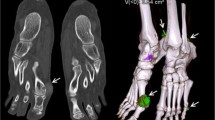
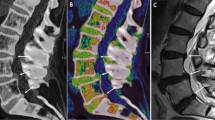
Abstract
Dual energy CT (DECT) is becoming increasingly popular and valuable in the domain of musculoskeletal imaging. Gout maps and crystal detection have been predominant indications for about a decade. Other important indications of bone marrow maps and metal artifact reduction are also frequent with added advantages of detection and characterization of bone marrow lesions similar to MR imaging and diagnosis of hardware related complications, respectively. This article discusses technical considerations and physics of DECT imaging and its role in musculoskeletal indications apart from crystal imaging with respective case examples and review of the related literature. DECT pitfalls in these domains are also highlighted and the reader can gain knowledge of above concepts for prudent use of DECT in their musculoskeletal and general practices.












Similar content being viewed by others
References
Kramer H, Pickhardt PJ, Kliewer MA, et al. Accuracy of liver fat quantification with advanced CT, MRI, and ultrasound techniques: prospective comparison with MR spectroscopy. Am J Roentgenol. 2017;208(1):92–100.
Chhana A, Doyle A, Sevao A, et al. Advanced imaging assessment of gout: comparison of dual-energy CT and MRI with anatomical pathology. Ann Rheum Dis. 2018;77(4):629–30.
Sanghavi PS, Jankharia BG. Applications of dual energy CT in clinical practice: A pictorial essay. Indian J Radiol Imaging. 2019;29(3):289. https://doi.org/10.4103/ijri.IJRI_241_19.
Ko SM, Song MG, Chee HK, Hwang HK, Feuchtner GM, Min JK. Diagnostic performance of dual-energy CT stress myocardial perfusion imaging: direct comparison with cardiovascular MRI. Am J Roentgenol. 2014;203(6):W605–13.
Ohta Y, Kishimoto J, Kitao S, et al. Investigation of myocardial extracellular volume fraction in heart failure patients using iodine map with rapid-kV switching dual-energy CT: Segmental comparison with MRI T1 mapping. J Cardiovasc Comput Tomogr. 2020;14(4):349–55.
Ko SM, Choi JW, Song MG, et al. Myocardial perfusion imaging using adenosine-induced stress dual-energy computed tomography of the heart: comparison with cardiac magnetic resonance imaging and conventional coronary angiography. Eur Radiol. 2011;21(1):26–35.
Hounsfield GN. Computerized transverse axial scanning (tomography) Part 1. Description of system Br J Radiol. 1973;46(552):1016–22.
Millner MR, McDavid WD, Waggener RG, Dennis MJ, Payne WH, Sank VJ. Extraction of information from CT scans at different energies. Med Phys. 1979;6(1):70–1. https://doi.org/10.1118/1.594555.
Di Chiro G, Brooks RA, Kessler RM, et al. Tissue Signatures with Dual-Energy Computed Tomography. Radiology. 1979;131(2):521–3. https://doi.org/10.1148/131.2.521.
Marin D, Boll DT, Mileto A, Nelson RC. State of the art: dual-energy CT of the abdomen. Radiology. 2014;271(2):327–42.
Mallinson PI, Coupal TM, McLaughlin PD, Nicolaou S, Munk PL, Ouellette HA. Dual-energy CT for the musculoskeletal system. Radiology. 2016;281(3):690–707.
Ghazi Sherbaf F, Sair HI, Shakoor D, et al. DECT in Detection of Vertebral Fracture–associated Bone Marrow Edema: A Systematic Review and Meta-Analysis with Emphasis on Technical and Imaging Interpretation Parameters. Radiology. 2021;300(1):110–9.
D’Angelo T, Albrecht MH, Caudo D, et al. Virtual non-calcium dual-energy CT: clinical applications. Eur Radiol Exp. 2021;5(1):1–13.
McCollough CH, Leng S, Yu L, Fletcher JG. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology. 2015;276(3):637–53. https://doi.org/10.1148/radiol.2015142631.
Johnson TR. Dual-energy CT general principles. Am J Roentgenol. 2012;199(5_supplement):S3–8.
Nicolaou S, Liang T, Murphy DT, Korzan JR, Ouellette H, Munk P. Dual-energy CT a promising new technique for assessment of the musculoskeletal system. Am J Roentgenol. 2012;199(5_supplement):S78–86.
Ascenti G, Krauss B, Mazziotti S, et al. Dual-energy computed tomography (DECT) in renal masses: nonlinear versus linear blending. Acad Radiol. 2012;19(10):1186–93.
Yu L, Leng S, McCollough CH. Dual-energy CT–based monochromatic imaging. Am J Roentgenol. 2012;199(5_supplement):S9–15.
Mahgerefteh S, Blachar A, Fraifeld S, Sosna J. Dual-energy derived virtual nonenhanced computed tomography imaging: current status and applications. In: Seminars in Ultrasound, CT and MRI, vol 31. Elsevier; 2010:321–7. https://doi.org/10.1053/j.sult.2010.06.001.
Gosangi B, Mandell JC, Weaver MJ, et al. Bone Marrow Edema at Dual-Energy CT A Game Changer in the Emergency Department. Radiogr Rev Publ Radiol Soc N Am Inc. 2020;40(3):859–74. https://doi.org/10.1148/rg.2020190173.
Rajiah P, Sundaram M, Subhas N. Dual-energy CT in musculoskeletal imaging: what is the role beyond gout? Am J Roentgenol. 2019;213(3):493–505. https://doi.org/10.2214/AJR.19.21095.
Wortman JR, Uyeda JW, Fulwadhva UP, Sodickson AD. Dual-energy CT for abdominal and pelvic trauma. Radiographics. 2018;38(2):586–602.
Pache G, Krauss B, Strohm P, et al. Dual-energy CT virtual noncalcium technique: detecting posttraumatic bone marrow lesions—feasibility study. Radiology. 2010;256(2):617–24.
Reddy T, McLaughlin PD, Mallinson PI, et al. Detection of occult, undisplaced hip fractures with a dual-energy CT algorithm targeted to detection of bone marrow edema. Emerg Radiol. 2015;22(1):25–9.
Dominguez S, Liu P, Roberts C, Mandell M, Richman PB. Prevalence of traumatic hip and pelvic fractures in patients with suspected hip fracture and negative initial standard radiographs—a study of emergency department patients. Acad Emerg Med. 2005;12(4):366–9.
Gotis-Graham I, McGuigan L, Diamond T, et al. Sacral insufficiency fractures in the elderly. J Bone Joint Surg Br. 1994;76(6):882–6.
Grangier C, Garcia J, Howarth NR, May M, Rossier P. Role of MRI in the diagnosis of insufficiency fractures of the sacrum and acetabular roof. Skeletal Radiol. 1997;26(9):517–24.
Shivanna D, Manjunath D, Amaravathi R. Greater arch injuries. J Hand Microsurg. 2014;6(02):69–73.
Ali IT, Wong WD, Liang T, et al. Clinical utility of dual-energy CT analysis of bone marrow edema in acute wrist fractures. Am J Roentgenol. 2018;210(4):842–7.
Kellock TT, Nicolaou S, Kim SS, et al. Detection of bone marrow edema in nondisplaced hip fractures: utility of a virtual noncalcium dual-energy CT application. Radiology. 2017;284(3):798–805.
Kim SJ, Ahn J, Kim HK, Kim JH. Is magnetic resonance imaging necessary in isolated greater trochanter fracture? A systemic review and pooled analysis. BMC Musculoskelet Disord. 2015;16(1):1–6.
Arshad R, Riaz O, Aqil A, Bhuskute N, Ankarath S. Predicting intertrochanteric extension of greater trochanter fractures of the hip on plain radiographs. Injury. 2017;48(3):692–4.
Narayanan A, Dettori N, Chalian M, Xi Y, Komarraju A, Chhabra A. Dual-energy CT-generated bone marrow oedema maps improve timely visualisation and recognition of acute lower extremity fractures. Clin Radiol: Published online; 2021.
Pham T, Azulay-Parrado J, Champsaur P, Chagnaud C, Legré V, Lafforgue P. “Occult” osteoporotic vertebral fractures: vertebral body fractures without radiologic collapse. Spine. 2005;30(21):2430–5.
Karaca L, Yuceler Z, Kantarci M, et al. The feasibility of dual-energy CT in differentiation of vertebral compression fractures. Br J Radiol. 2016;89(1057):20150300.
Cavallaro M, D’Angelo T, Albrecht MH, et al. Comprehensive comparison of dual-energy computed tomography and magnetic resonance imaging for the assessment of bone marrow edema and fracture lines in acute vertebral fractures. Eur Radiol. 2022 Jan;32(1):561–71. https://doi.org/10.1007/s00330-021-08081-8.
Lenchik L, Rogers LF, Delmas PD, Genant HK. Diagnosis of osteoporotic vertebral fractures: importance of recognition and description by radiologists. Am J Roentgenol. 2004;183(4):949–58.
Marshall RA, Weaver MJ, Sodickson A, Khurana B. Periprosthetic femoral fractures in the emergency department: what the orthopedic surgeon wants to know. Radiographics. 2017;37(4):1202–17.
Griffiths EJ, Cash DJW, Kalra S, Hopgood PJ. Time to surgery and 30-day morbidity and mortality of periprosthetic hip fractures. Injury. 2013;44(12):1949–52.
Fukuda T, Umezawa Y, Asahina A, Nakagawa H, Furuya K, Fukuda K. Dual energy CT iodine map for delineating inflammation of inflammatory arthritis. Eur Radiol. 2017;27(12):5034–40.
Diekhoff T, Scheel M, Hermann S, Mews J, Hamm B, Hermann KGA. Osteitis: a retrospective feasibility study comparing single-source dual-energy CT to MRI in selected patients with suspected acute gout. Skeletal Radiol. 2017;46(2):185–90.
Duer-Jensen A, Hørslev-Petersen K, Hetland ML, et al. Bone edema on magnetic resonance imaging is an independent predictor of rheumatoid arthritis development in patients with early undifferentiated arthritis. Arthritis Rheum. 2011;63(8):2192–202.
McQueen FM, Dalbeth N. Predicting joint damage in rheumatoid arthritis using MRI scanning. Arthritis Res Ther. 2009;11:124. https://doi.org/10.1186/ar2778.
Remuzgo-Martínez S, Genre F, López-Mejías R, et al. Expression of osteoprotegerin and its ligands, RANKL and TRAIL, in rheumatoid arthritis. Sci Rep. 2016;6(1):1–5.
Østergaard M, Emery P, Conaghan PG, et al. Significant improvement in synovitis, osteitis, and bone erosion following golimumab and methotrexate combination therapy as compared with methotrexate alone: A magnetic resonance imaging study of 318 methotrexate-naive rheumatoid arthritis patients. Arthritis Rheum. 2011;63(12):3712–22.
Krabbe S, Eshed I, Pedersen SJ, et al. Bone marrow oedema assessment by magnetic resonance imaging in rheumatoid arthritis wrist and metacarpophalangeal joints: the importance of field strength, coil type and image resolution. Rheumatology. 2014;53(8):1446–51.
Burke MC, Garg A, Youngner JM, Deshmukh SD, Omar IM. Initial experience with dual-energy computed tomography-guided bone biopsies of bone lesions that are occult on monoenergetic CT. Skeletal Radiol. 2019;48(4):605–13.
Zheng S, Dong Y, Miao Y, et al. Differentiation of osteolytic metastases and Schmorl’s nodes in cancer patients using dual-energy CT: advantage of spectral CT imaging. Eur J Radiol. 2014;83(7):1216–21.
Dong Y, Zheng S, Machida H, et al. Differential diagnosis of osteoblastic metastases from bone islands in patients with lung cancer by single-source dual-energy CT: advantages of spectral CT imaging. Eur J Radiol. 2015;84(5):901–7.
Kraus M, Weiss J, Selo N, et al. Spinal dual-energy computed tomography: improved visualisation of spinal tumorous growth with a noise-optimised advanced monoenergetic post-processing algorithm. Neuroradiology. 2016;58(11):1093–102.
Barrett JF, Keat N. Artifacts in CT: recognition and avoidance. Radiographics. 2004;24(6):1679–91.
Coupal TM, Mallinson PI, McLaughlin P, Nicolaou S, Munk PL, Ouellette H. Peering through the glare: using dual-energy CT to overcome the problem of metal artefacts in bone radiology. Skeletal Radiol. 2014;43(5):567–75.
Suh JS, Jeong EK, Shin KH, et al. Minimizing artifacts caused by metallic implants at MR imaging: experimental and clinical studies. AJR Am J Roentgenol. 1998;171(5):1207–13.
Bamberg F, Dierks A, Nikolaou K, Reiser MF, Becker CR, Johnson TRC. Metal artifact reduction by dual energy computed tomography using monoenergetic extrapolation. Eur Radiol. 2011;21(7):1424–9. https://doi.org/10.1007/s00330-011-2062-1.
Zhou C, Zhao YE, Luo S, et al. Monoenergetic imaging of dual-energy CT reduces artifacts from implanted metal orthopedic devices in patients with factures. Acad Radiol. 2011;18(10):1252–7.
Wang Y, Qian B, Li B, et al. Metal artifacts reduction using monochromatic images from spectral CT: evaluation of pedicle screws in patients with scoliosis. Eur J Radiol. 2013;82(8):e360–6.
Lewis M, Reid K, Toms AP. Reducing the effects of metal artefact using high keV monoenergetic reconstruction of dual energy CT (DECT) in hip replacements. Skeletal Radiol. 2013;42(2):275–82.
Patel BN, Marin D. Strategies to improve image quality on dual-energy computed tomography. Radiol Clin. 2018;56(4):641–7.
Kim YJ, Cha JG, Kim H, Yi JS, Kim HJ. Dual-energy and iterative metal artifact reduction for reducing artifacts due to metallic hardware: a loosening hip phantom study. Am J Roentgenol. 2019;212(5):1106–11.
Long Z, DeLone DR, Kotsenas AL, et al. Clinical assessment of metal artifact reduction methods in dual-energy CT examinations of instrumented spines. Am J Roentgenol. 2019;212(2):395–401.
Vande Berg BC, Lecouvet FE, Poilvache P, Dubuc JE, Maldague B, Malghem J. Anterior cruciate ligament tears and associated meniscal lesions: assessment at dual-detector spiral CT arthrography. Radiology. 2002;223(2):403–9.
Sun C, Miao F, ming WX, et al. An initial qualitative study of dual-energy CT in the knee ligaments. Surg Radiol Anat. 2008;30(5):443–7.
Wong WD, Shah S, Murray N, Walstra F, Khosa F, Nicolaou S. Advanced musculoskeletal applications of dual-energy computed tomography. Radiol Clin. 2018;56(4):587–600.
Sandhu R, Aslan M, Obuchowski N, Primak A, Karim W, Subhas N. Dual-energy CT arthrography: a feasibility study. Skeletal Radiol. 2021;50(4):693–703.
Jarraya M, Roemer FW, Gale HI, Landreau P, D’Hooghe P, Guermazi A. MR-arthrography and CT-arthrography in sports-related glenolabral injuries: a matched descriptive illustration. Insights Imaging. 2016;7(2):167–77.
Acid S, Le Corroller T, Aswad R, Pauly V, Champsaur P. Preoperative imaging of anterior shoulder instability: diagnostic effectiveness of MDCT arthrography and comparison with MR arthrography and arthroscopy. Am J Roentgenol. 2012;198(3):661–7.
Christie-Large M, Tapp MJF, Theivendran K, James SLJ. The role of multidetector CT arthrography in the investigation of suspected intra-articular hip pathology. Br J Radiol. 2010;83(994):861–7.
Wang CK, Tsai JM, Chuang MT, Wang MT, Huang KY, Lin RM. Bone marrow edema in vertebral compression fractures: detection with dual-energy CT. Radiology. 2013;269(2):525–33.
Patel BN, Alexander L, Allen B, et al. Dual-energy CT workflow: multi-institutional consensus on standardization of abdominopelvic MDCT protocols. Abdom Radiol. 2017;42(3):676–87.
Pache G, Bulla S, Baumann T, et al. Dose reduction does not affect detection of bone marrow lesions with dual-energy CT virtual noncalcium technique. Acad Radiol. 2012;19(12):1539–45.
Palmer WE, Simeone FJ. Can dual-energy CT challenge MR imaging in the diagnosis of focal infiltrative bone marrow lesions? Radiology 2018;286:1, 214–6. https://doi.org/10.1148/radiol.2017172325.
Thomas C, Schabel C, Krauss B, et al. Dual-energy CT: virtual calcium subtraction for assessment of bone marrow involvement of the spine in multiple myeloma. Am J Roentgenol. 2015;204(3):W324–31.
Long Z, Bruesewitz MR, DeLone DR, et al. Evaluation of projection-and dual-energy-based methods for metal artifact reduction in CT using a phantom study. J Appl Clin Med Phys. 2018;19(4):252–60.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cheraya, G., Sharma, S. & Chhabra, A. Dual energy CT in musculoskeletal applications beyond crystal imaging: bone marrow maps and metal artifact reduction. Skeletal Radiol 51, 1521–1534 (2022). https://doi.org/10.1007/s00256-021-03979-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-021-03979-2