Abstract
Objective
The number of shoulder arthroplasties is increasing along with the need for revision surgeries. Determining the stability of the implant is crucial in preoperative planning. This study aims to investigate whether radiolucent lines (RLL) in preoperative radiographs predict component loosening.
Materials and methods
Preoperative radiographs of 93 cases in 88 patients who underwent shoulder arthroplasty revision were evaluated regarding the presence of RLL. Correlation analyses were performed for radiographic findings and demographic factors (age, gender, BMI, prior surgeries) compared to intraoperative findings.
Results
The presence of RLL around the humeral component correlated with loosening (p < 0.001, Phi 0.511), and the distal zones 3 and 5 showed the strongest correlation (Phi 0.536). While RLL in only one zone did not predict loosening (p = 0.337), RLL present in two or more zones showed correlation with loosening (p < 0.001). Risk factors associated with loosening were a higher age at the time of revision surgery (p = 0.030) and the number of zones with RLL (p < 0.001). The glenoid component was loose in 39.0% of the cases; 5.5% of the glenoid components with RLL were stable. Nevertheless, the presence of RLL was highly associated with loosening (p < 0.001, Phi 0.603). A longer time between implantation and revision correlated with loosening of the glenoid component (p = 0.046).
Conclusion
While RLL do not predict loosening of the implant in general, occurrence in more than one zone correlates with loosening. If located in distal zones and with increasing number of zones with RLL, the correlation becomes even stronger and loosening is more likely.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
From 2010 to 2019, the volume of primary shoulder arthroplasties in Germany increased by approximately 14% each year, and a sevenfold increase in case numbers is expected by 2040 [1]. A massive rise in primary implantations is predicted in the United States as well [2]. This will consequently lead to an increase in shoulder arthroplasty revisions.
The reasons necessitating revision surgery are various, including component loosening, periprosthetic fractures, instability, component wear, or periprosthetic infections [3]. The most common cause, according to registry data from the German Shoulder and Elbow Society, is loosening of the humeral or glenoid component [4]. Determining whether an implant is well integrated or loose not only affects the diagnosis but is also crucial for a well-planned strategy for revision surgery for any complications.
If there is consecutive imaging that shows migration over time, loosening seems obvious. Much more common, however, is the sole evidence of radiolucent lines (RLL) between implant and bone. RLL are described in more than 75% of shoulder arthroplasties after 40 months [5] and over 80% after 10 years [6]. The extent to which these RLL are associated with the need for revision surgery is a subject of ongoing debate. The definitions of loosening based on radiographic findings vary in the literature. Kahn et al. defined a “humerus at risk” if RLL are present in three or more zones [7]. Gonzales et al. defined loosening of the shaft as either migration or RLL of > 2 mm around the whole shaft [8]. Loosening was hereby reported in 6% of the cases; however, revision surgery was only required in 1%. Gazielly et al. reported definite radiographic loosening of the glenoid in 15.5%, but only 2.5% required revision surgery [9]. In line with this, the asymptomatic presence of RLL is not necessarily an indication for revision surgery [7-10].
To date, the diagnostic value of RLL to predict the actual clinical stability of an implant in case of a planned revision surgery, regardless of the underlying diagnosis, remains unclear.
This study was designed to answer the following research questions: Can RLL in preoperative radiographs predict loosening of a shoulder arthroplasty prior to revision surgery? What are further risk factors predicting a loose component in shoulder arthroplasty revision?
Materials and methods
Patient cohort
This is a retrospective study on patients who underwent an exchange shoulder arthroplasty at the authors’ institution between 2007 and 2021. Details on the enrollment with in- and exclusion criteria are provided in the flowchart (Fig. 1). 93 shoulders in 88 patients were eligible for inclusion (74% females, 26% males, 72% right shoulders). Implants were 45 reverse shoulder arthroplasties (RSA), 14 anatomic total shoulder arthroplasties (TSA), 31 hemi-arthroplasties (HSA), and 3 proximal humerus replacements (PHR). Further details on demographics are shown in Table 1 and Fig. 2.
Radiographic protocol
Radiographs of the shoulder were obtained prior to revision surgery in all cases (mean 1.8 weeks prior to surgery, range 0–12, standard deviation (SD) 3.0). The radiographic assessment included:
-
1)
True antero-posterior view of the shoulder: The patient is standing, with the arm in neutral-zero-position. The scapula is placed flat on the radiographic cassette, and the body is rotated 30–45° in the frontal plane to the direction of the affected shoulder. The beam path is declined 20°. This radiograph provides the orthogonal view of the joint space and shows the greater tubercle on the margin of the radiograph [11].
-
2)
Outlet-view of the shoulder: The patient is sitting, with the arm hanging down. The affected side is rotated 30° away from the stative. The beam path is aligned tangential to the shoulder plate and in the 15–20° cranio-caudal direction [11].
Definition of radiolucency and loosening
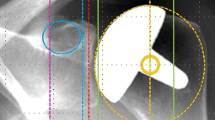
The minimum width of RLL was defined as 2 mm, the localization around the humeral component was classified according to the zones described by Boileau et al. [5]. An example is presented in Fig. 3. The glenoid component was divided into four zones (superior baseplate, inferior baseplate, central peg, screws) in RSA and into three zones (superior, medial, inferior third) in TSA. Loosening of a component was stated according to the intraoperative records.
Antero-posterior radiograph of a right shoulder with RSA. The humeral zones according to the classification described by Boileau et al. are labeled 1–7 [5]. In the presented case, RLL are present in all zones
Imaging analysis
All images and measurements were obtained from a picture archiving and communication system with planning software (AGFA, Mortsel, Belgium).
All radiographs were initially reviewed by two of the authors (one senior attending and one resident). The senior author repeated assessment of 21 radiographs to detect intra-rater reliability. Cases where the reviewers disagreed were discussed by four of the authors (LS, CG, MR, KL).
Statistical analysis
Descriptive statistics were used for demographics and frequencies. A contingence analysis with Pearson chi-square test was performed to test for correlation between categorical variables (dependency loosening on prior implant exchange, type of prosthesis, and type of fixation; presence of RLL). The strength of the correlation was determined with correlation coefficient Phi. Fisher’s exact test was performed to test whether the presence of RLL in one zone correlates with loosening. Binary logistic regression with forward inclusion (Wald) was performed to test for the influence of ordinal/metric variables (age at implantation, age at revision, number of prior surgeries, longevity) on loosening of a component.
Kohen’s Kappa κ was used to measure intra- and interrater reliability on a sample of 21 randomly selected cases. Both intra- and interrater reliability were good, with κ = 0.829, respectively.
Significance level was set at α = 0.050. Statistical analysis was performed with SPSS Statistics 22 (IBM, Armonk, New York, USA). Diagrams were built with Excel version 2202 (Microsoft, Redmont, Washington, USA).
The study has been reviewed by the authors’ Institutional Ethics Committee, and the necessity of approval has been waived due to the study’s retrospective character.
Results
Humeral component
The humeral component was found to be stable intraoperatively in 61 cases (65.6%) and was loose in 32 cases (34.4%). There was no difference between an anatomic design (TSA and HSA) and RSA (p = 0.378). Uncemented humeral components were loose in 35.5% versus 33.9% of cemented components; this difference was not significant (p = 0.877). A history of prior implant exchange was not a risk factor for loosening (p = 0.906). Risk factors associated with loosening of the humeral component were a higher age at the time of revision surgery (p = 0.030) and the number of zones with RLL (p < 0.001). A higher age at the time of implantation showed a tendency to a higher risk for loosening, though this was not significant (p = 0.050). The longevity of the implant (time between implantation and revision) did not correlate with loosening (p = 0.864). The indication for revision had an impact on the rate of loosening of the humeral component (p = 0.018). It was highest for septic loosening (71.4%), followed by aseptic loosening (50.0%), dislocation (33.3%), rotator-cuff related diagnoses (30%), periprosthetic shoulder infections (14.3%), and chronic instability (12.5%).
RLL around the humeral component were present in 28 cases (30.1%). The most common localization was the proximal part of the component where RLL were present in zones 1 and 7 in 25.8%, respectively. Figure 4 depicts the distribution of RLL according to the zones described by Boileau et al. [5] in all cases and only cases with intraoperative loosening. The presence of RLL, in general, correlated with loosening (p < 0.001, Phi 0.511); however, RLL in only one zone did not predict loosening (p = 0.337), but as soon as RLL were present in two or more zones, the correlation with loosening was highly significant (p < 0.001).
Percentage of cases with a loose humeral component (blue)/all cases (green) with radiographic evidence of RLL for each zone according to the classification described by Boileau et al. [5]
Moreover, the strength of this correlation varied depending on the localization of the RLL. The distal zones 3 and 5 showed a slightly stronger correlation (Phi 0.536) than the proximal zones 1 and 7 (Phi 0.504).
Glenoid component
The glenoid component was intraoperatively loose in 23 (39.0%) of the cases. 71.4% of the anatomic glenoids but only 28.9% of the RSA baseplates were loose; this difference was significant (p = 0.004). A longer time between implantation and revision (longevity) correlated with loosening (p = 0.046). Age at implantation, age at revision, history of a prior implant exchange, or the number of prior surgeries did not correlate with loosening (p = 0.111/0.110/0.179/0.492). RLL were present in 17 cases (28.8%). Figure 5 depicts the distribution of RLL to the zones. 94.5% of the glenoids with RLL were intraoperatively loose and 5.5% were stable. Therefore, the presence of glenoidal RLL is not an absolute predictor for glenoid loosening; however, it did correlate with high significance (p < 0.001, Phi 0.603).
Discussion
RLL are a common radiographic finding after shoulder arthroplasty. Rates up to 80% after 10 years are described [6]. This study, specifically reporting on cases prior to arthroplasty revision, detected RLL in 29% of the glenoid components and 30% of the humeral components. The relatively low rate may likely be explained by a shorter period between implantation and radiographic evaluation in the current study design. Furthermore, this study defiened RLL at a minimum width of 2 mm [8, 12]; this was not consistant in early studies on this topic [13, 14].
Most publications on RLL focused on a consecutive cohort of patients following shoulder arthroplasties. The current study stands out by directly comparing radiographic to intraoperative findings.
To date, it remains unclear how radiographic findings are associated with actual loosening of the implant. Furthermore, the radiographic definition of loosening is inconsistent in the literature. For example, Melis et al. defined loosening as RLL > 2 mm in more than three zones [12], Gonzales et al. as RLL > 2 mm around the whole shaft, and Kahn et al. saw the “humerus at risk” if there were any RLL in more than three zones [7]. However, only a small percentage of the patients with RLL seems to present symptoms or require revision surgery. In the cohort reported by Gonzales et al., only 2% of the patients underwent revision while 6% where classified as loose according to radiographic evaluation [8]. Therefore, the asymptomatic presence of RLL cannot generally be equated with loosening or an indication for revision surgery [7-10].
The current study focused on revision cases. Even though there was a significant correlation between RLL and loosening, the study showed that 13.1% of the humeral components, positive for RLL were still well fixed, which is a clinically relevant amount. Thus, the appearance of RLL correlates with loosening but does not guarantee for loosening of an implant. The predictive value of RLL was dependent on its localization and magnitude. RLL in only one zone were not associated with loosening (p = 0.337); in contrast, RLL in two or more zones were (p < 0.001). Furthermore, an increasing number of zones with RLL was associated with a higher risk for a loose implant (p < 0.001). While RLL in the proximal zones (1 and 7) were more common in general, RLL in the distal zones (3 and 5) showed an even stronger correlation with loosening (Phi 0.536). Thus, loosening of the humeral component is most likely if the RLL are located distally and involve multiple zones.
As with the humeral component, there is no consensus on the classification of radiographic findings and the definition of loosening in glenoid components. Due to the different fixation techniques in anatomic glenoids versus reverse baseplates, it stands to reason to use different systems for both implant types. However, the huge variety of classifications in the current literature reduces the comparability of the results even more [2, 12, 15]. Over all, loosening rates of up to 65% after 15 years are described [16]. In the current study, RLL were present in 29% of the cases. In TSA, RLL were most likely to occur in the superior and inferior, rather than the central areas. This is in line with the typical loosening mechanism described by Franklin et al. in 1988 as the rocking horse phenomenon [17]. A more even distribution of RLL was found in RSA baseplates, with only the cranial baseplate being affected slightly less frequently. Regardless of the type of implant, RLL were strongly associated with loosening. However, 5.5% of the cases with RLL turned out well fixed.
It is under debate if other imaging techniques might improve the non-invasive detection of implant loosening. Gregory et al. reported RLL in 54 of 55 observations from computer tomography (CT) scans (11 shoulders, 5 observers) and a higher intra-observer reliability compared to radiographs [18]. Yian et al. reported on 47 TSA, 47% of the RLL visualized by CT scans were not seen on plain radiographs [19]. However, none of the studies compared the imaging findings to intraoperative proof of loosening. Mallo et al. compared CT scans of painful shoulder arthroplasties to arthroscopic findings and reported a sensitivity of 70% and a specificity of 75% for the detection of loosening by CT evaluation [20]. Newer techniques such as SPECT/CT are only reported in case series, which are too small to provide reasonable evidence [21]. A recent study by Broden et al. investigated CT micromotion analysis; at a 24-month follow-up, RLL were detected in all glenoid components [22]. However, the functional outcome, assessed by Constant- and Oxford-Shoulder Score, improved over time [22]. Thus, those imaging techniques might provide a more reliable and sensitive option to detect radiographic signs of potential loosening in shoulder arthroplasties, but the clinical relevance of this finding remains questionable.
The current study also aimed to assess patient-related factors predictive of a loose component at revision surgery. Bacle et al. described a significant association between RLL and failed previous arthroplasty [23]. In contrast, our data showed no correlation between a previous exchange of implant and loosening (p = 906). The only factors identified to correlate with loosening were a higher age at the time of revision surgery for loosening of the humeral component (p = 0.030) and longevity of the implant for loosening of the glenoid component (p = 0.046).
The results presented in this study need to be interpreted against the background that only patients that underwent revision surgery were included. Another limitation is the variety in revision diagnoses and the inclusion of different implant designs.
Conclusion
The findings of this study confirm that RLL are correlated with implant loosening. However, a clinically relevant amount of implants were intraoperatively stable despite the presence of RLL; therefore, RLL do not guarantee loosening. The current results revealed the importance of a detailed analysis of RLL. Implants were more likely to be loose with the presence of RLL in an increasing number of zones and with humeral RLL being located more distally. Having this in mind, conventional radiographs still play a major role in the diagnostic workup and preoperative preparation in revision shoulder arthroplasty. It may be beneficial to also apply this study design of comparing radiographic results to intraoperative findings to alternate imaging techniques and/or combinations of those in order to investigate their clinical relevance.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CT:
-
Computed tomography
- HAS:
-
Hemi shoulder arthroplasty
- PHR:
-
Proximal humerus replacement
- RLL:
-
Radiolucent line/s
- RSA:
-
Reverse shoulder arthroplasty
- SD:
-
Standard deviation
- SPECT:
-
Single photon emission computed tomography
- TSA:
-
Anatomic total shoulder arthroplasty
References
Klug A, Herrmann E, Fischer S, Hoffmann R, Gramlich Y. Projections of primary and revision shoulder arthroplasty until 2040: facing a massive rise in fracture-related procedures. J Clin Med. 2021. https://doi.org/10.3390/jcm10215123.
Padegimas EM, Maltenfort M, Lazarus MD, Ramsey ML, Williams GR, Namdari S. Future patient demand for shoulder arthroplasty by younger patients: national projections. Clin Orthop Relat Res. 2015;473:1860–7. https://doi.org/10.1007/s11999-015-4231-z.
Bohsali KI, Bois AJ, Wirth MA. Complications of Shoulder Arthroplasty. J Bone Joint Surg Am. 2017;99:256–69. https://doi.org/10.2106/JBJS.16.00935.
Kircher J, Ohly B, Fal MF, Magosch P, Mauch F. Analysis of revision shoulder arthroplasty in the German nationwide registry from 2014 to 2018. JSES Int. 2021;5:382–90. https://doi.org/10.1016/j.jseint.2020.12.003.
Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: The Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15:527–40. https://doi.org/10.1016/j.jse.2006.01.003.
Bohsali KI, Wirth MA, Rockwood CA, JR. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88:2279–92. https://doi.org/10.2106/JBJS.F.00125.
Khan A, Bunker TD, Kitson JB. Clinical and radiological follow-up of the Aequalis third-generation cemented total shoulder replacement: a minimum ten-year study. J Bone Joint Surg Br. 2009;91:1594–600. https://doi.org/10.1302/0301-620X.91B12.22139.
Gonzalez J-F, Alami GB, Baque F, Walch G, Boileau P. Complications of unconstrained shoulder prostheses. J Shoulder Elbow Surg. 2011;20:666–82. https://doi.org/10.1016/j.jse.2010.11.017.
Gazielly DF, Scarlat MM, Verborgt O. Long-term survival of the glenoid components in total shoulder replacement for arthritis. Int Orthop. 2015;39:285–9. https://doi.org/10.1007/s00264-014-2637-y.
Grob A, Freislederer F, Marzel A, Audigé L, Schwyzer H-K, Scheibel M. Glenoid component loosening in anatomic total shoulder arthroplasty: association between radiological predictors and clinical parameters-an observational study. J Clin Med. 2021. https://doi.org/10.3390/jcm10020234.
Mauch F, Rickert C, Mauch B, Liem D. Nativradiologische Bildgebung des Schultergelenks. In: Scheibel M, Brunner U, editors. Schulter. Stuttgart, Germany: Georg Thieme Verlag; 2021. p. 86–8.
Melis B, DeFranco M, Lädermann A, Molé D, Favard L, Nérot C, et al. An evaluation of the radiological changes around the Grammont reverse geometry shoulder arthroplasty after eight to 12 years. J Bone Joint Surg Br. 2011;93:1240–6. https://doi.org/10.1302/0301-620X.93B9.25926.
Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6:495–505. https://doi.org/10.1016/s1058-2746(97)90081-1.
Stewart MP, Kelly IG. Total shoulder replacement in rheumatoid disease: 7- to 13-year follow-up of 37 joints. J Bone Joint Surg Br. 1997;79:68–72. https://doi.org/10.1302/0301-620x.79b1.6645.
Walch G, Edwards TB, Boulahia A, Boileau P, Mole D, Adeleine P. The influence of glenohumeral prosthetic mismatch on glenoid radiolucent lines: results of a multicenter study. J Bone Joint Surg Am. 2002;84:2186–91. https://doi.org/10.2106/00004623-200212000-00010.
Young A, Walch G, Boileau P, Favard L, Gohlke F, Loew M, Mole D. A multicentre study of the long-term results of using a flat-back polyethylene glenoid component in shoulder replacement for primary osteoarthritis. J Bone Joint Surg Br. 2011;93:210–6. https://doi.org/10.1302/0301-620X.93B2.25086.
Franklin JL, Barrett WP, Jackins SE, Matsen FA3. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3:39–46.
Gregory T, Hansen U, Khanna M, Mutchler C, Urien S, Amis AA, et al. A CT scan protocol for the detection of radiographic loosening of the glenoid component after total shoulder arthroplasty. Acta Orthop. 2014;85:91–6. https://doi.org/10.3109/17453674.2013.869653.
Yian EH, Werner CML, Nyffeler RW, Pfirrmann CW, Ramappa A, Sukthankar A, Gerber C. Radiographic and computed tomography analysis of cemented pegged polyethylene glenoid components in total shoulder replacement. J Bone Joint Surg Am. 2005;87:1928–36. https://doi.org/10.2106/JBJS.D.02675.
Mallo GC, Burton L, Coats-Thomas M, Daniels SD, Sinz NJ, Warner JJP. Assessment of painful total shoulder arthroplasty using computed tomography arthrography. J Shoulder Elbow Surg. 2015;24:1507–11. https://doi.org/10.1016/j.jse.2015.06.027.
Hirschmann MT, Schmid R, Dhawan R, Skarvan J, Rasch H, Friederich NF, Emery R. Combined single photon emission computerized tomography and conventional computerized tomography: Clinical value for the shoulder surgeons? Int J Shoulder Surg. 2011;5:72–6. https://doi.org/10.4103/0973-6042.86242.
Brodén C, Reilly P, Khanna M, Popat R, Olivecrona H, Griffiths D, et al. CT-based micromotion analysis method can assess early implant migration and development of radiolucent lines in cemented glenoid components: a clinical feasibility study. Acta Orthop. 2022;93:277–83. https://doi.org/10.2340/17453674.2022.1976.
Bacle G, Nove-Josserand L, Garaud P, Walch G. Long-Term Outcomes of Reverse Total Shoulder Arthroplasty: A Follow-up of a Previous Study. J Bone Joint Surg Am. 2017;99:454–61. https://doi.org/10.2106/JBJS.16.00223.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KL is a consultant for Arthrex and Stryker; this did not affect the current work. LS, CG, FG, and MR declare that they have no conflict of interest.
Ethics statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Approval from the Institutional Review Board was obtained and in keeping with the policies for a retrospective review, informed consent was not required.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Streck, L.E., Gaal, C., Gohlke, F. et al. Does radiolucency really predict loose components in revision shoulder arthroplasty?. Skeletal Radiol 52, 1759–1765 (2023). https://doi.org/10.1007/s00256-023-04330-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-023-04330-7