Abstract
Objective
This study investigated the distribution and characteristics of various bone and joint lesions on 18F-FAPI PET/CT in lung cancer patients.
Methods
Seventy-four lung cancer patients who underwent 18F-FAPI PET/CT were reviewed. Bone and joint lesions with elevated 18F-FAPI uptake were recorded and analyzed. The distribution and maximum uptake value (SUVmax) of different benign lesions or bone metastases were presented. In addition, the SUVmax of bone metastases on 18F-FDG and 18F-FAPI-04 PET/CT were also compared.
Results
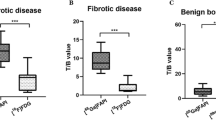
In 53 patients, a total of 262 lesions presented 18F-FAPI accumulation. Bone metastases were mainly in vertebrae, pelvis, and ribs, while benign lesions were in vertebral margins, alveolar bone, and shoulder joints. The SUVmax of bone metastases was significantly higher than that of benign lesions (\(6.6\pm 2.8\) vs. \(4.3\pm 1.6\), \(P<0.001\)), with NSCLC cases having higher SUVmax values than SCLC cases (\(7.3\pm 2.7\) vs. \(3.7\pm 1.1\), \(P<0.001\)). Among benign lesions, arthritis and periodontitis demonstrated higher SUVmax than degenerative lesions (arthritis: \(5.2\pm 2.3\); periodontitis: \(4.8\pm 1.5\); degenerative diseases: \(3.6\pm 0.9\); \(P<0.01\) and \(P<0.05\), respectively). The SUVmax of bone metastases was comparable between 18F-FDG and 18F-FAPI PET/CT. However, 18F-FAPI PET/CT was found to be superior in identifying cranial metastases compared to 18F-FDG PET/CT (TBRmet/brain: \(54.2\pm 13.5\) vs. \(0.6\pm 0.2\), \(P=0.02\)).
Conclusion
This study demonstrated that 18F-FAPI PET/CT is a valuable imaging modality for detecting bone and joint lesions in lung cancer patients. The SUVmax of malignant lesions was higher than that of benign lesions, but cannot accurately distinguish benign and malignant lesions. The uptake of FAPI differs among lesions with different pathological types.




Similar content being viewed by others
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Popper HH. Progression and metastasis of lung cancer. Cancer Metastasis Rev. 2016;35(1):75–91. https://doi.org/10.1007/s10555-016-9618-0.
Bai SB, Liu DZ, Cheng Y, Cui H, Liu M, Cui MX, Zhang BL, Mei QB, Zhou SY. Osteoclasts and tumor cells dual targeting nanoparticle to treat bone metastases of lung cancer. Nanomedicine-Uk. 2019;21:102054. https://doi.org/10.1016/j.nano.2019.102054.
Hernandez RK, Wade SW, Reich A, Pirolli M, Liede A, Lyman GH. Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States. BMC Cancer. 2018;18(1):44. https://doi.org/10.1186/s12885-017-3922-0.
Zhang L, Gong Z. Clinical characteristics and prognostic factors in bone metastases from lung cancer. Med Sci Monit. 2017;23:4087–94. https://doi.org/10.12659/msm.902971.
Cook G, Goh V. Molecular imaging of bone metastases and their response to therapy. J Nucl Med. 2020;61(6):799–806. https://doi.org/10.2967/jnumed.119.234260.
Blake GM, Park-Holohan SJ, Cook GJ, Fogelman I. Quantitative studies of bone with the use of 18F-fluoride and 99mTc-methylene diphosphonate. Semin Nucl Med. 2001;31(1):28–49. https://doi.org/10.1053/snuc.2001.18742.
Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS, Vessella R, Corey E, Padalecki S, Suva L, Chirgwin JM. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res. 2006;12(20 Pt 2):6213s–6s. https://doi.org/10.1158/1078-0432.CCR-06-1007.
Cook GJ, Goh V. Functional and hybrid imaging of bone metastases. J Bone Miner Res. 2018;33(6):961–72. https://doi.org/10.1002/jbmr.3444.
Cook GJ, Azad G, Padhani AR. Bone imaging in prostate cancer: the evolving roles of nuclear medicine and radiology. Clin Transl Imaging. 2016;4(6):439–47. https://doi.org/10.1007/s40336-016-0196-5.
Fitzgerald AA, Weiner LM. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev. 2020;39(3):783–803. https://doi.org/10.1007/s10555-020-09909-3.
Dendl K, Koerber SA, Kratochwil C, Cardinale J, Finck R, Dabir M, Novruzov E, Watabe T, Kramer V, Choyke PL, Haberkorn U, Giesel FL. FAP and FAPI-PET/CT in malignant and non-malignant diseases: a perfect symbiosis? Cancers (Basel) 2021;13(19):4946. https://doi.org/10.3390/cancers13194946
Stein S, Weber J, Nusser-Stein S, Pahla J, Zhang HE, Mohammed SA, Oppi S, Gaul DS, Paneni F, Tailleux A, Staels B, von Meyenn F, Ruschitzka F, Gorrell MD, Lüscher TF, Matter CM. Deletion of fibroblast activation protein provides atheroprotection. Cardiovasc Res. 2021;117(4):1060–9. https://doi.org/10.1093/cvr/cvaa142.
Windisch P, Zwahlen DR, Giesel FL, Scholz E, Lugenbiel P, Debus J, Haberkorn U, Adeberg S. Clinical results of fibroblast activation protein (FAP) specific PET for non-malignant indications: systematic review. Ejnmmi Res. 2021;11(1):18. https://doi.org/10.1186/s13550-021-00761-2.
Song Y, Qin C, Liu F, Lan X. Fibrous dysplasia mimicking skeletal metastasis on 68Ga-FAPI PET imaging. Clin Nucl Med. 2021;46(9):774–5. https://doi.org/10.1097/RLU.0000000000003671.
Wang Y, Yang X, Tian M, Lv H, Liu H. Orbital granulomatous inflammation mimicking malignancy on 68Ga-FAPI PET/CT. Clin Nucl Med. 2022;47(4):380–1. https://doi.org/10.1097/RLU.0000000000003982.
Gungor S, Selçuk NA. Benign bone cyst mimicking bone metastasis demonstrated on 68Ga-FAPI. Clin Nucl Med. 2022;47(1):e95–7. https://doi.org/10.1097/RLU.0000000000003796.
Ji Y, Shao C, Cui Y, Shi D, Su N, Wang Y, Zheng J. Sacral insufficiency fracture after radiotherapy for cervical cancer: appearance and dynamic changes on 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Contrast Media Mol Imaging. 2021;2021:5863530. https://doi.org/10.1155/2021/5863530.
Xu X, Sun ZY, Wu HW, Zhang CP, Hu B, Rong L, Chen HY, Xie HY, Wang YM, Lin HP, Bai YR, Ye Q, Ma XM. Diffusion-weighted MRI and (18)F-FDG PET/CT in assessing the response to neoadjuvant chemoradiotherapy in locally advanced esophageal squamous cell carcinoma. Radiat Oncol. 2021;16(1):132. https://doi.org/10.1186/s13014-021-01852-z.
Qin C, Song Y, Liu X, Gai Y, Liu Q, Ruan W, Liu F, Hu F, Lan X. Increased uptake of (68)Ga-DOTA-FAPI-04 in bones and joints: metastases and beyond. Eur J Nucl Med Mol Imaging. 2022;49(2):709–20. https://doi.org/10.1007/s00259-021-05472-3.
Kessler L, Ferdinandus J, Hirmas N, Zarrad F, Nader M, Kersting D, Weber M, Kazek S, Sraieb M, Hamacher R, Lueckerath K, Umutlu L, Fendler WP, Rischpler C. Pitfalls and common findings in (68)Ga-FAPI PET: a pictorial analysis. J Nucl Med. 2022;63(6):890–6. https://doi.org/10.2967/jnumed.121.262808.
Qin C, Song Y, Liu X, Gai Y, Liu Q, Ruan W, Liu F, Hu F, Lan X. Increased uptake of (68)Ga-DOTA-FAPI-04 in bones and joints: metastases and beyond. Eur J Nucl Med Mol Imaging. 2021. https://doi.org/10.1007/s00259-021-05472-3.
Blüml S, Redlich K, Smolen JS. Mechanisms of tissue damage in arthritis. Semin Immunopathol. 2014;36(5):531–40. https://doi.org/10.1007/s00281-014-0442-8.
Hurnakova J, Filippucci E, Cipolletta E, Di Matteo A, Salaffi F, Carotti M, Draghessi A, Di Donato E, Di Carlo M, Lato V, Horvath R, Komarc M, Pavelka K, Grassi W. Prevalence and distribution of cartilage damage at the metacarpal head level in rheumatoid arthritis and osteoarthritis: an ultrasound study. Rheumatology (Oxford). 2019;58(7):1206–13. https://doi.org/10.1093/rheumatology/key443.
Papadakis GZ, Millo C, Bagci U, Sadowski SM, Stratakis CA. Schmorl Nodes can cause increased 68Ga DOTATATE activity on PET/CT, mimicking metastasis in patients with neuroendocrine malignancy. Clin Nucl Med. 2016;41(3):249–50. https://doi.org/10.1097/RLU.0000000000001065.
Chen YK, Chen HY, Kao CH. Schmorl’s node may cause an increased FDG activity. Clin Nucl Med. 2011;36(6):494–5. https://doi.org/10.1097/RLU.0b013e3182173967.
Mattei TA, Rehman AA. Schmorl’s nodes: current pathophysiological, diagnostic, and therapeutic paradigms. Neurosurg Rev. 2014;37(1):39–46. https://doi.org/10.1007/s10143-013-0488-4.
Levine BD, Motamedi K, Chow K, Gold RH, Seeger LL. CT of rib lesions. AJR Am J Roentgenol. 2009;193(1):5–13. https://doi.org/10.2214/AJR.08.1216.
Smith PC, Martínez C, Martínez J, McCulloch CA. Role of fibroblast populations in periodontal wound healing and tissue remodeling. Front Physiol. 2019;10:270. https://doi.org/10.3389/fphys.2019.00270.
Jeffery AK. Osteophytes and the osteoarthritic femoral head. J Bone Joint Surg Br. 1975;57(3):314–24.
van der Kraan PM, van den Berg WB. Osteophytes: relevance and biology. Osteoarthritis Cartilage. 2007;15(3):237–44. https://doi.org/10.1016/j.joca.2006.11.006.
Liu H, Wang Y, Zhang W, Cai L, Chen Y. Elevated [(68)Ga]Ga-DOTA-FAPI-04 activity in degenerative osteophyte in a patient with lung cancer. Eur J Nucl Med Mol Imaging. 2021;48(5):1671–2. https://doi.org/10.1007/s00259-020-05090-5.
Pang Y, Zhao L, Luo Z, Hao B, Wu H, Lin Q, Sun L, Chen H. Comparison of (68)Ga-FAPI and (18)F-FDG uptake in gastric, duodenal, and colorectal cancers. Radiology. 2021;298(2):393–402. https://doi.org/10.1148/radiol.2020203275.
Funding
The study was supported by funds from the National Natural Science Foundation of China (81872475 and 82073345), Natural Science Innovation and Development Joint Foundation of Shandong (ZR202209010002) and Jinan Clinical Medicine Science and Technology Innovation Plan (202019060), the Natural Science Foundation of Shandong Province (ZR2021QH008), and the Bethune Charitable Foundation (flzh202116).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This retrospective study was approved by the local ethics committee of Shandong Cancer Hospital and Institute (reference number: SDZLEC2021-112–02).
Consent for publication
All the personal data involved in this article have been signed with informed consent.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Parts of this work have been accepted as oral presentation at the 2022 SNMMI Annual Meeting.
Yuchun Wei and Shuanghu Yuan are co-corresponding authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, X., Yan, S., Qin, X. et al. Increased 18F-FAPI uptake in bones and joints of lung cancer patients: characteristics and distributions. Skeletal Radiol 52, 2377–2386 (2023). https://doi.org/10.1007/s00256-023-04335-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-023-04335-2