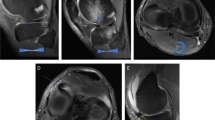
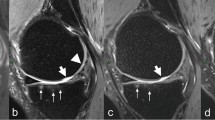
Abstract
Objectives
This study aimed to assess the diagnostic image quality and compare the knee cartilage segmentation results using a controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA)-accelerated 3D-dual echo steady-state (DESS) research package sequence in the knee.
Materials and Methods
A total of 64 subjects underwent both two- and fourfold CAIPIRINHA-accelerated 3D-DESS and DESS without parallel acceleration technique of the knee on a 3.0 T system. Two musculoskeletal radiologists evaluated the images independently for image quality and diagnostic capability following randomization and anonymization. The consistency of automatic segmentation results between sequences was explored using an automatic knee cartilage segmentation research application. The descriptive statistics and inter-observer and inter-method concordance of various acceleration sequences were investigated. P values < .05 were considered significant.
Results
For image quality evaluation, the image signal-to-noise ratio and contrast-to-noise ratio decreased with the decrease in scanning time. However, it is accompanied by the reduction of artifacts. Using 3D-DESS without parallel acceleration technique as the standard for cartilage grading diagnosisand the diagnostic agreement of two- and fourfold CAIPIRINHA-accelerated 3D-DESS was good, kappa value was 0.860 (P < .001) and 0.804 (p < 0.001), respectively. Regarding cartilage defects, the sensitivity and specificity of the twofold acceleration 3D-CAIPIRINHA-DESS were 95.56% and 97.70%, and the fourfold CAIPIRINHA-accelerated 3D-DESS were 91.49% and 97.65%, respectively. The intraclass correlation coefficients of various sequences in cartilage segmentation were almost all greater than 0.9.
Conclusion
The CAIPIRINHA-accelerated 3D-DESS sequence maintained comparable diagnostic and segmentations performance of knee cartilage after a 60% scan time reduction.







Similar content being viewed by others
Data availability
The data are available from the corresponding author on reasonable request.
Abbreviations
- CAIPIRINHA:
-
Controlled aliasing in parallel imaging results in higher acceleration
- DESS:
-
Dual echo steady-state
- 2D:
-
2-Dimensional
- 3D:
-
3-Dimensional
- OA:
-
Osteoarthritis
- MRI:
-
Magnetic resonance imaging
- FISP:
-
Fast imaging with steady-state precession
- PSIF:
-
Reverse fast imaging with steady state precession
- TE:
-
Echo time
- TR:
-
Repetition time
- FSE:
-
Fast spin echo
- VIBE:
-
Volumetric interpolated breath-hold examination
- TSE:
-
Turbo spin echo
- SNR:
-
Signal noise ratio
- CNR:
-
Contrast noise ratio
- ROI:
-
Region of interest
- FOV:
-
Field of view
- SI:
-
Signal intensity
- SIT:
-
Signal intensity of each other tissue
- SD:
-
Standard deviation of background noise
- DICOM:
-
Digital imaging and communications in medicine
- ICC:
-
Intraclass correlation coefficient
References
Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H, et al. Osteoarthritis. Lancet (London, England). 2015;386(9991):376–87.
Fransen M, Bridgett L, March L, Hoy D, Penserga E, Brooks P. The epidemiology of osteoarthritis in Asia. Int J Rheum Dis. 2011;14(2):113–21.
Li X, Benjamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007;15(7):789–97.
Gold GE, Burstein D, Dardzinski B, Lang P, Boada F, Mosher T. MRI of articular cartilage in OA: novel pulse sequences and compositional/functional markers. Osteoarthritis Cartilage. 2006;14 Suppl A:A76-86.
Kijowski R. 3D MRI of articular cartilage. Semin Musculoskelet Radiol. 2021;25(3):397–408.
Oei EHG, van Zadelhoff TA, Eijgenraam SM, Klein S, Hirvasniemi J, van der Heijden RA. 3D MRI in osteoarthritis. Semin Musculoskelet Radiol. 2021;25(3):468–79.
Wirth W, Nevitt M, Hellio Le Graverand MP, Benichou O, Dreher D, Davies RY, et al. Sensitivity to change of cartilage morphometry using coronal FLASH, sagittal DESS, and coronal MPR DESS protocols–comparative data from the Osteoarthritis Initiative (OAI). Osteoarthritis Cartilage. 2010;18(4):547–54.
Eckstein F, Hudelmaier M, Wirth W, Kiefer B, Jackson R, Yu J, et al. Double echo steady state magnetic resonance imaging of knee articular cartilage at 3 Tesla: a pilot study for the Osteoarthritis Initiative. Ann Rheum Dis. 2006;65(4):433–41.
Wright KL, Harrell MW, Jesberger JA, Landeras L, Nakamoto DA, Thomas S, et al. Clinical evaluation of CAIPIRINHA: comparison against a GRAPPA standard. J Magnet Reson Imaging. 2014;39(1):189–94.
Hu J, Xu B, Cao J, Yang R, Zhang H, Guo H, et al. Application value of CAIPIRINHA-VIBE with MOCO in liver magnetic resonance examination. Eur J Radiol. 2021;140: 109739.
Fritz B, Bensler S, Thawait GK, Raithel E, Stern SE, Fritz J. CAIPIRINHA-accelerated 10-min 3D TSE MRI of the ankle for the diagnosis of painful ankle conditions: performance evaluation in 70 patients. Eur Radiol. 2019;29(2):609–19.
Fritz J, Fritz B, Thawait GG, Meyer H, Gilson WD, Raithel E. Three-dimensional CAIPIRINHA SPACE TSE for 5-minute high-resolution MRI of the knee. Invest Radiol. 2016;51(10):609–17.
Van Dyck P, Smekens C, Roelant E, Vande Vyvere T, Snoeckx A, De Smet E. 3D CAIPIRINHA SPACE versus standard 2D TSE for routine knee MRI: a large-scale interchangeability study. Eur Radiol. 2022;32(9):6456–67.
Kalia V, Fritz B, Johnson R, Gilson WD, Raithel E, Fritz J. CAIPIRINHA accelerated SPACE enables 10-min isotropic 3D TSE MRI of the ankle for optimized visualization of curved and oblique ligaments and tendons. Eur Radiol. 2017;27(9):3652–61.
Hou B, Li Y, Xiong Y, Morelli JN, Wang J, Liu C, et al. Comparison of CAIPIRINHA-accelerated 3D fat-saturated-SPACE MRI with 2D MRI sequences for the assessment of shoulder pathology. Eur Radiol. 2022;32(1):593–601.
Zhang P, Yu B, Zhang R, Chen X, Shao S, Zeng Y, et al. Longitudinal study of the morphological and T2* changes of knee cartilages of marathon runners using prototype software for automatic cartilage segmentation. Br J Radiol. 2021;94(1119):20200833.
Liu L, Liu H, Zhen Z, Zheng Y, Zhou X, Raithel E, et al. Analysis of knee joint injury caused by physical training of freshmen students based on 3T MRI and automatic cartilage segmentation technology: a prospective study. Front Endocrinol. 2022;13: 839112.
Hou W, Zhao J, He R, Li J, Ou Y, Du M, et al. Quantitative measurement of cartilage volume with automatic cartilage segmentation in knee osteoarthritis. Clin Rheumatol. 2021;40(5):1997–2006.
Zhang P, Zhang RX, Chen XS, Zhou XY, Raithel E, Cui JL, et al. Clinical validation of the use of prototype software for automatic cartilage segmentation to quantify knee cartilage in volunteers. BMC Musculoskelet Disord. 2022;23(1):19.
Juras V, Szomolanyi P, Schreiner MM, Unterberger K, Kurekova A, Hager B, et al. Reproducibility of an automated quantitative MRI assessment of low-grade knee articular cartilage lesions. Cartilage. 2021;13(1_suppl):646s–57s.
Ramme AJ, Guss MS, Vira S, Vigdorchik JM, Newe A, Raithel E, et al. Evaluation of automated volumetric cartilage quantification for hip preservation surgery. J Arthroplasty. 2016;31(1):64–9.
Recht MP, Kramer J, Marcelis S, Pathria MN, Trudell D, Haghighi P, et al. Abnormalities of articular cartilage in the knee: analysis of available MR techniques. Radiology. 1993;187(2):473–8.
Fripp J, Crozier S, Warfield SK, Ourselin S. Automatic segmentation and quantitative analysis of the articular cartilages from magnetic resonance images of the knee. IEEE Trans Med Imaging. 2010;29(1):55–64.
Ebrahimkhani S, Jaward MH, Cicuttini FM, Dharmaratne A, Wang Y, de Herrera AGS. A review on segmentation of knee articular cartilage: from conventional methods towards deep learning. Artif Intell Med. 2020;106: 101851.
Eckstein F, Cicuttini F, Raynauld JP, Waterton JC, Peterfy C. Magnetic resonance imaging (MRI) of articular cartilage in knee osteoarthritis (OA): morphological assessment. Osteoarthritis Cartilage. 2006;14 Suppl A:A46-75.
Martel-Pelletier J, Paiement P, Pelletier JP. Magnetic resonance imaging assessments for knee segmentation and their use in combination with machine/deep learning as predictors of early osteoarthritis diagnosis and prognosis. Ther Adv Musculoskelet Dis. 2023;15:1759720x231165560.
Friedrich KM, Reiter G, Kaiser B, Mayerhöfer M, Deimling M, Jellus V, et al. High-resolution cartilage imaging of the knee at 3T: basic evaluation of modern isotropic 3D MR-sequences. Eur J Radiol. 2011;78(3):398–405.
Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16(12):1433–41.
Liu L, Wu G. Three-dimensional SPACE MR with CAIPIRINHA fourfold acceleration for assessing long head of biceps tendon. Acta Radiol (Stockholm, Sweden : 1987). 2021:2841851211055324.
Chaudhari AS, Stevens KJ, Wood JP, et al. Utility of deep learning super-resolution in the context of osteoarthritis MRI biomarkers[J]. J Magn Reson Imaging. 2020;51(3):768–79.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
Xiaoyue Zhou is an employee of Siemens Healthineers Ltd., Shanghai, China. Qiong Zhang is an employee of Siemens Shenzhen Magnetic Resonance Ltd., Shenzhen, China., Esther Raithel is an employee of Siemens Healthcare GmbH, Erlangen, Germany. The remaining authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wen, D., Zhou, X., Hou, B. et al. 3D-DESS MRI with CAIPIRINHA two- and fourfold acceleration for quantitatively assessing knee cartilage morphology. Skeletal Radiol (2024). https://doi.org/10.1007/s00256-024-04605-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00256-024-04605-7