Abstract
Purpose
Small molecules targeting fibroblast activation protein (FAP) have emerged as a new group of tracers for positron emission tomography (PET) in 2018. While most of the existing literature has been focussed on the application of FAP-specific PET in various kinds of cancers, some researchers have, both intentionally or unintentionally, used FAP-specific PET in patients with non-cancerous diseases. The purpose of this systematic review is therefore to summarize the available evidence of FAP-specific PET for non-malignant indications.
Methods
The MEDLINE database was searched for studies presenting the clinical use of FAP-specific PET, the records were screened according to PRISMA guidelines and articles containing patients suffering from non-malignant diseases were included.
Results
Sixteen studies with 303 patients were included. FAP-specific PET has been used in cardiac imaging, IgG4-related disease, benign tumors as well as various kinds of inflammation. Two prospective studies on FAP-specific PET for IgG4-related disease show its potential to differentiate inflammatory from fibrotic lesions, which could be used to determine the management of these patients.
Conclusion
While publications on FAP-specific PET for non-malignant indications are mostly limited to case reports and incidental findings, the first retrospective and prospective studies present promising results for IgG4-related as well as cardiovascular disease that warrant further research. Several currently recruiting trials will add to the body evidence in the next few years.
Similar content being viewed by others
Introduction
The development of small molecules targeting fibroblast activation protein (FAP) as tracers for positron emission tomography (PET) in 2018 has sparked considerable interest mainly in the oncological community [1, 2].
FAP has dipeptidyl peptidase as well as endopeptidase activity and its expression is limited on normal adult tissue. The expression can, however, increase significantly during tissue modelling, wound healing as well as in diseases such as arthritis, atherosclerosis and different cancers.
While most studies therefore evaluate the application of FAP-specific PET in patients with various kinds of cancers, the use of FAP-specific PET in non-malignant indications has initially been limited to occasional case reports [3].
However, as studies on FAP tracers in cancer began to grow in number and size, the amount of incidental findings of non-malignant diseases began to increase [4]. In addition, the promising results from the early case reports have caused the first dedicated studies on FAP-specific PET for non-malignant indications to be conducted which in turn have been met with more interest [5, 6].
Herein, we therefore conducted a systematic review of the use of FAP-specific PET in non-malignant indications to summarize the available evidence, to indicate areas where the available evidence is limited and to highlight interesting case reports that might inspire future research.
Methods
The review was conducted according to the PRISMA guidelines as applicable [7]. Studies published in English not earlier than 2018 that used FAP-specific PET in humans for any kind of non-malignant indication, either intentionally or unintentionally, were included. No limits regarding size of the patient collective or length of follow-up were applied. The MEDLINE database was searched on September 16th 2020 via the freely accessible PubMed interface. The query was designed to show results whose titles contained either of the words “fibroblast”, “FAP” or “FAPI” in combination with either “positron”, “PET” or “Imaging” (example syntax: “((Fibroblast[Title]) OR (FAP[Title]) OR (FAPI[Title])) AND ((PET[Title]) OR (Positron[Title]) OR (Imaging[Title]))”). After exclusion of duplicates, the titles were screened and only original research publications proceeded to full-text screening. All articles that did not contain any patients suffering from non-malignant disease or did not provide any information in addition to the presence of the disease were excluded. Risk of bias in individual studies was assessed by gathering the conflict of interest (COI) with a concrete relation to the submitted work and funding statements as reported in each publication.
Results
The inclusion workflow is depicted in Fig. 1. The query returned 52 publications and no duplicates. During screening of the records, 18 results were excluded due to presenting only preclinical results [2, 8,9,10,11,12,13,14,15,16,17], being a dataset [18] or a commentary/editorial [19,20,21,22,23,24]. During screening of the full-text articles, 18 articles were excluded as they only provided information on FAP-specific PET in cancer patients [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. Ultimately, 16 articles were included [4,5,6, 43,44,45,46,47,48,49,50,51,52,53,54,55] whose characteristics as well as the respective number of patients are depicted in Table 1.
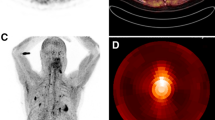
Comparison of 6 patients with IgG4-related disease (IgG4-RD) undergoing FDG and FAP-specific PET. FAP-specific PET detected IgG4-RD in the pancreas (patient #1, 2, 3, 4) bile duct/liver (patient #1, 2, 3), retroperitoneal fibrosis (patient #5), lung/pleura (patient #6), and salivary gland (patient #1, 3). Positive Iymph nodes on FDG PET show no enhancement on FAP-specific PET (patient #2, 3) (Fig. 2). This research was originally published in JNM. Luo et al. Fibroblast activation protein targeted PET/CT with 68Ga-FAPI for imaging IgG4-related disease: comparison to 18F-FDG PET/CT. J Nucl Med. 2020. ©SNMMI [47]
Sources of bias
A conflict of interest (COI) related to the submitted work was present in three publications (19%). A patent application for quinoline-based FAP targeting agents for imaging and therapy in nuclear medicine was the most frequent COI and present in two publications (13%). A funding source was named in nine publications (56%). The most frequent funding source was the Chinese Academy of Medical Sciences (CAMS) Initiative for Innovative Medicine in five publications (31%).
Cardiac imaging
Totzeck et al. published the first case report on cardiac imaging using FAP-specific PET in 2019 [54]. The patient previously diagnosed with metastasized pancreatic adenocarcinoma had a history of coronary artery disease and had received several systemic antineoplastic therapies (gemcitabine, Nab-Paclitaxel, modified FOLFIRINOX). Though he presented without signs of acute or chronic coronary syndromes, FAP-specific PET showed a strong uptake of the left ventricular myocardium which at that time had an ejection fraction of 41%. The authors hypothesize that this enhancement might be a possible sign of FAP activation due to cardiotoxic tissue damage.
Shi et al. performed FAP-specific PET on a patient with nonischemic chronic heart failure (CHF) induced by inflammation and fibrosis activation [44]. The strongest uptake was found in the left ventricular inferior wall and the left atrium (SUVmax = 2.60 and 2.39 respectively). The uptake in the right ventricle and atrium was slightly less (SUVmax = 2.10). The ejection fraction (EF) was slightly better for the right ventricle (left ventricular ejection fraction = 12.7%, right ventricular ejection fraction = 18.2%).
The first study that retrospectively analyzed the activity of FAP in the hearts of a larger collective of patients was published by Heckmann et al. and comprised a total of 229 patients with metastasized cancer in a modeling (185 patients) and a confirmatory cohort (44 patients) [5]. In addition to undergoing FAP-specific PET, patients were screened for multiple cardiovascular risk factors, cardiac medication, antineoplastic systemic therapies and prior radiotherapy to the chest. The multivariate modelling found an association between an increased uptake on FAP-specific PET and a hypothyroid metabolic state, overweight, diabetes mellitus as well as prior radiotherapy to the chest. Focal accumulation of the tracer was associated with coronary artery disease, the presence of cardiovascular risk factors and aspirin intake. While the uptake in patients with only a single cardiovascular risk factor was limited, the combination of risk factors caused a pronounced increase. In addition, a patient scanned while currently undergoing radiotherapy to the thorax showed a strong increase in myocardial tracer uptake.
IgG4-related disease
Luo et al. published a case report of a patient with IgG4-related disease (IgG4-RD), a systemic inflammation associated with the infiltration of IgG4-secreting plasma cells in the inflamed tissue, who underwent FAP-specific PET in 2019 [55]. On FDG PET, the patient exhibited enhancement in multiple lymph nodes in the head and neck as well as the mediastinum, the right lung, the parotid and submandibular gland. While FAP-specific PET also showed enhancement in the submandibular and parotid gland as well as the pulmonary nodules, the FDG-avid lymph nodes were negative but instead there was uptake in the uncinate process of the pancreas.
A case report by Pan et al. reported similar findings regarding FAP-negative FDG-avid lymph nodes, but an enhancement in the lacrimal glands that had not been spotted on FDG PET [52].
Two studies then compared FDG to FAP-specific PET in a prospective setting:
Luo et al. recruited 26 patients with IgG4-RD who underwent both PET imaging with both tracers within one week [47]. The study confirmed the propensity of FAP-specific PET to show involvement in the pancreas, bile duct, liver and lacrimal glands while FDG-avid lymph nodes were mostly negative.
The most recent study by Schmidkonz et al. enrolled 27 patients with IgG4-RD who underwent FDG and FAP-specific PET within two days [6]. In addition, a pathologist scored the extent of inflammation and fibrosis from previous biopsies using a semi-quantitative scale. Seven patients underwent their PET/CTs within two days prior to the initiation of rituximab therapy and repeated them within two days of therapy completion roughly seven months later. While lesions whose histopathology results showed strong lymphoplasmacytic infiltration of IgG4-positive cells had a stronger uptake on FDG PET, lesions with lots of activated fibroblasts expressing FAP were more positive on FAP-specific PET. In addition, antiinflammatory therapy showed only a partial reduction of enhancement in fibrotic lesions.
Tuberculosis
Hao et al. published a case report on a patient with a history of pulmonary tuberculosis and previous anti-tuberculous therapy [46]. When the patient presented with headache, lower back pain as well as limited mobility, a brain MRI revealed multiple enhancing lesions and Mycobacterium tuberculosis was found in the cerebrospinal fluid. FDG PET showed hypermetabolic lesions in the lumbar spine and hilar lymph nodes. Due to high background enhancement of the brain tissue, the lesions discovered on MRI were not visible. FAP-specific PET showed lesions in both lungs, the lower abdomen, lower spine and brain consistent with the findings from the MRI.
In a study by Chen et al., FAP-specific PET was conducted for a variety of indications with inconclusive findings on FDG PET [4]. The two patients with tuberculosis also exhibited a strong uptake in several lesions.
Benign tumors
The description of benign tumors on FAP-specific PET has been limited to occasional cases.
Chen et al. published a case report on a patient with a history of rectal cancer who underwent FDG PET prior to surgery which showed multiple pleural nodules as well as a solitary pulmonary nodule in the left lung with low uptake [51]. FAP-specific PET showed equally low uptake in the pleural nodules but an increased uptake in the pulmonary nodule. A subpleural nodule was biopsied but diagnosed as benign while the pulmonary nodule was found to be a primary adenocarcinoma of the lung.
Zhao et al. published a case report on a patient with cirrhosis and three liver nodules that were found on contrast-enhanced MRI but not visible on FDG PET [50]. While the cirrhotic liver showed an increased background on FAP-specific PET, the nodules showed only low activity and were therefore clearly visible. One of the nodules was diagnosed via biopsy as hepatic adenoma and none of the nodules showed change on a follow-up MRI at three months.
Shi et al. conducted a study on 17 patients presenting with suspicious hepatic lesions on CT, MRI or ultrasound and ultimately underwent surgery or biopsy [48]. The only patient who was confirmed to have ‘benign nodules with granulomatous tissue’ presented with negligible uptake (SUVmax = 0.72). The same applies to patients with hepatic adenoma and pancreatic cystadenoma in the study by Chen et al. [4].
The only publication that showed a moderate uptake for a benign tumor is a case report by Hayrapetian et al. on an infrascapular Elastofibroma dorsi in a patient with esophageal cancer [45].
Other inflammation
Luo et al. published a case report on a patient with pancreatic cancer and tumor-associated pancreatitis [53]. While FDG PET revealed a nodular lesion in the uncinate process, FAP-specific PET showed intense activity in the whole enlarged pancreas masking the lesion that was later confirmed as pancreatic ductal carcinoma.
Pang et al. published a case where FAP-specific PET was able to detect gastric signet cell carcinoma in a patient with a history of prostate cancer [49]. The authors note a bilateral uptake in the adrenal glands which they hypothesize to be hormonotherapy-induced chronic inflammation associated with androgen blockage.
The study by Chen et al. describes high uptake in another patient with pancreatitis and two patients with unspecified “post-treatment inflammatory reaction” but low uptake in two patients with esogastritis [4].
Xu, Zhao, Ding et al. presented a patient with prostate cancer with known arthritis of the left shoulder that also showed intense uptake on FDG as well as FAP-specific PET [43].
Discussion
As FAP-specific PET is a fairly novel imaging modality, the number of prospective studies is still low.
The cases where FAP-specific PET has been used in patients with benign tumors hint at a low tracer uptake. However, benign tumors that contain a large amount of active fibroblasts can exhibit stronger enhancement which is illustrated by the patient with elastofibroma dorsi [45]. Larger, prospective studies are warranted to provide information regarding the accuracy of FAP-specific PET for differentiating malignant from benign and potentially fibrotic lesions in different regions of the body.
Since even the role of FDG PET in tuberculosis is still being discussed, the cases of FAP-specific PET for tuberculosis are insufficient to draw meaningful conclusions. While FAP-specific PET could have an advantage in areas such as the brain where FDG PET shows a high background enhancement, the uptake of FDG in tissue with increased metabolism could be beneficial to reveal new lesions that have not yet undergone fibrotic remodelling. A head-to-head comparison between FAP-specific PET and FDG, ideally with follow-up imaging after the application of anti-tuberculous therapy, could help to clarify the role of FAP-specific PET in this disease. The same applies to other tracers that have been tested for this indication without demonstrating clear superiority or inferiority such as 68Ga-citrate [56].
The fact that FAP-specific PET can show a strong uptake in inflamed tissue has been viewed as a possible obstacle for its application in oncology since determining the extent of a tumor can be difficult when the surrounding tissue is inflamed which is demonstrated by the case report of the patient with tumor-associated pancreatitis [53]. Since increased metabolism is thought to precede the recruitment of activated fibroblasts, future studies could investigate if there is a possible application for FAP-specific PET to differentiate between acute and chronic inflammation and thereby predict the response to antiinflammatory therapy.
In IgG4-RD, FAP-specific PET was able to identify lesions characterized by fibrosis that subsequently responded worse to antiinflammatory therapy compared to lesions that were characterized by inflammation. If adequate treatment options were available for both types of lesions, FAP-specific PET could be used in a trial setting to determine whether it is able to improve the management of these patients.
In cardiac imaging, FAP-specific PET showed an association with various risk factors, especially in combination with each other. Similar results regarding an association of FAPI uptake with coronary artery disease, age and left ventricular ejection fraction were obtained in another retrospective study by Siebermair et al. on 32 cancer patients [57]. Together with preclinical data highlighting the role of FAP in myocardial infarction and FAP-specific PET of small animals, the application of FAP-specific PET in humans might provide more insights into the role of fibroblasts in acute as well as chronic heart disease [17, 58, 59]. Another question of interest could be whether the cardiac FAP signal intensity can serve as another prognostic, maybe even predictive, biomarker in addition to the already established cardiovascular risk factors.
Establishing the role of FAP-specific PET would also benefit from comparisons to other tracers that are mostly being investigated in the role of acute myocardial infarction such as the CXCR4 ligand 68Ga-pentixafor [60,61,62].
The findings that prior radiotherapy to the chest was associated with increased uptake and that a patient currently undergoing radiotherapy to the chest showed an even stronger uptake are particularly interesting from a radiation oncology perspective to better understand and potentially manage treatment-related side effects..
While it has been known for several decades that higher doses to the heart of 30 Gy or more can cause cardiotoxicity within the first years following radiotherapy, investigating the effects of lower doses to the heart is considerably more difficult as the latency period between the application of radiotherapy and the development of heart disease is longer [63]. Radiation-related heart disease can manifest itself as pericarditis, pericardial fibrosis, myocardial fibrosis as well as coronary artery disease and several cardiac avoidance techniques such as heart blocks, prone breast boards and respiratory gating methods have been implemented to reduce its incidence. Being able to non-invasively analyze the activation of fibroblasts during or after radiotherapy could provide more insight into the pathophysiology of radiation-related heart disease and in turn enable radiation oncologists to tailor their therapy to the individual patient. Since sparing organs at risk in close proximity to the target volume requires compromise to the target coverage or the constraints of other organs at risk, one might, for example, investigate if cardiac toxicity could also be reduced by sparing parts of the heart that already show increased fibroblast activation prior to radiotherapy instead of the heart as a whole.
Considering that radiotherapy is a mainstay of treatment for breast cancer as well as in functionally inoperable patients and that many systemic therapies have cardiotoxic side effects as well, progress in the understanding of radiation-related heart disease has the potential to improve outcomes for many patients [64].
With the expanding application of stereotactic body radiation therapy (SBRT) for smaller primary tumors of the lung or in the oligometastatic setting, this potential is likely to increase [65].
In addition to the heart, FAP-specific PET could be used to image fibrotic activity in a variety of organs. A case report by Sonni et al. describes a 36-year-old patient with cervical cancer who underwent FAP-specific PET that showed “symmetric, diffuse, peripheral bilateral breast uptake” that the authors hypothesize to be caused by hormonal stimulation since the patient had recently received gonadotropin injections for oocyte retrieval [66].
The capability of FAP-specific PET to detect fibrotic remodelling non-invasively and potentially earlier than other imaging modalities could be used in a variety of different organs and diseases. Future studies could try to assess whether FAP-specific PET at baseline is better at predicting radiation pneumonitis than conventional pretreatment imaging. In addition, FAP-specific PET might be used to monitor other diseases that are primarily characterized by inflammation and/or fibrotic remodelling such as systemic sclerosis and vasculitis.
In addition to imaging and monitoring disease, molecules targeting FAP have always been of interest for delivering therapies in a targeted fashion. While this interest has historically been focussed on oncology, bringing antiinflammatory drugs to the areas that are the most affected by inflammation and subsequent fibrosis could provide additional treatment options in non-malignant diseases as well by limiting side effects associated with the systemic distribution of antiinflammatory drugs.
Possible limitations at study level include the presence of a patent and/or equity COI in several of the included publications. A possible limitation at review level is that the search was limited to the MEDLINE database which is, however, mitigated by the fact that FAP-specific tracers are currently only used by a limited number of research groups whose results are published in MEDLINE-indexed journals. Another limitation is that the total number of patients who received FAP-specific PET for a given indication cannot be determined exactly as it cannot be excluded that some publications from the same group contain at least a subset of patients that is analyzed more than once. Lastly, co-authors of this review are in part represented as co-authors on one of the included articles [5].
As of September 2020, searching clinicaltrials.gov yielded five recruiting or not yet recruiting prospective trials on FAP-specific PET for non-malignant indications (Table 2). Four studies on rheumatoid arthritis, inflammatory bowel disease, IgG4-RD and Crohn’s disease are estimated to be completed by October 2021, while another trial on FAP-specific PET in liver fibrosis is estimated to be completed by December 2023.
Conclusion
While the research on FAP-specific PET for non-malignant indications is still in the stage of generating hypotheses and not changing practice, the studies that have been published show promising results that warrant further research, especially in cardiac imaging and immunology/rheumatology. Several studies on FAP-specific PET for non-malignant indications will provide additional evidence in the next few years.
Availability of data and materials
All included manuscripts are provided in Table 1. The results of the PubMed query are provided as a txt-file in the supplement.
References
Loktev A, Lindner T, Mier W, Debus J, Altmann A, Jäger D, et al. A Tumor-imaging method targeting cancer-associated fibroblasts. J Nucl Med. 2018;59:1423–9.
Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, Debus J, et al. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med. 2018;59:1415–22.
Windisch P, Zwahlen DR, Koerber SA, Giesel FL, Debus J, Haberkorn U, et al. Clinical results of fibroblast activation protein (FAP) specific PET and implications for radiotherapy planning: systematic review. Cancers. 2020;12:2629.
Chen H, Zhao L, Ruan D, Pang Y, Hao B, Dai Y, et al. Usefulness of [68Ga]Ga-DOTA-FAPI-04 PET/CT in patients presenting with inconclusive [18F]FDG PET/CT findings. Eur J Nucl Med Mol Imaging. 2020. https://doi.org/10.1007/s00259-020-04940-6.
Heckmann MB, Reinhardt F, Finke D, Katus HA, Haberkorn U, Leuschner F, et al. Relationship between cardiac fibroblast activation protein activity by positron emission tomography and cardiovascular disease. Circ Cardiovasc Imaging. 2020;13:e010628.
Schmidkonz C, Rauber S, Atzinger A, Agarwal R, Götz TI, Soare A, et al. Disentangling inflammatory from fibrotic disease activity by fibroblast activation protein imaging. Ann Rheum Dis. 2020. https://doi.org/10.1136/annrheumdis-2020-217408.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Pandya DN, Sinha A, Yuan H, Mutkus L, Stumpf K, Marini FC, et al. Imaging of fibroblast activation protein alpha expression in a preclinical mouse model of glioma using positron emission tomography. Molecules. 2020. https://doi.org/10.3390/molecules25163672.
Moon ES, Elvas F, Vliegen G, De Lombaerde S, Vangestel C, De Bruycker S, et al. Targeting fibroblast activation protein (FAP): next generation PET radiotracers using squaramide coupled bifunctional DOTA and DATA5m chelators. EJNMMI Radiopharm Chem. 2020;5:19.
Hintz HM, Gallant JP, Vander Griend DJ, Coleman I, Nelson PS, LeBeau AM. Imaging fibroblast activation protein alpha improves diagnosis of metastatic prostate cancer with positron emission tomography. Clin Cancer Res. 2020. https://doi.org/10.1158/1078-0432.CCR-20-1358.
Roy J, Hettiarachchi SU, Kaake M, Mukkamala R, Low PS. Design and validation of fibroblast activation protein alpha targeted imaging and therapeutic agents. Theranostics. 2020;10:5778–89.
Schröder MS, Harwardt M-L, Rahm JV, Li Y, Freund P, Dietz MS, et al. Imaging the fibroblast growth factor receptor network on the plasma membrane with DNA-assisted single-molecule super-resolution microscopy. Methods. 2020. https://doi.org/10.1016/j.ymeth.2020.05.004.
Xing J, Gong Q, Zou R, Li Z, Xia Y, Yu Z, et al. A novel fibroblast activation protein-targeted near-infrared fluorescent off-on probe for cancer cell detection, in vitro and in vivo imaging. J Mater Chem B Mater Biol Med. 2018;6:1449–51.
Arakawa A, Jakubowski N, Koellensperger G, Theiner S, Schweikert A, Flemig S, et al. Quantitative imaging of silver nanoparticles and essential elements in thin sections of fibroblast multicellular spheroids by high resolution laser ablation inductively coupled plasma time-of-flight mass spectrometry. Anal Chem. 2019a;91:10197–203.
Arakawa A, Jakubowski N, Koellensperger G, Theiner S, Schweikert A, Flemig S, et al. Imaging of Ag NP transport through collagen-rich microstructures in fibroblast multicellular spheroids by high-resolution laser ablation inductively coupled plasma time-of-flight mass spectrometry. Analyst. 2019b;144:4935–42.
van der Geest T, Roeleveld DM, Walgreen B, Helsen MM, Nayak TK, Klein C, et al. Imaging fibroblast activation protein to monitor therapeutic effects of neutralizing interleukin-22 in collagen-induced arthritis. Rheumatology. 2018;57:737–47.
Varasteh Z, Mohanta S, Robu S, Braeuer M, Li Y, Omidvari N, et al. Molecular imaging of fibroblast activity after myocardial infarction using a 68Ga-labeled fibroblast activation protein inhibitor, FAPI-04. J Nucl Med. 2019;60:1743–9.
Röhrich M, Loi L, Floca R, Haberkorn U, Paech D. Dataset of voxelwise correlated signal values of ADC, rCBV and FAP-specific PET of 13 Glioblastoma patients. Data in Brief. 2020;31:105712.
Calais J, Mona CE. Will FAPI PET/CT replace FDG PET/CT in the next decade?-Point: an important diagnostic, phenotypic and biomarker role. AJR Am J Roentgenol. 2020. https://doi.org/10.2214/AJR.20.24302.
Moradi F, Iagaru A. Will FAPI PET/CT Replace FDG PET/CT in the Next Decade?-Counterpoint: No, not so fast! AJR Am J Roentgenol. 2020. https://doi.org/10.2214/AJR.20.23794.
Thackeray JT. Sound and fibroblast activation protein inhibitor: imaging fibroblast activation in the heart. Circ Cardiovasc Imaging. 2020;13:e011603.
Chen H, Wu H. Reply: [68Ga]Ga-DOTA-FAPI-04 and [18F]FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with hepatic cancer. Eur J Nucl Med Mol Imaging. 2020;47:2080–2.
Zheng J, Yao S. [68Ga]Ga-DOTA-FAPI-04 and [18F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with hepatic cancer. Eur J Nucl Med Mol Imaging. 2020;47:2078–9.
Laffon E, Marthan R. Reversibility of 68Ga-FAPI-2 trapping might prove an asset for PET quantitative imaging. J Nucl Med. 2020;61:620.
Pang Y, Zhao L, Chen H. 68Ga-FAPI outperforms 18F-FDG PET/CT in identifying bone metastasis and peritoneal carcinomatosis in a patient with metastatic breast cancer. Clin Nucl Med. 2020. https://doi.org/10.1097/RLU.0000000000003263.
Liu Q, Shi S, Xu X, Yu X, Song S. The superiority of [68Ga]-FAPI-04 over [18F]-FDG PET/CT in imaging metastatic esophageal squamous cell carcinoma. Eur J Nucl Med Mol Imaging. 2020. https://doi.org/10.1007/s00259-020-04997-3.
Fan C, Guo W, Su G, Chen B, Chen H. Widespread metastatic gastric signet-ring cell carcinoma shown by 68Ga-FAPI PET/CT. Clin Nucl Med. 2020. https://doi.org/10.1097/RLU.0000000000003245.
Hao B, Wu J, Pang Y, Sun L, Chen H. 68Ga-FAPI PET/CT in Assessment of leptomeningeal metastases in a patient with lung adenocarcinoma. Clin Nucl Med. 2020;45:784–6.
Wang G, Jin X, Zhu H, Wang S, Ding J, Zhang Y, et al. 68Ga-NOTA-FAPI-04 PET/CT in a patient with primary gastric diffuse large B cell lymphoma: comparisons with [18F] FDG PET/CT. Eur J Nucl Med Mol Imaging. 2020. https://doi.org/10.1007/s00259-020-04946-0.
Windisch P, Röhrich M, Regnery S, Tonndorf-Martini E, Held T, Lang K, et al. Fibroblast activation protein (FAP) specific PET for advanced target volume delineation in glioblastoma. Radiother Oncol. 2020;150:159–63.
Röhrich M, Floca R, Loi L, Adeberg S, Windisch P, Giesel FL, et al. FAP-specific PET signaling shows a moderately positive correlation with relative CBV and no correlation with ADC in 13 IDH wildtype glioblastomas. Eur J Radiol. 2020;127:109021.
Röhrich M, Loktev A, Wefers AK, Altmann A, Paech D, Adeberg S, et al. IDH-wildtype glioblastomas and grade III/IV IDH-mutant gliomas show elevated tracer uptake in fibroblast activation protein-specific PET/CT. Eur J Nucl Med Mol Imaging. 2019. https://doi.org/10.1007/s00259-019-04444-y.
Syed M, Flechsig P, Liermann J, Windisch P, Haberkorn U, Debus J, et al. Fibroblast activation protein (FAPI) specific PET for advanced target volume delineation in head and neck cancer. Int J Radiat Oncol Biol Phys. 2019;105:383.
Pang Y, Hao B, Shang Q, Sun L, Chen H. Comparison of 68Ga-FAPI and 18F-FDG PET/CT in a patient with cholangiocellular carcinoma: a case report. Clin Nucl Med. 2020;45:566–7.
Chen H, Pang Y, Wu J, Zhao L, Hao B, Wu J, et al. Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur J Nucl Med Mol Imaging. 2020;47:1820–32.
Meyer C, Dahlbom M, Lindner T, Vauclin S, Mona C, Slavik R, et al. Radiation dosimetry and biodistribution of 68Ga-FAPI-46 PET imaging in cancer patients. J Nucl Med. 2019. https://doi.org/10.2967/jnumed.119.236786.
Koerber SA, Staudinger F, Kratochwil C, Adeberg S, Haefner MF, Ungerechts G, et al. The role of FAPI-PET/CT for patients with malignancies of the lower gastrointestinal tract - first clinical experience. J Nucl Med. 2020. https://doi.org/10.2967/jnumed.119.237016.
Giesel F, Adeberg S, Syed M, Lindner T, Jimenez LD, Mavriopoulou E, et al. FAPI-74 PET/CT using either 18F-AlF or cold-kit 68Ga-labeling: biodistribution, radiation dosimetry and tumor delineation in lung cancer patients. J Nucl Med. 2020. https://doi.org/10.2967/jnumed.120.245084.
Khreish F, Rosar F, Kratochwil C, Giesel FL, Haberkorn U, Ezziddin S. Positive FAPI-PET/CT in a metastatic castration-resistant prostate cancer patient with PSMA-negative/FDG-positive disease. Eur J Nucl Med Mol Imaging. 2020;47:2040–1.
Giesel FL, Heussel CP, Lindner T, Röhrich M, Rathke H, Kauczor H-U, et al. FAPI-PET/CT improves staging in a lung cancer patient with cerebral metastasis. Eur J Nucl Med Mol Imaging. 2019;46:1754–5.
Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. 2019;60:801–5.
Giesel F, Kratochwil C, Lindner T, Marschalek M, Loktev A, Lehnert W, et al. FAPI-PET/CT: biodistribution and preliminary dosimetry estimate of two DOTA-containing FAP-targeting agents in patients with various cancers. J Nucl Med. 2018. https://doi.org/10.2967/jnumed.118.215913.
Xu T, Zhao Y, Ding H, Cai L, Zhou Z, Song Z, et al. [68Ga]Ga-DOTA-FAPI-04 PET/CT imaging in a case of prostate cancer with shoulder arthritis. Eur J Nucl Med Mol Imaging. 2020. https://doi.org/10.1007/s00259-020-05028-x.
Shi X, Lin X, Huo L, Li X. Cardiac fibroblast activation in dilated cardiomyopathy detected by positron emission tomography. J Nucl Cardiol. 2020. https://doi.org/10.1007/s12350-020-02315-w.
Hayrapetian A, Girgis MD, Yanagawa J, French SW, Schelbert HR, Auerbach MS, et al. Incidental detection of elastofibroma dorsi with 68Ga-FAPI-46 and 18F-FDG PET/CT in a patient with esophageal cancer. Clin Nucl Med. 2020. https://doi.org/10.1097/RLU.0000000000003218.
Hao B, Wu X, Pang Y, Sun L, Wu H, Huang W, et al. [18F]FDG and [68Ga]Ga-DOTA-FAPI-04 PET/CT in the evaluation of tuberculous lesions. Eur J Nucl Med Mol Imaging. 2020. https://doi.org/10.1007/s00259-020-04941-5.
Luo Y, Pan Q, Yang H, Peng L, Zhang W, Li F. Fibroblast activation protein targeted PET/CT with 68Ga-FAPI for imaging IgG4-related disease: comparison to 18F-FDG PET/CT. J Nucl Med. 2020. https://doi.org/10.2967/jnumed.120.244723.
Shi X, Xing H, Yang X, Li F, Yao S, Zhang H, et al. Fibroblast imaging of hepatic carcinoma with 68Ga-FAPI-04 PET/CT: a pilot study in patients with suspected hepatic nodules. Eur J Nucl Med Mol Imaging. 2020. https://doi.org/10.1007/s00259-020-04882-z.
Pang Y, Huang H, Fu L, Zhao L, Chen H. 68Ga-FAPI PET/CT detects gastric signet-ring cell carcinoma in a patient previously treated for prostate cancer. Clin Nucl Med. 2020;45:632–5.
Zhao L, Gu J, Fu K, Lin Q, Chen H. 68Ga-FAPI PET/CT in assessment of liver nodules in a cirrhotic patient. Clin Nucl Med. 2020. https://doi.org/10.1097/RLU.0000000000003015.
Chen H, Zhao L, Ruan D, Sun L, Lin Q. 68Ga-FAPI PET/CT improves therapeutic strategy by detecting a second primary malignancy in a patient with rectal cancer. Clin Nucl Med. 2020;45:468–70.
Pan Q, Luo Y, Zhang W. Recurrent immunoglobulin G4-related disease shown on 18F-FDG and 68Ga-FAPI PET/CT. Clin Nucl Med. 2020;45:312–3.
Luo Y, Pan Q, Zhang W, Li F. Intense FAPI uptake in inflammation may mask the tumor activity of pancreatic cancer in 68Ga-FAPI PET/CT. Clin Nucl Med. 2020;45:310–1.
Totzeck M, Siebermair J, Rassaf T, Rischpler C. Cardiac fibroblast activation detected by positron emission tomography/computed tomography as a possible sign of cardiotoxicity. Eur Heart J. 2020;41:1060.
Luo Y, Pan Q, Zhang W. IgG4-related disease revealed by 68Ga-FAPI and 18F-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2019;46:2625–6.
Ankrah AO, Lawal IO, Boshomane TMG, Klein HC, Ebenhan T, Dierckx RAJO, et al. Comparison of fluorine(18)-fluorodeoxyglucose and Gallium(68)-citrate PET/CT in patients with tuberculosis. Nuklearmedizin. 2019;58:371–8.
Siebermair J, Köhler MI, Kupusovic J, Nekolla SG, Kessler L, Ferdinandus J, et al. Cardiac fibroblast activation detected by Ga-68 FAPI PET imaging as a potential novel biomarker of cardiac injury/remodeling. J Nucl Cardiol. 2020. https://doi.org/10.1007/s12350-020-02307-w.
Tillmanns J, Hoffmann D, Habbaba Y, Schmitto JD, Sedding D, Fraccarollo D, et al. Fibroblast activation protein alpha expression identifies activated fibroblasts after myocardial infarction. J Mol Cell Cardiol. 2015;87:194–203.
Kaur H, Takefuji M, Ngai CY, Carvalho J, Bayer J, Wietelmann A, et al. Targeted ablation of periostin-expressing activated fibroblasts prevents adverse cardiac remodeling in mice. Circ Res. 2016;118:1906–17.
Lapa C, Reiter T, Werner RA, Ertl G, Wester H-J, Buck AK, et al. [68Ga]Pentixafor-PET/CT for imaging of chemokine receptor 4 expression after myocardial infarction. JACC Cardiovasc Imaging. 2015;8:1466–8.
Reiter T, Kircher M, Schirbel A, Werner RA, Kropf S, Ertl G, et al. Imaging of C-X-C motif chemokine receptor CXCR4 expression after myocardial infarction with [68Ga]Pentixafor-PET/CT in correlation with cardiac MRI. JACC Cardiovasc Imaging. 2018;11:1541–3.
Thackeray JT, Derlin T, Haghikia A, Napp LC, Wang Y, Ross TL, et al. Molecular imaging of the chemokine receptor CXCR4 after acute myocardial infarction. JACC Cardiovasc Imaging. 2015;8:1417–26.
Darby SC, Cutter DJ, Boerma M, Constine LS, Fajardo LF, Kodama K, et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 2010;76:656–65.
Alarid-Escudero F, Blaes AH, Kuntz KM. Trade-offs between efficacy and cardiac toxicity of adjuvant chemotherapy in early-stage breast cancer patients: do competing risks matter? Breast J. 2017;23:401–9.
Gomez DR, Tang C, Zhang J, Blumenschein GR Jr, Hernandez M, Lee JJ, et al. Local consolidative therapy vs maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II randomized study. J Clin Oncol. 2019;37:1558.
Sonni I, Lee-Felker S, Memarzadeh S, Quinn MM, Mona CE, Lückerath K, et al. 68Ga-FAPi-46 diffuse bilateral breast uptake in a patient with cervical cancer after hormonal stimulation. Eur J Nucl Med Mol Imaging. 2020. https://doi.org/10.1007/s00259-020-04947-z.
Funding
No funding was received for this project.
Author information
Authors and Affiliations
Contributions
Data collection and analysis: PW. Writing of the original draft: PW, SA. Review and editing: DZ, FG, ES, PL, JD, UH. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
As this research does not involve human participants and is only aggregating the results of other, previously published studies, an ethics approval was not needed.
Consent for publication
As this research does not involve any identifying information, no consent was needed.
Competing interests
P.W. has a patent application titled ‘Method for detection of neurological abnormalities’ outside of the submitted work. UH and FG have a patent application for quinolone based FAP-targeting agents for imaging and therapy in nuclear medicine. UH and FG also have shares of a consultancy-group for iTheranostics. S.A and J.D. received grants from Accuray International Sàrl outside the submitted work. S.A. and J.D. received grants from Merck Serono GmbH outside the submitted work. S.A. received grants from Novocure GmbH, MSD and Astra Zeneca outside the submitted work, S.A. Novocure GmbH, Actinium Pharmaceuticals, Telix Pharmaceuticals shareholding. J.D. received grants from The Clinical Research Institute GmbH (CRI), View Ray Inc., Accuray Incorporated, RaySearch Laboratories AB, Vision RT limited, Astellas Pharma GmbH, Astra Zeneca GmbH, Solution Akademie GmbH, Ergomed PLC Surrey Research Park, Siemens Healthcare GmbH, Quintiles GmbH, Pharmaceutical Research Associates GmbH, Boehringer Ingelheim Pharma GmbH Co, PTW-Freiburg Dr. Pychlau GmbH and Nanobiotix A.A. outside the submitted work. The other authors declare no conflict of interest. P.L. reports receiving lecture fees from Bayer Vital and Pfizer Pharma and educational support from Boston Scientific and Johnson & Johnson outside the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Windisch, P., Zwahlen, D.R., Giesel, F.L. et al. Clinical results of fibroblast activation protein (FAP) specific PET for non-malignant indications: systematic review. EJNMMI Res 11, 18 (2021). https://doi.org/10.1186/s13550-021-00761-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13550-021-00761-2