Abstract:
Purpose:
The aim of this study was to analyse the impact of FDG-PET staging on treatment results of neo-adjuvant radiochemotherapy in patients with advanced non-small cell lung cancer (NSCLC). We compared prospectively the outcome of two patient groups with stage III NSCLC undergoing the same neo-adjuvant radio-chemotherapy (NARCT). In one group, FDG-PET was part of the pretherapeutic staging, whereas in the other group, no PET scans were performed.
Methods:
One hundred and eighty-eight patients with advanced stage III NSCLC were selected for a phase II trial of NARCT. The first 115 patients underwent conventional workup (CWU) and FDG-PET before inclusion (group I); the remaining 73 patients underwent CWU only (group II). All patients were followed up according to a standardised protocol for at least 11 months (up to 64 months). Overall survival and disease-free survival were used as parameters of therapeutic success and analysed statistically.
Results:
After staging, 157/188 patients were included in the clinical trial. Thirty-one were excluded owing to the results of FDG-PET, in most cases because of the detection of previously unknown distant metastases. Overall survival and metastasis-free survival were significantly longer in patients of group I stratified by FDG-PET than in group II (p=0.006 and 0.02 respectively). Another significant factor for survival was complete tumour resection (p=0.02). Gender, histological tumour type, tumour grade and UICC stage had no significant influence.
Conclusion:
Pretherapeutic staging by FDG-PET significantly influences the results of NARCT and subsequent surgery by identifying patients not eligible for curative treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-small cell lung cancer (NSCLC) is the leading cause of cancer death in both men and women. Patients with stage I or II NSCLC may be cured by surgery, whereas those with stage IIIA or IIIB are considered inoperable. In the past the only therapeutic option for these patients was radiotherapy and/or palliative chemotherapy. Recently a new therapeutic strategy was introduced, aiming at tumour reduction in order to reach an operable tumour stage. Since then, several new agents and many different regimens consisting of various combinations of chemotherapy and radiotherapy have been studied in large clinical trials [1–5].
The PLUS study [6], a randomised study by van Tinteren et al., demonstrated that 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) has the potential to avoid futile surgery in patients with advanced NSCLC. In accordance with other studies [7–15], the authors demonstrated superior diagnostic accuracy of FDG-PET staging prior to surgery as compared with the standard staging procedures [computed tomography (CT) of thorax and chest, ultrasonography of the abdomen, bone scintigraphy and mediastinoscopy]. PET staging revealed previously unknown M1 or N3 stage in about 30% of all patients who were subsequently excluded from surgery. The PLUS study confirmed that FDG-PET is suitable for selection of those patients who will profit from aggressive treatment regimens. Therefore it can be assumed that improved patient selection might lead to better results, in particular longer survival in patients undergoing aggressive treatment.
No such outcome data are as yet available. A recent study by Hoekstra et al. analysed the prognostic relevance of response evaluation using FDG-PET in 47 patients [16]. Her group was able to show that certain FDG-PET findings prior to and after neoadjuvant therapy were highly predictive for patient outcome. However, no direct comparison of treatment results for patients staged by PET and those staged without use of PET has been published to date. The aim of the presented prospective study was therefore to find out whether patients with advanced NSCLC (IIIA or IIIB) staged by FDG-PET have an improved clinical outcome compared with those staged by conventional procedures only, when the same treatment protocol was employed.
Materials and methods
Patients
An overview of our study population, including the results of surgery and clinical follow-up, is given in Fig. 1. From August 1998 to March 2004, 188 consecutive patients with histologically proven NSCLC were selected for inclusion in a neo-adjuvant phase II trial [17]. Inclusion criteria were as follows: NSCLC of UICC stage III, age between 18 and 70 years, presence of macroscopic tumour manifestations. Patients in poor condition (Karnofsky index <70%) were excluded, as were patients with supraclavicular lymph node or distant metastases (stage IV) shown by conventional workup (CWU) and patients who had previously been treated because of other malignant diseases. The study was approved by the local ethics committee; all patients gave informed consent to participation.
One hundred and fifty-seven of the patients (121 male, 36 female, mean age 57.9 years, range 31–70 years) were finally enrolled in the study. Whether or not a patient received FDG-PET depended solely on the time of inclusion: between August 1998 amd January 2002, patients selected for radiochemotherapy were routinely referred for FDG-PET (group I: CWU+PET, n=115). After January 2002, FDG-PET was no longer part of the routine staging protocol for budgetary reasons (group II: CWU only,n=73).
All patients underwent the same neoadjuvant treatment protocol: Induction chemotherapy (weeks 0–4) consisted of 100 mg/m2 paclitaxel (Taxol) and carboplatin (Carboplat) per infusion applied weekly, followed by a second treatment cycle (weeks 6–9) consisting of radiotherapy (2×1.5 Gy/day, 5×/week up to 45 Gy) in combination with paclitaxel (50 mg/m2) and carboplatin [area under the curve (AUC) 2] on days 1, 8 and 15 of radiation.
Routine staging
CWU consisted of bronchoscopy with biopsy, X-ray and CT of the chest, mediastinoscopy, bone scintigraphy, and ultrasonography or CT of the abdomen. Patients were assigned to stage IIIA or IIIB according to the system developed by the American Joint Committee on Cancer [18].
FDG-PET
As part of the staging procedure in patient group I, FDG-PET was performed after conventional staging, but prior to mediastinoscopy to avoid secondary (postoperative) changes. A whole-body PET scanner (Advance, General Electric Medical Systems, Milwaukee, WI, USA) operated in 2D mode was used for all PET scans. After a 12-h overnight fast, patients were injected with 350–450 MBq 18F-FDG intravenously. Blood glucose levels were lower than 6.7 mmol/l in all patients (mean 5.2±1.3 mmol/l). One hour after injection, static emission scans were obtained covering the neck, thorax, abdomen and pelvis (six fields of view=90 cm). PET scans were processed using iterative reconstruction, and images were corrected for attenuation. FDG uptake was calculated as average SUV (standardised uptake value, SUVavg) derived from regions of interest using an isocontour threshold defined as: threshold=(SUVmax+SUVbackground)/2. Image analysis was done by two experienced nuclear medicine specialists who evaluated the extent of the primary tumour, mediastinal lymph node spread and presence of distant metastases (S.M.E., M.R.), without being aware of the other results of conventional staging. For assessment of mediastinal foci, a SUV cut-off of 2.5 was applied to discriminate between benign (<2.5) and malignant (>2.5) lesions.
Surgery
The decision to perform surgery was based on local findings of CT and re-mediastinoscopy or video thoracoscopy after completion of neoadjuvant therapy. Results of pretherapeutic staging PET were integrated whenever distant metastases identified by PET could be verified by another imaging method. “Successful surgery” was defined as complete resection of all visible or previously detected tumour parts, including corresponding mediastinal lymph node stations. “No or no successful surgery” was defined as failure to perform an operation owing to the results of post-therapeutic re-staging or because intraoperative findings of exploratory thoracotomy indicated an inoperable situation.
Outcome analysis
Standardised follow-up of all patients (physical and laboratory examinations, repeated CT or X-ray of the chest at 3-monthly intervals; detailed follow-up protocols for the period of radiochemotherapy and after surgery have been published previously [19]) was organised by the Schillerhoehe Hospital as the responsible study centre. A last update was obtained in November 2005.
Statistical analysis
The Kaplan-Meier method was used to estimate the survival probability for groups I and II. Survival time was defined as the time between tumour verification by histology and death or last follow-up. Disease-free survival was defined as the interval between surgery and onset of local recurrence or detection of distant metastases. The relationship between PET staging and survival was tested by the log-rank test. Univariate Cox analysis was applied to assess the effects of the following variables on survival: age, gender, histological tumour type, tumour grade and tumour stage. Interactions and joint effects of the variables “PET staging” and “successful operation” were examined in a Cox proportional hazards model.
Results
Patients
From August 1998 to March 2004 a total of 188 patients with suspected stage III NSCLC were assessed for eligibility for neoadjuvant treatment; as indicated above,115 of them underwent CWU and FDG-PET, while 73 only conventional workup (Fig. 1).
Thirty-one patients of group I (27%) were excluded from neoadjuvant treatment after FDG-PET. In 28 of these patients (24%), PET detected distant metastases and these cases were thus UICC stage IV. Three further patients were excluded owing to absence of tumour (n=2) or change in histological diagnosis (n=1). In the latter case an atypical pattern found at FDG-PET (mediastinal bulk without localised lung lesion) initiated histological re-evaluation, which finally revealed seminoma instead of NSCLC. Thus, in total 157 patients were included in this analysis, 84 belonging to group I (PET staging) and 73 to group II (CWU). Of the 28 patients with distant metastases shown by PET, ten nevertheless received neoadjuvant treatment because metastases could not yet be identified by other methods at that time point (n=8) or because surgical resection of adrenal metastases was planned (n=2). Survival of those patients with distant metastases was not taken into account.
Surgery was performed in 103/157 patients, including 59/84 (69%) in group I and 44/73 (60%) in group II. Complete tumour resection could be achieved in 48 of group I (57%) and 37 of group II (51%) (Table 1). Nine patients died postoperatively, corresponding to an in-hospital operative mortality of 8.7%.
Comparison of groups I and II
The two patient groups were compared statistically with regard to other factors which might have an influence on outcome: age, gender, tumour stage, T stage assessed by CT and N stage assessed by mediastinoscopy. Results are shown in Table 1. The groups showed a significant difference with regard to tumour stage. No other significant differences could be identified.
Univariate survival analysis
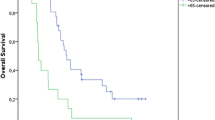
Follow-up data were available for all 157 patients. One hundred and three patients had died. Of the remaining 54 patients, 40 are alive at present (November 2005). No recent follow-up data are available in 14 patients owing to refusal of further follow-up (n=8) or the patient moving away from the area (n=6). The median overall survival of the whole patient group was 14.2 months (group I, 22.3 months vs group II, 11.3 months). The median follow-up of the 40 patients who are still alive is 35.3 months [16–50.6 months (25–75% quantiles)] in group I and 16.4 (8.4–22.4) in group II. Univariate outcome analysis was performed concerning the following criteria: staging by PET, complete tumour resection, UICC stage, N stage, T stage, histological tumour type, age and gender. Outcome in terms of overall survival (Fig. 2, p=0.006) or time interval until onset of distant metastases (Fig. 3, p=0.02) was significantly better in patients of group I. Distant metastases occurred in 61 patients during follow-up: 22 patients developed brain metastases, 17 bone metastases, seven liver metastases, six lung metastases, four adrenal metastases, three abdominal metastases and two cervical metastases.
The interval until the onset of local recurrence in patients who underwent surgery was not significantly different between the two groups (p=0.6).
Complete resection led to a significantly better outcome in the whole patient group (p=0.02). The influence of UICC stage was not significant: patients with stage IIIA had only a slightly better outcome (p=0.2). Survival was not significantly affected by age, gender, N stage as determined by mediastinoscopy, T stage as determined by CT, histological tumour type or tumour grade.
Multivariate survival analysis
Interactions and joint effects of the variables “PET staging” and “successful operation” were examined in a Cox proportional hazards model. The only variable significantly correlated with survival in all subgroups was staging by PET. An interesting additional result of subgroup analysis was a highly significant difference between the patients who underwent resection after PET staging versus patients who underwent resection without PET staging (Fig. 4,p=0.002).
Discussion
Prognosis and survival in non-small cell lung cancer (NSCLC) are mainly determined by two factors: the local resectability of the tumour and the presence of systemic tumour spread. For operable stages (no contralateral lymph node metastases, T1–3, no distant metastases), a 5-year disease-free survival of up to 60% has been reported [20]. Nowadays, in primary, inoperable NSCLC (stage IIIA and B), new neoadjuvant treatment protocols, consisting of combined chemotherapy and radiation, have been developed with the aim of reducing tumour extent in order to reach an operable stage. These therapeutic regimens are aggressive and under permanent clinical evaluation. This study was part of an ongoing phase II trial for the evaluation of a new neoadjuvant radiochemotherapy protocol [17]. In the first part of the study, PET staging was prescribed in addition to conventional workup with the aim of evaluating the accuracy of PET staging. After inclusion of 115 patients, the trial was prolonged, but without FDG-PET. In the first part of the trial, PET detected distant metastases in 24% of the patients. After NARCT, a comparable proportion of patients remained inoperable in both groups (45% vs 49%). This reflects the fact that pretherapeutic PET is of limited value for the assessment of local resectability because it does not show the exact tumour extent or infiltration of neighbouring structures. Accordingly, local recurrences occurred with similar frequencies in both groups. In contrast, the frequency of new distant metastases during ongoing NARCT and after NARCT was significantly higher in patients who were not staged by PET (p=0.02, mean time to onset of metastases: 2.7 years in group I vs 1.0 years in group II). As to the overall survival, we were able to demonstrate that patients selected for NARCT by PET staging had a highly significant longer survival than those who underwent conventional workup only. This survival advantage presumably is not based on improved local tumour control but rather on a lower frequency and later onset of distant metastases. An interesting and surprising result was that even when patients with distant metastases were included in the analysis, patients staged by PET still had a better outcome. Of course, this finding has to be interpreted with care, since patient inclusion was not randomised and this result may reflect a difference in characteristics between the two groups even when it was not significantly detectable in univariate analysis. One explanation for this finding might be the advantage of avoiding unnecessary and aggressive therapy, taking into account the high morbidity and mortality of extensive surgery and intense radiation and chemotherapy: in our study group, in 9/188 patients death was caused by surgery or postoperative morbidity and in four further patients by radiotherapy.
We are aware that one limitation of this study is that it was not initially designed for the evaluation of PET staging. The primary endpoint was the assessment of the new neoadjuvant treatment. Thus, the data are prospective, but not randomised as to the performance of FDG-PET. The two groups are quite comparable concerning demographic data, T and N stage, and pretherapeutic lung function. Only the UICC stage is significantly different, which nonetheless turned out to have no significant influence on overall survival. A second limitation is that the follow-up in the second group is considerably shorter because of later patient inclusion. However, we found this difference irrelevant, because at the time of the last follow-up more than half of the patients were already dead in both groups. On the other hand, it is a strength of this study that the clinical setting was strictly standardised in many respects: All patients underwent the same staging (X-ray and CT of the thorax, bronchoscopy, ultrasonography, bone scintigraphy and mediastinoscopy) at the same hospital, completed in one group by the addition of FDG-PET staging. We included only patients with UICC stage IIIA and B. All patients were selected for a phase II trial with a neoadjuvant treatment protocol consisting of a combination of paclitaxel and carboplatin, followed by hyperfractionated radiotherapy and, in the event of a favourable response, curative surgery. Mediastinoscopy and surgery were performed by the same physicians, as was the follow-up, and all PET investigations were performed on the same scanner by the same two physicians. So, inter-observer variability and influence of different therapeutic approaches did not play a role in the results.
Some aspects of the value of PET staging for patient selection for neoadjuvant therapy remain unclear. FDG-PET was performed in addition to all established conventional staging procedures. A question to be addressed by future controlled studies could be whether it is possible to replace one or more diagnostic procedures (for example, bone scintigraphy or mediastinoscopy in patients with negative PET findings in the mediastinum) without loss of diagnostic accuracy.
Conclusion
We conclude from this study that FDG-PET staging contributes to optimal treatment planning in patients with UICC stage III NSCLC by identifying patients with previously unknown N3 and M1 stage disease that warrants exclusion from unnecessary radiochemotherapy as well as from surgery. This patient selection results in improved outcome despite the fact that it does not improve local tumour control by surgery. It appears to be justified in order to restrict aggressive neoadjuvant treatment to patients who will presumably benefit from the procedure.
References
Burkes RL, Ginsberg RJ, Shepherd FA, Blackstein ME, Goldberg ME, Waters PF, et al. Induction chemotherapy with mitomycin, vindesine, and cisplatin for stage III unresectable non-small cell lung cancer: results of the Toronto phase II trial. J Clin Oncol 1992;10:580–96
Johnson DH, Strupp J, Greco FA, Stewart J, Merrill W, Malcolm A, et al. Neoadjuvant cisplatin plus vinblastin chemotherapy in locally advanced non-small cell lung cancer. Cancer 1991;68:1216–20
Rosell R, Gomez-Codina J, Camps C, Maestre J, Padille J, Canto A, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small cell lung cancer. N Engl J Med 1994;330:153–8
Roth JA, Fossella F, Komaki R, Ryan MB, Putnam JB Jr, Lee JS, et al. A randomized trial comparing preoperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small cell lung cancer. J Natl Cancer Inst 1994;86:673–80
Weiden PL, Piantadosi S. Preoperative chemotherapy (cisplatin and fluorouracil) and radiation therapy in stage III non-small cell lung cancer: a phase-II-study of the Lung Cancer Study Group. J Natl Cancer Inst 1991;83:266–72
van Tinteren H, Hoekstra OS, Smit EF, van den Bergh JH, Schreurs AJ, Stallaert RA, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet 2002;359:1388–93
Saunders CA, Dussek JE, O’Doherty MJ, Maisey MN. Evaluation of fluorine-18-fluorodeoxyglucose whole body positron emission tomography imaging in the staging of lung cancer. Ann Thorac Surg 1999;67:790–7
Schrevens L, Lorent N, Dooms C, Vansteenkiste J. The role of PET scan in diagnosis, staging, and management of non-small cell lung cancer. Oncologist 2004;9:633–43
Bury T, Dowlati A, Paulus P, Corhay JL, Hustinx R, Ghaye B, et al. Whole-body 18FDG positron emission tomography in the staging of non-small cell lung cancer. Eur Respir J 1997;10:2529–34
Lewis P, Griffin S, Marsden P, Gee T, Nunan T, Malsey M, et al. Whole-body18F-fluorodeoxyglucose positron emission tomography in preoperative evaluation of lung cancer. Lancet 1994;344:1265–6
Pieterman RM, Van Putten JW, Meuzelaar JJ, Mooyaart EL, Vaalburg W, Koeter GH, et al. Preoperative staging of non-small cell lung cancer with positron emission tomography. N Engl J Med 2000;343:254–61
Hicks RJ, Kalff V, MacManus MP, Ware RE, Hogg A, McKenzie AF, et al.18F-FDG PET provides high-impact and powerful prognostic stratification in staging newly diagnosed non-small cell lung cancer. J Nucl Med 2001;42:1596–604
Hoekstra CJ, Stroobants SG, Hoekstra OS, Vansteenkiste J, Biesma B, Schramel FJ, et al. The value of [18F]fluoro-2-deoxy-D-glucose positron emission tomography in the selection of patients with stage IIIA-N2 non-small cell lung cancer for combined modality treatment. Lung Cancer 2003;39:151–7
Schmucking M, Baum RP, Griesinger F, Presselt N, Bonnet R, Przetak C, et al. Molecular whole-body cancer staging using positron emission tomography: consequences for therapeutic management and metabolic radiation treatment planning. Recent Results Cancer Res 2003;162:195–202
Eschmann SM, Friedel G, Paulsen F, Budach W, Harer-Mouline C, Dohmen BM, et al. FDG-PET for staging in patients with advanced non- small cell lung cancer (NSCLC) scheduled for neoadjuvant radio-chemotherapy. Eur J Nucl Med Mol Imaging 2002;29:804–8
Hoekstra CJ, Stroobants SG, Smit EF, Vansteenkiste J, van Tinteren H, Postmus PE, et al. Prognostic relevance of response evaluation using [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography in patients with locally advanced non-small-cell lung cancer. J Clin Oncol 2005;23:8362–70
Friedel G, Hruska D, Budach W, Wolf M, Kyriss T, Hurtgen M, et al. Neoadjuvant chemoradiotherapy of stage III non-small-cell lung cancer. Lung Cancer 2000;30:175–85
AJCC cancer staging manual. 6th edn. Berlin Heidelberg New York: Springer; 2002; p. 165–77
Eschmann SM, Friedel G, Paulsen F, Reimold M, Hehr T, Budach W, et al. Is standardised 18F-FDG uptake value an outcome predictor in patients with stage III non-small cell lung cancer? Eur J Nucl Med Mol Imaging 2006;33:263–9
Martini N, Bains MS, Burt ME, Zakowski MF, McCormack P, Rusch VW, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 1995;109:120–9
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Eschmann, S.M., Friedel, G., Paulsen, F. et al. Impact of staging with 18F-FDG-PET on outcome of patients with stage III non-small cell lung cancer: PET identifies potential survivors. Eur J Nucl Med Mol Imaging 34, 54–59 (2007). https://doi.org/10.1007/s00259-006-0197-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-006-0197-0