety of injurious noxae, including hypertension, dyslipidaemia, shear stress and infections (a). Leaky damaged endothelium allows the passage of leukocytes and lipids into the subendothelial space. Activated endothelial cells increase the expression of adhesion molecules and inflammatory genes. Circulating monocytes migrate into the subendothelial space and differentiate into macrophages (b). Macrophages take up lipids deposited in the intima by a number of receptors, including scavenger receptor A (SR-A) and CD36. Deregulated uptake of modified LDL through these receptors leads to cholesterol accumulation and “foam cell” formation. Multiple foam cells form the fatty streak and secrete proinflammatory cytokines and MMPs. Both factors amplify the local inflammatory response in the lesion and in the local matrix. Repeated cycles of inflammation (c) lead to accumulation of macrophages, some of which can die in this location producing the so-called necrotic core. Living macrophages induce SMC proliferation and migration in the lesion to form the fibrous cap of the advanced complicated stable atherosclerotic lesion (“stable” plaque). T cells also play a major role in the path of inflammation. T cells may encounter antigens such as oxidized LDL and HSPs of endogenous or microbial origin. Several different effector mechanisms can be elicited by immune responses (d). The combination of IFN-γ and TNF-β upregulates the expression of CXCR3 promoting the development of the Th1 lymphocyte ()pathway which is strongly proinflammatory. The selective recruitment and activation of Th1 T cells determines a potent inflammatory cascade leading to the transition from stable to “unstable/ruptured” plaque. During this transition we postulated the existence of a theoretical plaque structure known as “vulnerable” plaque, very similar to the unstable plaque except for plaque erosion/rupture. In this context, IFN-γ strongly inhibits the proliferation of SMCs and the production of interstitial collagens by vascular SMCs, thereby affecting the stability of the fibrous cap (e). Activated macrophages secrete procoagulant proteins and MMPs that can degrade collagen. In addition, ligation of CD40 expressed by macrophages increases the production of matrix-degrading proteases. All this leading to an unstable or ruptured plaque (f)
Atherosclerotic plaque development (http://www.resverlogix.com/product_development/nexvas_platform/nexvas_vascular_inflammation.html)
Non-nuclear imaging techniques for detecting atherosclerotic plaques
For almost 50 years, contrast angiography (“luminogram”) has been the gold standard imaging technique for atherosclerosis. It provides high-resolution definition of the site and severity of luminal stenosis. However, assessment of coronary lumen integrity is of limited value for the detection of subclinical cardiovascular disease because, as stated before, the artery lumen is preserved in the beginning by outward (positive) arterial remodelling [23, 60]. Another limitation of angiography is that diffuse atherosclerotic disease may narrow the entire lumen of the artery and as a result underestimate the degree of local stenosis. Furthermore it is an invasive technique which can cause minor and major complications. In the following we describe other non-nuclear invasive and non-invasive techniques for detecting atherosclerosis. After that we will focus on the nuclear techniques that have been or are currently being used. Table 1 shows the different imaging modalities and their role in the different stages of the development of atherosclerosis.
Non-nuclear invasive imaging techniques
Intravascular ultrasound (IVUS) permits direct arterial vascular wall imaging and delineates the thickness and echogenicity of vessel wall structures. The standard techniques for intravascular coronary delivery are used for this intravascular examination. It can be a valuable supplement to angiography. Angiography depicts only a 2-D silhouette of the lumen, whereas IVUS allows tomographic assessment of lumen area, plaque size, distribution and composition of the plaque. An important potential application of IVUS is the identification of atheromas at risk of rupture [61]. IVUS-derived residual plaque burden is a useful predictor of clinical outcome [62]. Recent advances in IVUS technology, such as the use of high-frequency transducers [63], radiofrequency signal analysis [64, 65], integrated backscatter [66] and elastography [67], have improved the capability of IVUS for plaque characterization. However, there are limitations of IVUS. Interpretation still relies on simple visual inspection, and different tissue components appear quite similar. Artefacts can adversely affect the ultrasound images and the physical size of ultrasound catheters (∼1 mm) constitutes an important limitation in imaging severe stenoses [61].
Another non-nuclear invasive technique is intravascular MRI (IVMRI) using catheter-based imaging coils. Studies have demonstrated its capability of differentiating plaque components in ex vivo human and in in vivo animal lesions [68–70]. A limitation of both aforementioned techniques is the limited resolution of both IVUS (100–250 µm) [63–65, 67] and IVMRI (120–440 µm) [68, 69]. This precludes accurate evaluation of relevant microstructural features, such as thin fibrous caps (<65 µm).
Intracoronary angioscopy allows direct visualization of the plaque surface, presence of thrombus and macroscopic features, such as ulcerations and fissures [71, 72]. However, this technique remains a research tool because of the inability to examine small-caliber vessels or cross-stenotic lesions or the different layers within the arterial wall [71].
Given its high resolution (10–15 µm, due to the use of infrared light rather than acoustic waves), intravascular optical coherence tomography (OCT) has great promise for the assessment of the microstructure of the plaque [73–75] and potentially for the quantification of macrophage content within fibrous caps. This unique capability for fibrous cap characterization suggests that this technology may be well suited for identifying vulnerable plaques in patients [76]. Yabushita et al. established OCT criteria for characterizing atherosclerotic plaques in vitro by correlating OCT images with histology. They found a strong correlation with the corresponding histopathology in vitro [77]. OCT exhibited superior delineation of structural detail compared with IVUS [78]. Limited penetration depth (1–2 mm) allows only partial examination of larger vessels with advanced atherosclerosis. Other limitations are the reduction of image quality when imaging through blood or large volumes of tissue and lack of an adequate portable source for in vivo imaging [71, 78].
Several other new technologies, such as thermography, Raman spectroscopy and near infrared (NIR) spectroscopy, may have diagnostic and therapeutic implications only when combined with other catheter-based imaging techniques [71]. All of the above-mentioned techniques are invasive and therefore not suited for screening or serial studies.
Non-nuclear non-invasive imaging techniques
High-resolution, B-mode ultrasound (US) with Doppler flow imaging is the modality of choice for examining the carotid arteries and the aorta. Measurements of wall thickness and quantitative analysis of plaque mass and area as well as plaque characteristics, reflected by echogenicity, can be determined [79]. The carotid intima-media thickness (IMT) has been recognized as a surrogate measure of atherosclerosis. Associated with CVD risk factors and with coronary artery disease (CAD), it is a useful index of subclinical CVD and predicts CVD outcome [80]. Ultrasound detection of carotid plaque helped identify asymptomatic patients with advanced subclinical atherosclerosis [81]. A study of a very large population, called the Atherosclerosis Risk in Communities (ARIC) study, showed that the hazard rate ratio comparing extreme mean IMT (>1 mm) to not extreme (<1 mm) was 5.1 in women and 1.9 in men. The relation was graded. Mean carotid IMT was called a non-invasive predictor of future CVD incidence [82]. For each 0.03 mm increase per year in carotid IMT the relative risk for non-fatal myocardial infarction or coronary death was 2.2 and the relative risk for any coronary event was 3.1 [83]. Finally, the carotid IMT is a marker for early, preclinical atherosclerosis in high-risk children and adults [84].
This technique is non-invasive, inexpensive and easily applied, but is highly operator dependent and has low reproducibility. Furthermore, it has no role in detecting atherosclerosis in small arteries directly.
Where magnetic resonance imaging (MRI), X-ray angiography and US can identify calcified deposits in blood vessels, only electron-beam computed tomography (EBCT) and fast-gated helical or spiral (i.e. multidetector row or MD) CT can quantitate the amount or volume of calcium [85]. This has a strong correlation with the extent of atherosclerosis burden.
Histological and ultrafast CT studies support the association of tissue densities >130 HU with calcified plaques [86]. However, high-risk plaques often lack calcium. Although controversy exists over the relation of calcification to plaque vulnerability, pathology data indicate that atherosclerotic calcifications are frequent in acute lesions and are associated with plaque healing after rupture, thus being a possible marker of susceptibility to ischaemic events [87].
In the coronary arteries, the amount of coronary calcium could be a predictor of risk of coronary events. A high calcium score is sensitive but not a specific marker for coronary stenosis. A negative calcium score may exclude CVD; it has been demonstrated that in a population with predominately intermediate likelihood of CAD, a coronary artery calcium score (CACS) of zero excludes inducible ischaemia on myocardial perfusion PET [88], but non-calcified plaques can be eventually missed. The greatest potential for CACS appears to be in the detection of advanced coronary atherosclerosis in patients who are apparently at intermediate risk [89, 90]. It could also be used as an additional tool to the risk stratification of asymptomatic individuals [91]. It was recently stated that a stepwise approach including history, CACS and CT angiography (CTA) can identify about 50% of the patients with normal myocardial perfusion imaging who have a higher risk and may benefit from aggressive medical management [92].
Coronary CTA is an emerging technology to evaluate the lumen of the coronary arteries. Plaque burden can be either assessed quantitatively or semi-quantitatively with CTA; plaque location should be considered as well, as vulnerable plaques are most often observed in proximal segments of the coronary artery tree. To some extent, CTA also allows assessment of plaque composition since non-calcified plaques have low attenuation, calcified plaques high attenuation, and mixed plaques have both non-calcified and calcified elements. Plaque remodelling, a marker of vulnerability, can also be appreciated by CTA [93]. The burden of angiographic disease detected by CTA provides both independent and incremental value in predicting all-cause mortality in symptomatic patients independent of age, gender, conventional risk factors and CACS [94]. The operating characteristics of CTA support its potential role as a tool useful in ruling out obstructive CAD and its clinical implementation appears to positively impact conventional coronary angiography by reducing the frequency of normal invasive examinations [95]. Due to its excellent negative predictive value, coronary CTA is a suitable test to exclude significant CAD. However, given its high rate of false-positive results, particularly in the presence of significant coronary calcification, CTA only rarely is a real alternative to invasive coronary angiography in clinical practice. This was recently reported in a paper indicating that contrast-enhanced CTA can exclude significant CAD in patients with a low-intermediate CACS but is of limited value in patients with a high CACS [96]. In the evaluation if carotid plaques it has been demonstrated that CTA with multidetector capabilities is able to quantify total plaque area, calcifications and fibrous tissue in good correlation with histology whereas lipid core can only be adequately quantified in mildly calcified plaques [97]. The proportion of carotid plaque calcification, rather than absolute volume, was associated with stability. Highly calcified carotid plaques (>45%) were demonstrated to be more stable [98]. This is in accordance with the finding that in contrast to lipid pools, which dramatically increase stress, calcification is supposed to reduce fibrous cap stress in atherosclerotic lesions [99].
Concluding, CTA is an useful tool; however, use of CTA in asymptomatic patients as a screening test is currently not recommended because it has both significant radiation and contrast administration.
Magnetic resonance imaging (MRI) has been applied in the study of atherosclerotic disease in different ways: with high-resolution sequences, with contrast-enhanced techniques and with MR angiography (MRA) with and without contrast injection. MRI is definitely superior to other imaging modalities in distinguishing soft tissue contrast. High-resolution MRI has the potential to non-invasively image the human coronary artery wall, but coronary imaging by MR has been limited by artefacts related to blood flow, motion and low spatial resolution. Fayad et al. developed an in vivo high-resolution black-blood magnetic resonance method to investigate the morphological features of both normal and atherosclerotic human coronary arteries and they found a significant difference in the average maximum coronary wall thickness between the two [100]. However, this technique uses breath-holding strategies, which are often difficult to implement in patients, especially those with pulmonary or CAD. Botnar et al. used a free-breathing MR method that demonstrated a significantly greater coronary wall thickness and wall area in patients with angiographic CAD [101]. Likewise, Kim et al. assessed increased coronary vessel wall thickness with preservation of lumen size in patients with non-significant CAD using free-breathing black-blood three-dimensional cardiovascular magnetic resonance, consistent with the Glagov principal [102]. This could be of use in quantifying subclinical disease.
Cai et al. showed that high-resolution multi-contrast MRI is capable of classifying intermediate to advanced human atherosclerotic lesions in the human carotid artery and is also capable of distinguishing advanced lesions from early and intermediate plaques (according to the American Heart Association classification) [103]. Hatsukami et al. introduced the use of bright blood imaging (i.e. 3-D fast time-of-flight imaging) for the visualization of fibrous cap thickness and morphological integrity. In a prospective in vivo and in vitro serial examination of human carotid artery lesions they found a high level of agreement between MR imaging and histological findings in distinguishing between thin intact, thick intact and ruptured fibrous caps [104]. Compared with asymptomatic plaques, symptomatic plaques had a higher incidence of fibrous cap rupture and juxtaluminal haemorrhage or thrombus [105].
The reproducibility of MRI was assessed by the HIgh-Resolution magnetic resonance Imaging in atherosclerotic Stenosis of the Carotid artery study group (HIRISC). Reproducibility of MRI for identifying and quantifying carotid plaque components is overall acceptable, but there is still significant variability that should be taken into account in the design of prognostic studies and clinical trials; in particular reproducibility for fibrous cap identification needs to be improved [106]. Serial MRI scans proved feasibility in monitoring changes in atherosclerotic plaque composition. This could have potential for following patients with known or suspected atherosclerotic CAD or for serial evaluation after pharmacological intervention [107–109].
Other studies suggested a link between enhancement patterns in contrast-enhanced MRI (ce-MRI) and neovasculature [110, 111]. Kerwin et al. used dynamic ce-MRI for quantitative measurement of the extent of neovasculature within carotid plaques, providing a potential means to identify plaque vulnerability [112]. Novel MR contrast agents were developed, like fibrin-targeted paramagnetic nanoparticles, that could allow sensitive detection and quantification of occult microthrombi within the intimal surface of atherosclerotic vessels, therefore allowing in an early stage the localization of fibrin and direct identification of vulnerable plaques [113, 114]. The first reports seem promising, but in the last few years no further results were mentioned.
MR angiography has been studied for years and has undergone numerous technical improvements and innovations. Coronary MRA techniques were reviewed by Fayad et al. The sensitivity and specificity was persistently lower as compared to traditional contrast coronary X-ray angiography and each of the techniques suffers from artefacts due to cardiac motion and limitations in spatial and temporal resolution [115]. Methods, like routine administration of an oral β-blocker before scanning, were applied to minimize motion artefacts [116]. Till now, the results are not satisfying enough. MRA with gadolinium injection is routinely applied in clinical practice for the study of larger vessels such as the carotid artery, aorta and peripheral vessels. It represents, in most cases, the second diagnostic tool after Doppler ultrasound. For carotid atherosclerotic disease MRA with contrast injection represents the best diagnostic tool for visualization of the vascular tree from the aortic arch to the intracranial vessels. It is frequently performed with MRI of the brain for a complete vascular evaluation.
All of the above-mentioned non-nuclear imaging modalities allow early detection of plaque formation, but they do not provide any information on the actual risk of plaque rupture, the main cause of acute ischaemic events. These vulnerable plaques are often only mildly stenotic and histologically characterized by a large lipid or necrotic core, a thin fibrous cap, intraplaque haemorrhage and presence of activated macrophages [32, 37, 59]. Moreover, a study by Ojio et al. revealed that microthrombus formation precedes acute myocardial infarction or stroke by days to months, providing an opportunity to intervene and prevent serious complications [117].
These items emphasizes the need to detect the most vulnerable plaques at an early stage. Non-nuclear imaging modalities are not capable of doing so.
Nuclear imaging techniques for detecting atherosclerotic plaques
Many radiopharmaceuticals have been developed on the basis of molecules and cells involved in atherogenesis. These radiopharmaceuticals can be divided into three major groups, based on their target cells (see Table 2).
Radiopharmaceuticals targeting atherosclerotic lesion components
Already in the 1980s the feasibility of localizing human atherosclerotic plaques with 99mTc-labelled LDL was tested in patients. It did accumulate in some atherosclerotic plaques (found in carotid endarterectomy specimens) and in a few patients the accumulation was sufficient for detection by gamma camera imaging. However, the amount of LDL that accumulated was very low, depended on the lesion composition and coronary lesions could not be visualized because of residual blood pool activity [118].
Later on, reports suggested that oxidized LDL (oxLDL, an atherogenic lipid) played a major role in the pathogenesis of atherosclerosis. A technique was developed to oxidize autologous LDL and to label it with 99mTc. Biodistribution data demonstrated rapid clearing of 99m Tc-oxLDL from circulation, accumulation mostly by organs rich in macrophages, and it could be detected at the level of carotid plaques [119].
Activated macrophages are densely present in atherosclerotic lesions. They are known to express scavenger receptors for modified LDL. Acetylated LDL (ac-LDL) competes with oxLDL for access to these receptors. 99m Tc-ac-LDL visualized densely localized scavenger receptor sites constituted by activated macrophages at the site of inflammatory lesions and therefore appeared promising for the scintigraphy of atherosclerotic lesions [120].
Recently, lectin-like oxLDL receptor 1 (LOX-1) was found to be mediating the biological effects of oxLDL in the process of plaque formation. Moreover, animal studies demonstrated that LOX-1 expression in atherosclerotic plaques was positively correlated with plaque instability in vivo, thus suggesting that this receptor may play an important role in the destabilization of atherosclerotic plaques [121]. Therefore, 99m Tc-anti-LOX-1 monoclonal IgG was designed, prepared and its usefulness as an atherosclerosis imaging agent was tested in mice and rabbits. The atherosclerotic lesions were divided into four categories using the recommendations of the American Heart Association where type IV lesions contained thin fibrous connective tissue and a dense accumulation of extracellular lipid and foam cells, corresponding to vulnerable lesions in human atherosclerotic plaques. Indeed, the level of 99mTc-LOX-1 monoclonal IgG accumulation in grade IV atheroma was significantly higher than that in neointimal lesions or other, more stable lesions [122]. Nuclear imaging of LOX-1 expression may be useful for predicting atheroma at risk for rupture.
The free radicals O -2 and .NO are generated by blood vessels and can rapidly react to produce a peroxynitrite anion (ONOO-), a powerful oxidant that modifies lipoproteins making them more atherogenic. Peroxynitrite-modified β-VLDL labelled with 99m Tc was studied in vitro in rabbits. It was found to be an interesting tool to study lipoprotein accumulation in organs (liver, kidneys) and maybe also in atherosclerotic plaques [123].
MDA2 is a monoclonal mural antibody to epitopes that are present on oxLDL, but not on normal LDL. 125 I-labelled MDA2 can be used to detect atherosclerotic lesions in rabbit aortas and 99m Tc-labelled MDA2 to image atherosclerotic lesions in living rabbits [124].
Uptake of 99mTc-MDA2 in plaques was significantly higher than in normal aortas and it also correlated with aortic weight and percent atherosclerotic surface area in rabbits and mice. Moreover, it also reflected reduced plaque oxLDL content after hypocholesterolaemic intervention (diet) [125]. Because these oxidation-specific antibodies showed these results for atherosclerotic lesions, the authors developed a human oxidation-specific antibody, called IK17, which was labelled with 125I. Restricted immunologic reactions, higher in vivo plaque uptake and improved lesion to blood ratio was found, compared to the murine antibody [126].
The last tracer specific for atherosclerotic lesions components is 99m Tc-endothelin derivative. Vascular SMCs express endothelin receptors, reason to see if an endothelin derivative called ZK167054 could be used for imaging of experimentally induced atherosclerosis. The results indicated that in vivo imaging of atherosclerosis with an endothelin derivative is a feasible method for detecting and characterizing atherosclerotic arterial wall lesions at early stages in a rabbit restenosis model [127, 128].
Radiopharmaceuticals to detect atherosclerotic inflammation
Several cytokines, chemokines and their receptors, responsible for early events in atherosclerosis, can be directly or indirectly labelled with radionuclides and therefore visualized in vivo. These chemokines have low molecular weight, short half-life and rapid plasma clearance, all favourable radiopharmaceutical features [129].
Monocytes and macrophages, predominant cell types in acute and chronic inflammation, are attracted to and activated by monocyte chemotactic peptide 1 (MCP-1). 125 I-MCP-1 has been tested in normal mice and in atheroma-rich rabbits and the uptake correlated with the number of macrophages per unit area [130]. In another study in rats, 99m Tc-MCP-1 was found to localize only in zones of subacute inflammation, reflecting the density of macrophages and monocytes [131]. Radiolabelled MCP-1 may be a useful tracer for imaging monocyte/macrophage-rich atherosclerotic lesions in the stage of subacute inflammation.
IL-8 is a chemotactic cytokine that binds with a high affinity to receptors expressed on neutrophils. It plays an important role in cell recruitment during acute inflammation. Various animal models showed specific and rapid accumulation of 99m Tc-IL-8 in infectious and inflammatory foci [132, 133]. Labelling with 123I gave the same promising results [134]. Recently, 99mTc-IL-8 was also tested in 20 patients. Injection was well tolerated and 99mTc-IL-8 scintigraphy seemed to be a promising new tool for the detection of infections in patients as early as 4 h after injection [135]. It has not been tested in atherosclerosis yet.
IL-1 is also a proinflammatory cytokine with a high affinity to a specific receptor expressed on monocytes and lymphocytes. It was one of the first cytokines developed for imaging acute inflammation. Labelled with 123I or 125I, IL-1 was tested in different animal models of infection or sterile inflammation [136]. There was a highly specific uptake at the infection site, but due to side effects (hypotension, headache) even in low doses, the radioiodinated IL-1 has never been tried in humans.
Consequently, a radiolabelled IL-1 receptor antagonist (IL-1 RA) was developed, with the same binding affinity for IL-1 receptors but without any biological activity. However, in mice the abscess uptake of IL-1 RA was much lower than that of IL-1 because of interaction with serum proteins [137]. This was also seen in a rabbit infection model; however, the observation of radiolabelled IL-1 RA in the infectious focus was important [138]. After these results, 123 I-IL-1 RA was studied in patients with rheumatoid arthritis (RA) and to assess whether it is suitable for scintigraphic visualization of synovitis . 123I-IL-1 RA was able to image inflamed joints but autoradiographic studies did not indicate that the joint accumulation of radiolabelled IL-1 RA was due to specific IL-1 receptor targeting [139]. The uptake behaviour was similar to those of non-specific labelled agents, so it seems that radiolabelled IL-1 RA is not suitable for scintigraphic detection of inflammation and therefore it will play no role in imaging atherosclerosis.
IL-2 has a high affinity for IL-2 receptors expressed by activated lymphocytes during inflammation. IL-2 is a growth-promoting factor of T lymphocytes and has effects on a wide variety of cells such as CD4+ T cells, CD8+ T cells, B cells and natural killer cells. Most prominently, it is important in T-cell activation and inflammatory consequences thereof [140]. Both 99m Tc-- and 123 I-labelled IL-2 have been successfully used in several diseases characterized by a chronic lymphocytic infiltration, such as Crohn’s disease, coeliac disease, type 1 diabetes and autoimmune thyroid diseases [141].
Higher serum IL-2 levels are associated with increased carotid artery IMT, a predictor of stroke and vascular disease [142]. 99mTc-IL-2 is therefore also used for imaging carotid atherosclerosis in humans. Fourteen patients (16 plaques) eligible for endarterectomy underwent 99mTc-IL-2 scintigraphy before surgery. Another nine patients (13 plaques) received atorvastatin or a standard hypocholesterolaemic diet and scintigraphy was performed before and after 3 months of treatment. 99mTc-IL-2 accumulated in vulnerable carotid plaques and the accumulation correlated with the amount of IL-2R+ cells within the plaque (measured ex vivo by histology). Also, the amount of 99mTc-IL-2 within the plaque was influenced by lipid-lowering treatment with a statin [143].
99mTc-IL-2 is a very promising tracer that could provide useful information for the selection of infiltrated vulnerable plaques at risk of rupture. There are no significant biological side effects at the low dose used for imaging purposes. However, it is not yet commercially available and a major drawback is the complexity of the labelling procedure, although 99mTc-IL-2 can be easily prepared [141].
Other cytokines have been labelled with iodide, but the applications remain uncertain. 125I-IL-3 has only been tried for in vitro binding studies without further application in humans. 123I-IL-6 was compared to iodinated IL-1. The degree of IL-6 uptake in different animal models of acute inflammation was much lower than IL-2. Therefore, the use of radioiodinated IL-6 was abandoned. 123I-IL-12 was successfully tested in a mouse model of autoimmune colitis and could be a possible alternative to IL-2 [141].
All of the above-mentioned tracers are SPECT tracers. The well-known PET tracer 18 F-fluorodeoxyglucose ( 18 F-FDG) has the possible advantage that it can provide absolute quantification and better resolution than the SPECT tracers. FDG is a glucose analogue that is trapped in cells in proportion to the cells’ metabolic activity. 18F-FDG is retained within plaque macrophages more avidly than within other plaque elements [144]. A good reproducibility was reported in a group of patients that underwent carotid artery and aortic imaging, with high inter- and intraobserver agreement and low variability of 18F-FDG uptake over 2 weeks [145].
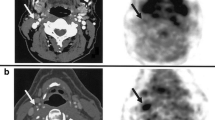
Results in the iliac and femoral arteries were also highly reproducible; however, the FDG uptake in the carotid arteries was significantly higher than in both iliac and femoral vessels [146]. In 30% of patients with documented carotid atherosclerosis inflammation was detected by FDG PET imaging. This raises the possibility for this non-invasive metabolic imaging modality to aid in the risk stratification and selection of appropriate therapy. Large prospective studies are necessary to determine if the detection of inflamed plaque by 18F-FDG PET is useful for predicting future CVD [147]. An example of FDG PET imaging in atherosclerosis can be seen in Fig. 3.
Upper left: CT image transverse view, atherosclerotic plaque in widened abdominal aorta. Upper right: fused FDG PET and CT, transverse view, uptake in atherosclerotic lesion in abdominal aorta. Lower left: CT image coronal view (same patient), multiple atherosclerotic lesions in abdominal aorta. Lower right: fused FDG PET and CT, coronal view, FDG uptake in atherosclerotic lesions in abdominal aorta
Radiopharmaceuticals to detect thrombosis
Radiolabelled platelets are theoretically the best pool to image the platelet-rich thrombus development in a lesion. 111 In-platelet scintigraphy has emerged as the technique of choice. Already in the 1980s there was enthusiasm about this technique because platelet accumulation was related to the surface characteristics and severity of carotid lesions, especially in the presence of ulceration [148]. However, the use of this technique is lowered because of the low signal to noise ratio with the circulating blood pool and the lack of documentation of the underlying pathological condition. In 2001, the performance of 111In-platelet scintigraphy with blood pool subtraction was compared to ultrasound Doppler techniques and carotid artery plaque specimens removed at the time of carotid endarterectomy in 22 patients.
111In-platelet scintigraphy was found to be an accurate non-invasive diagnostic tool to detect thrombotic complications in carotid plaques [149]. Despite these findings, prospective studies with this technique have not yet been performed.
99mTc-radiolabelled peptides have been developed for thrombosis imaging, targeting either activated platelets [the glycoprotein (GP) IIb/IIIa receptor and phosphatidylserine] or fibrin (C-terminal portion of the γ-chain of fibrin) [150].
99mTc-apcitide (also known as 99mTc-P280) is a radiolabelled peptide that binds with high affinity and specificity to the GP IIb/IIIa receptors expressed on the activated platelets that are involved in acute thrombosis [151]. It detects most acute thrombi and has potential utility in suspected recurrent deep venous thrombosis (DVT) [152]. It has been approved by the Food and Drug Administration (FDA) for the clinical detection of acute DVT.
Another agent developed for targeting activated platelets is 99m Tc-DMP444, which is another GP IIb/IIIa receptor antagonist. It has been shown to be actively incorporated into arterial and venous thrombi in DVT and pulmonary embolism (PE). It has also been used in imaging infective endocarditis.
A fibrin α-chain, N-terminal peptide that binds to the C-terminal portion of the γ-chain of fibrin has been modified and labelled with technetium. The resultant peptide 99m Tc-labelled fibrin α-chain binds to rabbit, dog and human fibrin in large quantities and delineates experimental DVT and PE in rabbits. This agent is worthy of further investigation [153].
Another agent for targeting fibrin that has been investigated is 99m Tc-fibrin-binding domain (FBD), a radiolabelled fibrin-binding domain of fibronectin. It has shown a high diagnostic accuracy in the detection of DVT [154]. The role in atherosclerosis still remains unclear.
Radiopharmaceuticals to detect apoptosis
Finally, annexin V, an endogenous human protein, binds with high affinity to phosphatidylserine. Labelling with technetium makes it possible to assess non-invasively the early stage of apoptosis and to provide information about disease progression or regression. The use of 99m Tc-annexin V has been described in monitoring therapies in acute myocardial infarction and accumulates in affected joints in an experimental model of rheumatoid arthritis [155]. Apoptosis is commonly observed in advanced atherosclerotic lesions. A recent study in a rabbit model showed higher 99mTc-annexin V accumulation in grade IV atheroma than in other more stable lesions. 99mTc-annexin V may be useful in identifying atheroma at risk for rupture and also possibly in assessing response to anti-atherosclerotic therapy [156].
Future perspectives
The identification of major, causal risk factors for the development of atherosclerosis provide physicians reliable tools to identify individuals who are at high risk for atherosclerosis-related clinical events. However, the quantitative risk assessment would be significantly improved if atherosclerotic plaque burden could be assessed more directly. Non-nuclear imaging modalities have a lot of possibilities, but are not able to look at early events and plaque vulnerability. The emerging area of nuclear imaging techniques can provide measures of biological activity of atherosclerotic plaques, thereby improving the prediction of clinical events. Many tracers already have been studied and are based on atherosclerotic lesion components, inflammation and thrombosis. In recent years there has been a major improvement in tracer development for inflammation with very promising results. Especially 99mTc-IL-2 has been successfully used in several inflammatory diseases. Till now all of these radionuclides are SPECT tracers. PET tracers have advantages over SPECT tracers. They can provide absolute quantification and a better resolution. SPECT/CT and PET/CT cameras can also add to better anatomical mapping. There is a lack of correlation between CCAS, coronary artery stenosis and vulnerability of the plaque. The integration of anatomical and physiological information of the plaque using PET/CT with specific plaque target agents may provide additional information for the clinician by the improved risk stratification and diagnostic accuracy of integrated techniques. Coupling cytokines with PET radionuclides seems to be very promising in the future. Especially 18F-IL-2 could have great potential. At present, there is no special tracer that can be called the diagnostic tool to diagnose or predict stroke or infarction in high-risk patients. Prospective randomized trials are necessary to evaluate more precisely the clinical usefulness of these nuclear techniques with these tracers. In future, these tracers could theoretically also be used as targeted therapy to combat CVD.
References
Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993;362(6423):801–9.
Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet 1999;353(9147):89–92.
Scott J. Pathophysiology and biochemistry of cardiovascular disease. Curr Opin Genet Dev 2004;14(3):271–9.
Williams K, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol 1995;15(5):551–61.
Rapp JH, Lespine A, Hamilton RL, Colyvas N, Chaumeton AH, Tweedie-Hardman J, et al. Triglyceride-rich lipoproteins isolated by selected-affinity anti-apolipoprotein B immunosorption from human atherosclerotic plaque. Arterioscler Thromb 1994;14(11):1767–74.
Coresh J, Kwiterovich PO Jr. Small, dense low-density lipoprotein particles and coronary heart disease risk: a clear association with uncertain implications. JAMA 1996;276(11):914–5.
Fielding CJ, Fielding PE. Molecular physiology of reverse cholesterol transport. J Lipid Res 1995;36(2):211–28.
Knopp RH. Drug treatment of lipid disorders. N Engl J Med 1999;341(7):498–511.
Buhman KK, Accad M, Novak S, Choi RS, Wong JS, Hamilton RL, et al. Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat Med 2000;6(12):1341–7.
Callahan A. Cerebrovascular disease and statins: a potential addition to the therapeutic armamentarium for stroke prevention. Am J Cardiol 2001;88(7B):33J-7J.
Smilde TJ, van Wissen S, Wollersheim H, Trip MD, Kastelein JJ, Stalenhoef AF. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet 2001;357(9256):577–81.
Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285(19):2486–97.
Dannenberg AL, Keller JB, Wilson PW, Castelli WP. Leisure time physical activity in the Framingham Offspring Study. Description, seasonal variation, and risk factor correlates. Am J Epidemiol 1989;129(1):76–88.
Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnoea with myocardial infarction in men. Lancet 1990;336(8710):261–4.
Grundy SM. Primary prevention of coronary heart disease: integrating risk assessment with intervention. Circulation 1999;100(9):988–98.
Davies MJ. A macro and micro view of coronary vascular insult in ischemic heart disease. Circulation 1990;82(3 Suppl):II38–46.
Emond M, Mock MB, Davis KB, Fisher LD, Holmes DR Jr, Chaitman BR, et al. Long-term survival of medically treated patients in the Coronary Artery Surgery Study (CASS) Registry. Circulation 1994;90(6):2645–57.
Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97(18):1837–47.
Insull W Jr. The pathology of atherosclerosis: plaque development and plaque responses to medical treatment. Am J Med 2009;122(1 Suppl):S3–14.
Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998;339(20):1415–25.
Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet 2004;363(9420):1491–502.
Nighoghossian N, Derex L, Douek P. The vulnerable carotid artery plaque: current imaging methods and new perspectives. Stroke 2005;36(12):2764–72.
Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med 1987;316(22):1371–5.
Burke AP, Kolodgie FD, Farb A, Weber DK, Malcom GT, Smialek J, et al. Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation 2001;103(7):934–40.
Schoenhagen P, Ziada K, Vince DG, Nissen S, Tuzcu EM. Arterial remodeling and coronary artery disease: the concept of “dilated” versus “obstructive” coronary atherosclerosis. J Am Coll Cardiol 2001;38(2):297–306.
Burke AP, Kolodgie FD, Farb A, Weber D, Virmani R. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation 2002;105(3):297–303.
Varnava AM, Mills PG, Davies MJ. Relationship between coronary artery remodeling and plaque vulnerability. Circulation 2002;105(8):939–43.
Little WC, Constantinescu M, Applegate RJ, Kutcher MA, Burrows MT, Kahl FR, et al. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation 1988;78(5 Pt 1):1157–66.
Ambrose JA, Tannenbaum MA, Alexopoulos D, Hjemdahl-Monsen CE, Leavy J, Weiss M, et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol 1988;12(1):56–62.
Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation 1995;92(3):657–71.
Schoenhagen P, Ziada KM, Kapadia SR, Crowe TD, Nissen SE, Tuzcu EM. Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: an intravascular ultrasound study. Circulation 2000;101(6):598–603.
Virmani R, Kolodgie F, Burke A, Farb A, Schwartz S. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2000;20(5):1262–75.
Mofidi R, Crotty TB, McCarthy P, Sheehan SJ, Mehigan D, Keaveny TV. Association between plaque instability, angiogenesis and symptomatic carotid occlusive disease. Br J Surg 2001;88(7):945–50.
de Boer OJ, van der Wal AC, Teeling P, Becker AE. Leucocyte recruitment in rupture prone regions of lipid-rich plaques: a prominent role for neovascularization? Cardiovasc Res 1999;41(2):443–9.
Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002;105(9):1135–43.
Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364(9438):937–52.
Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 1999;340(2):115–26.
Cybulsky MI, Gimbrone MA Jr. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 1991;251(4995):788–91.
Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, et al. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest 2001;107(10):1255–62.
Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol 2007;27(11):2292–301.
Dong ZM, Chapman SM, Brown AA, Frenette PS, Hynes RO, Wagner DD. The combined role of P- and E-selectins in atherosclerosis. J Clin Invest 1998;102(1):145–52.
Collins T, Cybulsky MI. NF-kappaB: pivotal mediator or innocent bystander in atherogenesis? J Clin Invest 2001;107(3):255–64.
Topper JN, Cai J, Falb D, Gimbrone MA Jr. Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci U S A 1996;93(19):10417–22.
Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2-/- mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 1998;394(6696):894–7.
Reape TJ, Rayner K, Manning CD, Gee AN, Barnette MS, Burnand KG, et al. Expression and cellular localization of the CC chemokines PARC and ELC in human atherosclerotic plaques. Am J Pathol 1999;154(2):365–74.
Boisvert WA, Santiago R, Curtiss LK, Terkeltaub RA. A leukocyte homologue of the IL-8 receptor CXCR-2 mediates the accumulation of macrophages in atherosclerotic lesions of LDL receptor-deficient mice. J Clin Invest 1998;101(2):353–63.
Lee H, Shi W, Tontonoz P, Wang S, Subbanagounder G, Hedrick CC, et al. Role for peroxisome proliferator-activated receptor alpha in oxidized phospholipid-induced synthesis of monocyte chemotactic protein-1 and interleukin-8 by endothelial cells. Circ Res 2000;87(6):516–21.
Chen XL, Tummala PE, Olbrych MT, Alexander RW, Medford RM. Angiotensin II induces monocyte chemoattractant protein-1 gene expression in rat vascular smooth muscle cells. Circ Res 1998;83(9):952–9.
Mach F, Sauty A, Iarossi AS, Sukhova GK, Neote K, Libby P, et al. Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells. J Clin Invest 1999;104(8):1041–50.
Schwarz JB, Langwieser N, Langwieser NN, Bek MJ, Seidl S, Eckstein HH, et al. Novel role of the CXC chemokine receptor 3 in inflammatory response to arterial injury: involvement of mTORC1. Circ Res 2009;104(2):189–200. 8p following 200.
Clinton SK, Underwood R, Hayes L, Sherman ML, Kufe DW, Libby P. Macrophage colony-stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. Am J Pathol 1992;140(2):301–16.
Qiao JH, Tripathi J, Mishra NK, Cai Y, Tripathi S, Wang XP, et al. Role of macrophage colony-stimulating factor in atherosclerosis: studies of osteopetrotic mice. Am J Pathol 1997;150(5):1687–99.
Irvine KM, Andrews MR, Fernandez-Rojo MA, Schroder K, Burns CJ, Su S, et al. Colony-stimulating factor-1 (CSF-1) delivers a proatherogenic signal to human macrophages. J Leukoc Biol 2009;85(2):278–88.
Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol 2006;6(7):508–19.
Hansson GK. Atherosclerosis–an immune disease: the Anitschkov Lecture 2007. Atherosclerosis 2009;202(1):2–10.
Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 1994;94(6):2493–503.
Lee RT, Libby P. The unstable atheroma. Arterioscler Thromb Vasc Biol 1997;17(10):1859–67.
Mach F, Schonbeck U, Bonnefoy JY, Pober JS, Libby P. Activation of monocyte/macrophage functions related to acute atheroma complication by ligation of CD40: induction of collagenase, stromelysin, and tissue factor. Circulation 1997;96(2):396–9.
Davies MJ. Stability and instability: two faces of coronary atherosclerosis. The Paul Dudley White Lecture 1995. Circulation 1996;94(8):2013–20.
Kashyap VS, Pavkov ML, Bishop PD, Nassoiy SP, Eagleton MJ, Clair DG, et al. Angiography underestimates peripheral atherosclerosis: lumenography revisited. J Endovasc Ther 2008;15(1):117–25.
Nissen SE, Yock P. Intravascular ultrasound: novel pathophysiological insights and current clinical applications. Circulation 2001;103(4):604–16.
Mintz GS, Popma JJ, Pichard AD, Kent KM, Salter LF, Chuang YC, et al. Intravascular ultrasound predictors of restenosis after percutaneous transcatheter coronary revascularization. J Am Coll Cardiol 1996;27(7):1678–87.
Prati F, Arbustini E, Labellarte A, Dal Bello B, Sommariva L, Mallus MT, et al. Correlation between high frequency intravascular ultrasound and histomorphology in human coronary arteries. Heart 2001;85(5):567–70.
Komiyama N, Berry GJ, Kolz ML, Oshima A, Metz JA, Preuss P, et al. Tissue characterization of atherosclerotic plaques by intravascular ultrasound radiofrequency signal analysis: an in vitro study of human coronary arteries. Am Heart J 2000;140(4):565–74.
Nair A, Kuban BD, Tuzcu EM, Schoenhagen P, Nissen SE, Vince DG. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation 2002;106(17):2200–6.
Okubo M, Kawasaki M, Ishihara Y, Takeyama U, Kubota T, Yamaki T, et al. Development of integrated backscatter intravascular ultrasound for tissue characterization of coronary plaques. Ultrasound Med Biol 2008;34(4):655–63.
de Korte CL, Sierevogel MJ, Mastik F, Strijder C, Schaar JA, Velema E, et al. Identification of atherosclerotic plaque components with intravascular ultrasound elastography in vivo: a Yucatan pig study. Circulation 2002;105(14):1627–30.
Rivas PA, Nayak KS, Scott GC, McConnell MV, Kerr AB, Nishimura DG, et al. In vivo real-time intravascular MRI. J Cardiovasc Magn Reson 2002;4(2):223–32.
Rogers WJ, Prichard JW, Hu YL, Olson PR, Benckart DH, Kramer CM, et al. Characterization of signal properties in atherosclerotic plaque components by intravascular MRI. Arterioscler Thromb Vasc Biol 2000;20(7):1824–30.
Worthley SG, Helft G, Fuster V, Fayad ZA, Shinnar M, Minkoff LA, et al. A novel nonobstructive intravascular MRI coil: in vivo imaging of experimental atherosclerosis. Arterioscler Thromb Vasc Biol 2003;23(2):346–50.
Fayad ZA, Fuster V. Clinical imaging of the high-risk or vulnerable atherosclerotic plaque. Circ Res 2001;89(4):305–16.
Uchida Y, Nakamura F, Tomaru T, Morita T, Oshima T, Sasaki T, et al. Prediction of acute coronary syndromes by percutaneous coronary angioscopy in patients with stable angina. Am Heart J 1995;130(2):195–203.
Brezinski ME, Tearney GJ, Weissman NJ, Boppart SA, Bouma BE, Hee MR, et al. Assessing atherosclerotic plaque morphology: comparison of optical coherence tomography and high frequency intravascular ultrasound. Heart 1997;77(5):397–403.
Fujimoto JG, Boppart SA, Tearney GJ, Bouma BE, Pitris C, Brezinski ME. High resolution in vivo intra-arterial imaging with optical coherence tomography. Heart 1999;82(2):128–33.
Jang IK, Bouma BE, Kang DH, Park SJ, Park SW, Seung KB, et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol 2002;39(4):604–9.
Tearney GJ, Yabushita H, Houser SL, Aretz HT, Jang IK, Schlendorf KH, et al. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation 2003;107(1):113–9.
Yabushita H, Bouma BE, Houser SL, Aretz HT, Jang IK, Schlendorf KH, et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation 2002;106(13):1640–5.
Patwari P, Weissman NJ, Boppart SA, Jesser C, Stamper D, Fujimoto JG, et al. Assessment of coronary plaque with optical coherence tomography and high-frequency ultrasound. Am J Cardiol 2000;85(5):641–4.
Weinberger J, Ramos L, Ambrose JA, Fuster V. Morphologic and dynamic changes of atherosclerotic plaque at the carotid artery bifurcation: sequential imaging by real time B-mode ultrasonography. J Am Coll Cardiol 1988;12(6):1515–21.
O’Leary DH, Polak JF. Intima-media thickness: a tool for atherosclerosis imaging and event prediction. Am J Cardiol 2002;90(10C):18L–21.
Wyman RA, Fraizer MC, Keevil JG, Busse KL, Aeschlimann SE, Korcarz CE, et al. Ultrasound-detected carotid plaque as a screening tool for advanced subclinical atherosclerosis. Am Heart J 2005;150(5):1081–5.
Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol 1997;146(6):483–94.
Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Liu CH, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med 1998;128(4):262–9.
Davis PH, Dawson JD, Riley WA, Lauer RM. Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: The Muscatine Study. Circulation 2001;104(23):2815–9.
Becker CR, Kleffel T, Crispin A, Knez A, Young J, Schoepf UJ, et al. Coronary artery calcium measurement: agreement of multirow detector and electron beam CT. AJR Am J Roentgenol 2001;176(5):1295–8.
Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation 1995;92(8):2157–62.
Burke AP, Taylor A, Farb A, Malcom GT, Virmani R. Coronary calcification: insights from sudden coronary death victims. Z Kardiol 2000;89 Suppl 2:49–53.
Esteves FP, Khan A, Correia LC, Nye JA, Halkar RK, Schuster DM, et al. Absent coronary artery calcium excludes inducible myocardial ischemia on computed tomography/positron emission tomography. Int J Cardiol 2009 Nov 3 [Epub ahead of print].
O’Rourke RA, Brundage BH, Froelicher VF, Greenland P, Grundy SM, Hachamovitch R, et al. American College of Cardiology/American Heart Association Expert Consensus document on electron-beam computed tomography for the diagnosis and prognosis of coronary artery disease. Circulation 2000;102(1):126–40.
Wong ND. Surrogate measures of atherosclerosis and implications for evaluating cardiovascular risk. Diabetes Obes Metab 2003;5(2):73–80.
Raggi P. Coronary-calcium screening to improve risk stratification in primary prevention. J La State Med Soc 2002;154(6):314–8.
Choudhary G, Shin V, Punjani S, Ritter N, Sharma SC, Wu WC. The role of calcium score and CT angiography in the medical management of patients with normal myocardial perfusion imaging. J Nucl Cardiol 2010;17:45–51.
Hoffmann U, Moselewski F, Nieman K, Jang IK, Ferencik M, Rahman AM, et al. Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol 2006;47(8):1655–62.
Ostrom MP, Gopal A, Ahmadi N, Nasir K, Yang E, Kakadiaris I, et al. Mortality incidence and the severity of coronary atherosclerosis assessed by computed tomography angiography. J Am Coll Cardiol 2008;52(16):1335–43.
Chow BJ, Abraham A, Wells GA, Chen L, Ruddy TD, Yam Y, et al. Diagnostic accuracy and impact of computed tomographic coronary angiography on utilization of invasive coronary angiography. Circ Cardiovasc Imaging 2009;2(1):16–23.
Nieman K, Galema TW, Neefjes LA, Weustink AC, Musters P, Moelker AD, et al. Comparison of the value of coronary calcium detection to computed tomographic angiography and exercise testing in patients with chest pain. Am J Cardiol 2009;104(11):1499–504.
de Weert TT, Ouhlous M, Meijering E, Zondervan PE, Hendriks JM, van Sambeek MR, et al. In vivo characterization and quantification of atherosclerotic carotid plaque components with multidetector computed tomography and histopathological correlation. Arterioscler Thromb Vasc Biol 2006;26(10):2366–72.
Nandalur KR, Hardie AD, Raghavan P, Schipper MJ, Baskurt E, Kramer CM. Composition of the stable carotid plaque: insights from a multidetector computed tomography study of plaque volume. Stroke 2007;38(3):935–40.
Huang H, Virmani R, Younis H, Burke AP, Kamm RD, Lee RT. The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation 2001;103(8):1051–6.
Fayad ZA, Fuster V, Fallon JT, Jayasundera T, Worthley SG, Helft G, et al. Noninvasive in vivo human coronary artery lumen and wall imaging using black-blood magnetic resonance imaging. Circulation 2000;102(5):506–10.
Botnar RM, Stuber M, Kissinger KV, Kim WY, Spuentrup E, Manning WJ. Noninvasive coronary vessel wall and plaque imaging with magnetic resonance imaging. Circulation 2000;102(21):2582–7.
Kim WY, Stuber M, Bornert P, Kissinger KV, Manning WJ, Botnar RM. Three-dimensional black-blood cardiac magnetic resonance coronary vessel wall imaging detects positive arterial remodeling in patients with nonsignificant coronary artery disease. Circulation 2002;106(3):296–9.
Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 2002;106(11):1368–73.
Hatsukami TS, Ross R, Polissar NL, Yuan C. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation 2000;102(9):959–64.
Saam T, Cai J, Ma L, Cai YQ, Ferguson MS, Polissar NL, et al. Comparison of symptomatic and asymptomatic atherosclerotic carotid plaque features with in vivo MR imaging. Radiology 2006;240(2):464–72.
Touze E, Toussaint JF, Coste J, Schmitt E, Bonneville F, Vandermarcq P, et al. Reproducibility of high-resolution MRI for the identification and the quantification of carotid atherosclerotic plaque components: consequences for prognosis studies and therapeutic trials. Stroke 2007;38(6):1812–9.
Corti R, Fuster V, Fayad ZA, Worthley SG, Helft G, Chaplin WF, et al. Effects of aggressive versus conventional lipid-lowering therapy by simvastatin on human atherosclerotic lesions: a prospective, randomized, double-blind trial with high-resolution magnetic resonance imaging. J Am Coll Cardiol 2005;46(1):106–12.
Helft G, Worthley SG, Fuster V, Fayad ZA, Zaman AG, Corti R, et al. Progression and regression of atherosclerotic lesions: monitoring with serial noninvasive magnetic resonance imaging. Circulation 2002;105(8):993–8.
Zhao XQ, Yuan C, Hatsukami TS, Frechette EH, Kang XJ, Maravilla KR, et al. Effects of prolonged intensive lipid-lowering therapy on the characteristics of carotid atherosclerotic plaques in vivo by MRI: a case-control study. Arterioscler Thromb Vasc Biol 2001;21(10):1623–9.
Wasserman BA, Smith WI, Trout HH III, Cannon RO III, Balaban RS, Arai AE. Carotid artery atherosclerosis: in vivo morphologic characterization with gadolinium-enhanced double-oblique MR imaging initial results. Radiology 2002;223(2):566–73.
Yuan C, Kerwin WS, Ferguson MS, Polissar N, Zhang S, Cai J, et al. Contrast-enhanced high resolution MRI for atherosclerotic carotid artery tissue characterization. J Magn Reson Imaging 2002;15(1):62–7.
Kerwin W, Hooker A, Spilker M, Vicini P, Ferguson M, Hatsukami T, et al. Quantitative magnetic resonance imaging analysis of neovasculature volume in carotid atherosclerotic plaque. Circulation 2003;107(6):851–6.
Flacke S, Fischer S, Scott MJ, Fuhrhop RJ, Allen JS, McLean M, et al. Novel MRI contrast agent for molecular imaging of fibrin: implications for detecting vulnerable plaques. Circulation 2001;104(11):1280–5.
Winter PM, Caruthers SD, Yu X, Song SK, Chen J, Miller B, et al. Improved molecular imaging contrast agent for detection of human thrombus. Magn Reson Med 2003;50(2):411–6.
Fayad ZA, Fuster V, Nikolaou K, Becker C. Computed tomography and magnetic resonance imaging for noninvasive coronary angiography and plaque imaging: current and potential future concepts. Circulation 2002;106(15):2026–34.
Ropers D, Baum U, Pohle K, Anders K, Ulzheimer S, Ohnesorge B, et al. Detection of coronary artery stenoses with thin-slice multi-detector row spiral computed tomography and multiplanar reconstruction. Circulation 2003;107(5):664–6.
Ojio S, Takatsu H, Tanaka T, Ueno K, Yokoya K,Matsubara T, et al. Considerable time from the onset of plaque rupture and/or thrombi until the onset of acute myocardial infarction in humans: coronary angiographic findings within 1 week before the onset of infarction. Circulation 2000;102(17):2063–9.
Lees AM, Lees RS, Schoen FJ, Isaacsohn JL, Fischman AJ, McKusick KA, et al. Imaging human atherosclerosis with 99mTc-labeled low density lipoproteins. Arteriosclerosis 1988;8(5):461–70.
Iuliano L, Signore A, Vallabajosula S, Colavita AR, Camastra C, Ronga G, et al. Preparation and biodistribution of 99m technetium labelled oxidized LDL in man. Atherosclerosis 1996;126(1):131–41.
Gurudutta GU, Babbar AK, Shailaja S, Soumya P, Sharma RK. Evaluation of potential tracer ability of (99m)Tc-labeled acetylated LDL for scintigraphy of LDL-scavenger receptor sites of macrophageal origin. Nucl Med Biol 2001;28(3):235–41.
Ishino S, Mukai T, Kume N, Asano D, Ogawa M, Kuge Y, et al. Lectin-like oxidized LDL receptor-1 (LOX-1) expression is associated with atherosclerotic plaque instability–analysis in hypercholesterolemic rabbits. Atherosclerosis 2007;195(1):48–56.
Ishino S, Mukai T, Kuge Y, Ogawa M, Takai N, Kamihashi J, et al. Targeting of lectinlike oxidized low-density lipoprotein receptor 1 (LOX-1) with 99mTc-labeled anti-LOX-1 antibody: potential agent for imaging of vulnerable plaque. J Nucl Med 2008;49(10):1677–85.
Silva EL, Meneghetti JC, Coelho IJ, Abdalla DS. Peroxynitrite-modified 99mTc-beta-VLDL: tissue distribution and plasma clearance rate. Free Radic Biol Med 2001;31(4):440–9.
Tsimikas S, Palinski W, Halpern SE, Yeung DW, Curtiss LK, Witztum JL. Radiolabeled MDA2, an oxidation-specific, monoclonal antibody, identifies native atherosclerotic lesions in vivo. J Nucl Cardiol 1999;6(1 Pt 1):41–53.
Tsimikas S, Shortal B, Witztum J, Palinski W. In vivo uptake of radiolabeled MDA2, an oxidation-specific monoclonal antibody, provides an accurate measure of atherosclerotic lesions rich in oxidized LDL and is highly sensitive to their regression. Arterioscler Thromb Vasc Biol 2000;20(3):689–97.
Tsimikas S. Noninvasive imaging of oxidized low-density lipoprotein in atherosclerotic plaques with tagged oxidation-specific antibodies. Am J Cardiol 2002;90(10C):22L–7.
Dinkelborg LM, Duda SH, Hanke H, Tepe G, Hilger CS, Semmler W. Molecular imaging of atherosclerosis using a technetium-99m-labeled endothelin derivative. J Nucl Med 1998;39(10):1819–22.
Tepe G, Duda SH, Meding J, Brehme U, Ritter J, Hanke H, et al. Tc-99m-labeled endothelin derivative for imaging of experimentally induced atherosclerosis. Atherosclerosis 2001;157(2):383–92.
Signore A, Annovazzi A, Corsetti F, Capriotti G, Chianelli M, De Winter F, et al. Biological imaging for the diagnosis of inflammatory conditions. BioDrugs 2002;16(4):241–59.
Ohtsuki K, Hayase M, Akashi K, Kopiwoda S, Strauss HW. Detection of monocyte chemoattractant protein-1 receptor expression in experimental atherosclerotic lesions: an autoradiographic study. Circulation 2001;104(2):203–8.
Blankenberg FG, Tait JF, Blankenberg TA, Post AM, Strauss HW. Imaging macrophages and the apoptosis of granulocytes in a rodent model of subacute and chronic abscesses with radiolabeled monocyte chemotactic peptide-1 and annexin V. Eur J Nucl Med 2001;28(9):1384–93.
Rennen HJ, Boerman OC, Oyen WJ, Corstens FH. Kinetics of 99mTc-labeled interleukin-8 in experimental inflammation and infection. J Nucl Med 2003;44(9):1502–9.
Rennen HJ, Boerman OC, Oyen WJ, van der Meer JW, Corstens FH. Specific and rapid scintigraphic detection of infection with 99mTc-labeled interleukin-8. J Nucl Med 2001;42(1):117–23.
van der Laken CJ, Boerman OC, Oyen WJ, van de Ven MT, van der Meer JW, Corstens FH. Radiolabeled interleukin-8: specific scintigraphic detection of infection within a few hours. J Nucl Med 2000;41(3):463–9.
Bleeker-Rovers CP, Rennen HJ, Boerman OC, Wymenga AB, Visser EP, Bakker JH, et al. 99mTc-labeled interleukin 8 for the scintigraphic detection of infection and inflammation: first clinical evaluation. J Nucl Med 2007;48(3):337–43.
van der Laken CJ, Boerman OC, Oyen WJ, van de Ven MT, Claessens RA, van der Meer JW, et al. Specific targeting of infectious foci with radioiodinated human recombinant interleukin-1 in an experimental model. Eur J Nucl Med 1995;22(11):1249–55.
van der Laken CJ, Boerman OC, Oyen WJ, van de Ven MT, Claessens RA, van der Meer JW, et al. Different behaviour of radioiodinated human recombinant interleukin-1 and its receptor antagonist in an animal model of infection. Eur J Nucl Med 1996;23(11):1531–5.
van der Laken CJ, Boerman OC, Oyen WJ, van de Ven MT, van der Meer JW, Corstens FH. Imaging of infection in rabbits with radioiodinated interleukin-1 (alpha and beta), its receptor antagonist and a chemotactic peptide: a comparative study. Eur J Nucl Med 1998;25(4):347–52.
Barrera P, van der Laken CJ, Boerman OC, Oyen, WJ, van de Ven MT, van Lent PL, et al. Radiolabelled interleukin-1 receptor antagonist for detection of synovitis in patients with rheumatoid arthritis. Rheumatology (Oxford) 2000;39(8):870–4.
Fayad ZA, Amirbekian V, Toussaint JF, Fuster V. Identification of interleukin-2 for imaging atherosclerotic inflammation. Eur J Nucl Med Mol Imaging 2006;33(2):111–6.
Signore A, Capriotti G, Scopinaro F, Bonanno E, Modesti A. Radiolabelled lymphokines and growth factors for in vivo imaging of inflammation, infection and cancer. Trends Immunol 2003;24(7):395–402.
Elkind MS, Rundek T, Sciacca RR, Ramas R, Chen HJ, Boden-Albala B, et al. Interleukin-2 levels are associated with carotid artery intima-media thickness. Atherosclerosis 2005;180(1):181–7.
Annovazzi A, Bonanno E, Arca M, D’Alessandria C, Marcoccia A, Spagnoli LG, et al. 99mTc-interleukin-2 scintigraphy for the in vivo imaging of vulnerable atherosclerotic plaques. Eur J Nucl Med Mol Imaging 2006;33(2):117–26.
Tawakol A, Migrino RQ, Bashian GG, Bedru S, Vermylen D, Cury RC, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol 2006;48(9):1818–24.
Rudd JH, Myers KS, Bansilal S, Machac J, Rafique A, Farkouh M, et al. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol 2007;50(9):892–6.
Rudd JH, Myers KS, Bansilal S, Machac J, Pinto CA, Tong C, et al. Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med 2008;49(6):871–8.
Tahara N, Kai H, Nakaura H, Mizoguchi M, Ishibashi M, Kaida H, et al. The prevalence of inflammation in carotid atherosclerosis: analysis with fluorodeoxyglucose-positron emission tomography. Eur Heart J 2007;28(18):2243–8.
Moriwaki H, Matsumoto M, Handa N, Isaka Y, Hashikawa K, Oku N, et al. Functional and anatomic evaluation of carotid atherothrombosis. A combined study of indium 111 platelet scintigraphy and B-mode ultrasonography. Arterioscler Thromb Vasc Biol 1995;15(12):2234–40.
Manca G, Parenti G, Bellina R, Boni G, Grosso M, Bernini W, et al. 111In platelet scintigraphy for the noninvasive detection of carotid plaque thrombosis. Stroke 2001;32(3):719–27.
Signore A, Annovazzi A, Chianelli M, Corsetti F, Van de Wiele C, Watherhouse RN. Peptide radiopharmaceuticals for diagnosis and therapy. Eur J Nucl Med 2001;28(10):1555–65.
Taillefer R, Edell S, Innes G, Lister-James J. Acute thromboscintigraphy with (99m)Tc-apcitide: results of the phase 3 multicenter clinical trial comparing 99mTc-apcitide scintigraphy with contrast venography for imaging acute DVT. Multicenter Trial Investigators. J Nucl Med 2000;41(7):1214–23.
Bates S, Lister-James J, Julian J, Taillefer R, Moyer B, Ginsberg J. Imaging characteristics of a novel technetium Tc 99m-labeled platelet glycoprotein IIb/IIIa receptor antagonist in patients with acute deep vein thrombosis or a history of deep vein thrombosis. Arch Intern Med 2003;163(4):452–6.
Thakur ML, Pallela VR, Consigny PM, Rao PS, Vessileva-Belnikolovska D, Shi R. Imaging vascular thrombosis with 99mTc-labeled fibrin alpha-chain peptide. J Nucl Med 2000;41(1):161–8.
Taillefer R. Radiolabeled peptides in the detection of deep venous thrombosis. Semin Nucl Med 2001;31(2):102–23.
Chianelli M, Parisella MG, D’Alessandria C, Corsetti F, Scopinaro F, Signore A. The developing role of peptide radiopharmaceuticals in the study of chronic inflammation: new techniques for novel therapeutic options. Q J Nucl Med 2003;47(4):256–69.
Ishino S, Kuge Y, Takai N, Strauss HW, Blankenberg FG, Shiomi M, et al. 99mTc-Annexin A5 for noninvasive characterization of atherosclerotic lesions: imaging and histological studies in myocardial infarction-prone Watanabe heritable hyperlipidemic rabbits. Eur J Nucl Med Mol Imaging 2007;34(6):889–99.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Glaudemans, A.W.J.M., Slart, R.H.J.A., Bozzao, A. et al. Molecular imaging in atherosclerosis. Eur J Nucl Med Mol Imaging 37, 2381–2397 (2010). https://doi.org/10.1007/s00259-010-1406-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-010-1406-4