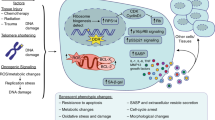
Abstract
Senescent cells play a vital role in the pathogenesis of musculoskeletal (MSK) diseases, such as chronic inflammatory joint disorders, rheumatoid arthritis (RA), and osteoarthritis (OA). Cellular senescence in articular joints represents a response of local cells to persistent stress that leads to cell-cycle arrest and enhanced production of inflammatory cytokines, which in turn perpetuates joint damage and leads to significant morbidities in afflicted patients. It has been recently discovered that clearance of senescent cells by novel “senolytic” therapies can attenuate the chronic inflammatory microenvironment of RA and OA, preventing further disease progression and supporting healing processes. To identify patients who might benefit from these new senolytic therapies and monitor therapy response, there is an unmet need to identify and map senescent cells in articular joints and related musculoskeletal tissues. To fill this gap, new imaging biomarkers are being developed to detect and characterize senescent cells in human joints and musculoskeletal tissues. This review article will provide an overview of these efforts. New imaging biomarkers for senescence cells are expected to significantly improve the specificity of state-of-the-art imaging technologies for diagnosing musculoskeletal disorders.


Similar content being viewed by others
References
Collaborators GBDO. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023;5(9):e508–22.
Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780–5.
Hunziker EB. Articular cartilage repair: problems and perspectives. Biorheology. 2000;37(1–2):163–4.
Kinner B, Capito RM, Spector M. Regeneration of articular cartilage. Adv Biochem Eng Biotechnol. 2005;94:91–123.
Xu M, Bradley EW, Weivoda MM, Hwang SM, Pirtskhalava T, Decklever T, et al. Transplanted senescent cells induce an osteoarthritis-like condition in mice. J Gerontol A Biol Sci Med Sci. 2017;72(6):780–5.
Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23(6):775–81.
Jordan JM, De Roos AJ, Renner JB, Luta G, Cohen A, Craft N, et al. A case-control study of serum tocopherol levels and the alpha- to gamma-tocopherol ratio in radiographic knee osteoarthritis: the Johnston County Osteoarthritis Project. Am J Epidemiol. 2004;159(10):968–77.
Diez-Villares S, Garcia-Varela L, Antas SG, Caeiro JR, Carpintero-Fernandez P, Mayan MD, et al. Quantitative PET tracking of intra-articularly administered (89)Zr-peptide-decorated nanoemulsions. J Control Release. 2023;356:702–13.
Faust HJ, Zhang H, Han J, Wolf MT, Jeon OH, Sadtler K, et al. IL-17 and immunologically induced senescence regulate response to injury in osteoarthritis. J Clin Invest. 2020;130(10):5493–507.
Nogueira-Recalde U, Lorenzo-Gomez I, Blanco FJ, Loza MI, Grassi D, Shirinsky V, et al. Fibrates as drugs with senolytic and autophagic activity for osteoarthritis therapy. EBioMedicine. 2019;45:588–605.
Yan S, Dong W, Li Z, Wei J, Han T, Wang J, et al. Metformin regulates chondrocyte senescence and proliferation through microRNA-34a/SIRT1 pathway in osteoarthritis. J Orthop Surg Res. 2023;18(1):198.
Yang H, Chen C, Chen H, Duan X, Li J, Zhou Y, et al. Navitoclax (ABT263) reduces inflammation and promotes chondrogenic phenotype by clearing senescent osteoarthritic chondrocytes in osteoarthritis. Aging (Albany NY). 2020;12(13):12750–70.
Min HK, Kim SH, Won JY, Kim KW, Lee JY, Lee SH, et al. Dasatinib, a selective tyrosine kinase inhibitor, prevents joint destruction in rheumatoid arthritis animal model. Int J Rheum Dis. 2023;26(4):718–26.
Javadi F, Ahmadzadeh A, Eghtesadi S, Aryaeian N, Zabihiyeganeh M, Rahimi Foroushani A, et al. The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: a double-blind, randomized controlled trial. J Am Coll Nutr. 2017;36(1):9–15.
Matsuno H, Nakamura H, Katayama K, Hayashi S, Kano S, Yudoh K, et al. Effects of an oral administration of glucosamine-chondroitin-quercetin glucoside on the synovial fluid properties in patients with osteoarthritis and rheumatoid arthritis. Biosci Biotechnol Biochem. 2009;73(2):288–92.
Fessler J, Husic R, Schwetz V, Lerchbaum E, Aberer F, Fasching P, et al. Senescent T-cells promote bone loss in rheumatoid arthritis. Front Immunol. 2018;9:95.
Tang B, Chen Y, Zhao P, Yan W, Huang X, Jiang W, et al. MiR-601-induced BMSCs senescence accelerates steroid-induced osteonecrosis of the femoral head progression by targeting SIRT1. Cell Mol Life Sci. 2023;80(9):261.
Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017;23(9):1072–9.
Farr JN, Rowsey JL, Eckhardt BA, Thicke BS, Fraser DG, Tchkonia T, et al. Independent roles of estrogen deficiency and cellular senescence in the pathogenesis of osteoporosis: evidence in young adult mice and older humans. J Bone Miner Res. 2019;34(8):1407–18.
Farr JN, Fraser DG, Wang H, Jaehn K, Ogrodnik MB, Weivoda MM, et al. Identification of senescent cells in the bone microenvironment. J Bone Miner Res. 2016;31(11):1920–9.
Saul D, Monroe DG, Rowsey JL, Kosinsky RL, Vos SJ, Doolittle ML, et al. Modulation of fracture healing by the transient accumulation of senescent cells. Elife. 2021; 10.
Bajada S, Marshall MJ, Wright KT, Richardson JB, Johnson WE. Decreased osteogenesis, increased cell senescence and elevated Dickkopf-1 secretion in human fracture non union stromal cells. Bone. 2009;45(4):726–35.
Marin I, Boix O, Garcia-Garijo A, Sirois I, Caballe A, Zarzuela E, et al. Cellular senescence is immunogenic and promotes antitumor immunity. Cancer Discov. 2023;13(2):410–31.
Schosserer M, Grillari J, Breitenbach M. The dual role of cellular senescence in developing tumors and their response to cancer therapy. Front Oncol. 2017;7:278.
Jeon OH, David N, Campisi J, Elisseeff JH. Senescent cells and osteoarthritis: a painful connection. J Clin Invest. 2018;128(4):1229–37.
Martin JA, Brown TD, Heiner AD, Buckwalter JA. Chondrocyte senescence, joint loading and osteoarthritis. Clin Orthop Relat Res. 2004;(427 Suppl):S96–103.
Li J, Pei M. Cell senescence: a challenge in cartilage engineering and regeneration. Tissue Eng Part B Rev. 2012;18(4):270–87.
Del Rey MJ, Valin A, Usategui A, Ergueta S, Martin E, Municio C, et al. Senescent synovial fibroblasts accumulate prematurely in rheumatoid arthritis tissues and display an enhanced inflammatory phenotype. Immun Ageing. 2019;16:29.
Carlo MD Jr, Loeser RF. Increased oxidative stress with aging reduces chondrocyte survival: correlation with intracellular glutathione levels. Arthritis Rheum. 2003;48(12):3419–30.
Martin JA, Buckwalter JA. Telomere erosion and senescence in human articular cartilage chondrocytes. J Gerontol A Biol Sci Med Sci. 2001;56(4):B172-179.
Montero-Melendez T, Nagano A, Chelala C, Filer A, Buckley CD, Perretti M. Therapeutic senescence via GPCR activation in synovial fibroblasts facilitates resolution of arthritis. Nat Commun. 2020;11(1):745.
Wang Y, Liu J, Ma X, Cui C, Deenik PR, Henderson PKP, et al. Real-time imaging of senescence in tumors with DNA damage. Sci Rep. 2019;9(1):2102.
Chen JA, Guo W, Wang Z, Sun N, Pan H, Tan J, et al. In vivo imaging of senescent vascular cells in atherosclerotic mice using a beta-galactosidase-activatable nanoprobe. Anal Chem. 2020;92(18):12613–21.
Biran A, Zada L, Abou Karam P, Vadai E, Roitman L, Ovadya Y, et al. Quantitative identification of senescent cells in aging and disease. Aging Cell. 2017;16(4):661–71.
Brandl A, Meyer M, Bechmann V, Nerlich M, Angele P. Oxidative stress induces senescence in human mesenchymal stem cells. Exp Cell Res. 2011;317(11):1541–7.
Ingrosso D, D’Angelo S, di Carlo E, Perna AF, Zappia V, Galletti P. Increased methyl esterification of altered aspartyl residues in erythrocyte membrane proteins in response to oxidative stress. Eur J Biochem. 2000;267(14):4397–405.
Lee BY, Han JA, Im JS, Morrone A, Johung K, Goodwin EC, et al. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006;5(2):187–95.
Suryadevara V, Hudgins AD, A. R, Pappalardo A, Karpova A, A.K. D, et al. SenGuiDe: SenNet guidelines for detecting senescent cells across tissues. . Nature Reviews Molecular Cell Biology. in press.
Gao SG, Zeng C, Li LJ, Luo W, Zhang FJ, Tian J, et al. Correlation between senescence-associated beta-galactosidase expression in articular cartilage and disease severity of patients with knee osteoarthritis. Int J Rheum Dis. 2016;19(3):226–32.
Li W, Xiong Y, Chen W, Wu L. Wnt/beta-catenin signaling may induce senescence of chondrocytes in osteoarthritis. Exp Ther Med. 2020;20(3):2631–8.
Fan Y, Cheng J, Zeng H, Shao L. Senescent cell depletion through targeting BCL-family proteins and mitochondria. Front Physiol. 2020;11:593630.
Wang B, Wang L, Gasek NS, Zhou Y, Kim T, Guo C, et al. An inducible p21-Cre mouse model to monitor and manipulate p21-highly-expressing senescent cells in vivo. Nat Aging. 2021;1(10):962–73.
Koo S, Won M, Li H, Kim WY, Li M, Yan C, et al. Harnessing alpha-l-fucosidase for in vivo cellular senescence imaging. Chem Sci. 2021;12(29):10054–62.
Yang L, Liu G, Chen Q, Wan Y, Liu Z, Zhang J, et al. An activatable NIR probe for the detection and elimination of senescent cells. Anal Chem. 2022;94(13):5425–31.
Liu J, Ma X, Cui C, Chen Z, Wang Y, Deenik PR, et al. Noninvasive NIR imaging of senescence via in situ labeling. J Med Chem. 2021;64(24):17969–78.
Lozano-Torres B, Blandez JF, Galiana I, Lopez-Dominguez JA, Rovira M, Paez-Ribes M, et al. A two-photon probe based on naphthalimide-styrene fluorophore for the in vivo tracking of cellular senescence. Anal Chem. 2021;93(5):3052–60.
Lilley LM, Kamper S, Caldwell M, Chia ZK, Ballweg D, Vistain L, et al. Self-immolative activation of beta-galactosidase-responsive probes for in vivo mr imaging in mouse models. Angew Chem Int Ed Engl. 2020;59(1):388–94.
Tang JH, Li H, Yuan C, Parigi G, Luchinat C, Meade TJ. Molecular engineering of self-immolative bioresponsive MR probes. J Am Chem Soc. 2023;145(18):10045–50.
Suryadevara V, Hajipour MJ, Habte FG, Morakote W, Malik N, Chang E, et al. A multimodal based imaging approach using a novel radiotracer, (18F)-PyGal to detect senescence in small and large animal models. Osteoarthr Imaging. 2023;3:100107.
He Z, Xu K, Li Y, Gao H, Miao T, Zhao R, et al. Molecularly targeted fluorescent sensors for visualizing and tracking cellular senescence. Biosensors (Basel). 2023; 13(9).
Han J, Han MS, Tung CH. A fluorogenic probe for beta-galactosidase activity imaging in living cells. Mol Biosyst. 2013;9(12):3001–8.
Dai H, Chen R, Gui C, Tao T, Ge Y, Zhao X, et al. Eliminating senescent chondrogenic progenitor cells enhances chondrogenesis under intermittent hydrostatic pressure for the treatment of OA. Stem Cell Res Ther. 2020;11(1):199.
Bauer ME. Accelerated immunosenescence in rheumatoid arthritis: impact on clinical progression. Immun Ageing. 2020;17:6.
Collins JA, Diekman BO, Loeser RF. Targeting aging for disease modification in osteoarthritis. Curr Opin Rheumatol. 2018;30(1):101–7.
Taghian T, Batista AR, Kamper S, Caldwell M, Lilley L, Li H, et al. Real-time MR tracking of AAV gene therapy with betagal-responsive MR probe in a murine model of GM1-gangliosidosis. Mol Ther Methods Clin Dev. 2021;23:128–34.
Brooks PM. Impact of osteoarthritis on individuals and society: how much disability? Social consequences and health economic implications. Curr Opin Rheumatol. 2002;14(5):573–7.
Novais EJ, Tran VA, Johnston SN, Darris KR, Roupas AJ, Sessions GA, et al. Long-term treatment with senolytic drugs Dasatinib and Quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nat Commun. 2021;12(1):5213.
Kanzaki N, Saito K, Maeda A, Kitagawa Y, Kiso Y, Watanabe K, et al. Effect of a dietary supplement containing glucosamine hydrochloride, chondroitin sulfate and quercetin glycosides on symptomatic knee osteoarthritis: a randomized, double-blind, placebo-controlled study. J Sci Food Agric. 2012;92(4):862–9.
Sierra-Ramirez A, Lopez-Aceituno JL, Costa-Machado LF, Plaza A, Barradas M, Fernandez-Marcos PJ. Transient metabolic improvement in obese mice treated with navitoclax or dasatinib/quercetin. Aging (Albany NY). 2020;12(12):11337–48.
Zheng W, Feng Z, You S, Zhang H, Tao Z, Wang Q, et al. Fisetin inhibits IL-1beta-induced inflammatory response in human osteoarthritis chondrocytes through activating SIRT1 and attenuates the progression of osteoarthritis in mice. Int Immunopharmacol. 2017;45:135–47.
Yousefzadeh MJ, Zhu Y, McGowan SJ, Angelini L, Fuhrmann-Stroissnigg H, Xu M, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28.
Nakagawa Y, Mukai S, Yamada S, Matsuoka M, Tarumi E, Hashimoto T, et al. Short-term effects of highly-bioavailable curcumin for treating knee osteoarthritis: a randomized, double-blind, placebo-controlled prospective study. J Orthop Sci. 2014;19(6):933–9.
Okamoto H, Kamatani N. Successful treatment with fenofibrate, a peroxisome proliferator activated receptor alpha ligand, for a patient with rheumatoid arthritis. Ann Rheum Dis. 2004;63(8):1002–3.
Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 2016;15(3):428–35.
Chaganti RK, Tolstykh I, Javaid MK, Neogi T, Torner J, Curtis J, et al. High plasma levels of vitamin C and E are associated with incident radiographic knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(2):190–6.
Wluka AE, Stuckey S, Brand C, Cicuttini FM. Supplementary vitamin E does not affect the loss of cartilage volume in knee osteoarthritis: a 2 year double blind randomized placebo controlled study. J Rheumatol. 2002;29(12):2585–91.
Funding
This work was funded by a grant from the National Institutes of Health and the Common Fund’s Cellular Senescence Network (SenNet) Program, grant number UG3/UH3CA268112.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
- Senescent cells are key mediators of many musculoskeletal (MSK) diseases, such as osteoarthritis, rheumatoid arthritis and osteoporosis, among others.
- The development and clinical approval of new senolytic therapies create an urgent need for biomarkers that can detect senescent cells with clinical imaging tools.
- New beta-gal-sensitive MRI and PET imaging agents can detect senescent cells in arthritic joints and are currently being translated to the clinic.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Daldrup-Link, H.E., Suryadevara, V., Tanyildizi, Y. et al. Musculoskeletal imaging of senescence. Skeletal Radiol (2024). https://doi.org/10.1007/s00256-024-04585-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00256-024-04585-8