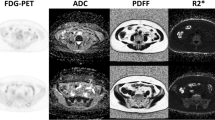
Abstract
Objective
To evaluate the correlation between bone marrow cellularity (BMC) and metabolic activity in healthy subjects and to see whether yellow marrow is indeed metabolically quiescent. Because metabolic activity can be assumed to reflect vascularity, we assessed the relationship between regional metabolic activity and geographic frequency of metastases as noted in the literature.
Materials and methods
Two hundred and twenty locations (ten in each side of the pelvis and proximal femur) were evaluated in 11 consecutive healthy volunteers with simultaneous PET/MR. BMC was calculated through precise water–fat fraction quantification with a 6-echo gradient echo. We analyzed correlations between cellularity and SUVr, age, and R2*. We also looked at the relation between our results and the reported prevalence of metastases.
Results
There was moderate but statistically significant correlation between BMC and metabolic activity (r = 0.636, p < 0.0001). Interestingly, the iliac and sacrum had higher metabolic activity relative to cellularity, whereas the femoral neck and lesser trochanter showed lower SUVr than other regions with the similar cellularity. The relatively lower metabolic status of the femoral neck conflicted with its reported high frequency of metastasis. Excluding regions with almost no remaining red marrow, cellularity showed inverse relationship with age (r = 0.476, p < 0.0001) and direct relationship with R2* (r = 0.532, p < 0.0010).
Conclusions
Metabolic activity of bone marrow was largely dependent on BMC while yellow marrow seems metabolically quiescent. The discrepancy between the assumed vascularity as determined by metabolic activity and reported sites of metastasis suggested that the process of bone metastasis may not depend entirely on vascularity.






Similar content being viewed by others
References
Hwang S, Panicek DM. Magnetic resonance imaging of bone marrow in oncology, part 1. Skelet Radiol. 2007;36(10):913–20.
Navarro SM, Matcuk GR, Patel DB, Skalski M, White EA, Tomasian A, et al. Musculoskeletal imaging findings of hematologic malignancies. Radiographics. 2017;37(3):881–900.
Basu S, Houseni M, Bural G, Chamroonat W, Udupa J, Mishra S, et al. Magnetic resonance imaging based bone marrow segmentation for quantitative calculation of pure red marrow metabolism using 2-deoxy-2-[F-18]fluoro-d-glucose-positron emission tomography: a novel application with significant implications for combined structure-function approach. Mol Imaging Biol. 2007;9(6):361–5.
Budzik JF, Lefebvre G, Forzy G, El Rafei M, Chechin D, Cotten A. Study of proximal femoral bone perfusion with 3D T1 dynamic contrast-enhanced MRI: a feasibility study. Eur Radiol. 2014;24(12):3217–23.
Krishnamurthy GT, Tubis M, Hiss J, Blahd WH. Distribution pattern of metastatic bone disease. A need for total body skeletal image. JAMA. 1977;237(23):2504–6.
Tubiana-Hulin M. Incidence, prevalence and distribution of bone metastases. Bone. 1991;12(Suppl 1):S9–10.
Choi J, Raghavan M. Diagnostic imaging and image-guided therapy of skeletal metastases. Cancer control. 2012;19(2):102–12.
Hardouin P, Rharass T, Lucas S. Bone marrow adipose tissue: to be or not to be a typical adipose tissue? Front Endocrinol (Lausanne). 2016;7:85.
Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012;50(2):546–52.
Schraml C, Schmid M, Gatidis S, Schmidt H, la Fougere C, Nikolaou K, et al. Multiparametric analysis of bone marrow in cancer patients using simultaneous PET/MR imaging: correlation of fat fraction, diffusivity, metabolic activity, and anthropometric data. J Magn Reson Imaging. 2015;42(4):1048–56.
Nakamura-Ishizu A, Takizawa H, Suda T. The analysis, roles and regulation of quiescence in hematopoietic stem cells. Development. 2014;141(24):4656–66.
Suva LJ, Washam C, Nicholas RW, Griffin RJ. Bone metastasis: mechanisms and therapeutic opportunities. Nat Rev Endocrinol. 2011;7(4):208–18.
Lafage-Proust MH, Roche B, Langer M, Cleret D, Vanden Bossche A, Olivier T, et al. Assessment of bone vascularization and its role in bone remodeling. Bonekey rep. 2015;4:662.
Heinonen I, Kemppainen J, Kaskinoro K, Langberg H, Knuuti J, Boushel R, et al. Bone blood flow and metabolism in humans: effect of muscular exercise and other physiological perturbations. J Bone Miner Res. 2013;28(5):1068–74.
MacEwan IJ, Glembotski NE, D'Lima D, Bae W, Masuda K, Rashidi HH, et al. Proton density water fraction as a biomarker of bone marrow cellularity: validation in ex vivo spine specimens. Magn Reson Imaging. 2014;32(9):1097–101.
Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984;153(1):189–94.
Reeder SB, McKenzie CA, Pineda AR, Yu H, Shimakawa A, Brau AC, et al. Water–fat separation with IDEAL gradient-echo imaging. J Magn Reson Imaging. 2007;25(3):644–52.
Ma J. Dixon techniques for water and fat imaging. J Magn Reson Imaging. 2008;28(3):543–58.
Hernando D, Kellman P, Haldar JP, Liang ZP. Robust water/fat separation in the presence of large field inhomogeneities using a graph cut algorithm. Magn Reson Med. 2010;63(1):79–90.
Gheysens O, Postnov A, Deroose CM, Vandermeulen C, de Hoon J, Declercq R, et al. Quantification, variability, and reproducibility of basal skeletal muscle glucose uptake in healthy humans using 18F-FDG PET/CT. J Nucl Med. 2015;56(10):1520–6.
Tsujikawa T, Tsuyoshi H, Kanno M, Yamada S, Kobayashi M, Narita N, et al. Selected PET radiomic features remain the same. Oncotarget. 2018;9(29):20734–46.
Picci P, Manfrini M, Fabbri N, Gambarotti M, Vanel D. Atlas of musculoskeletal tumors and tumorlike lesions. The Rizzoli case archive. Berlin: Springer; 2014. p. 251–2.
Feng H, Wang J, Xu J, Chen W, Zhang Y. The surgical management and treatment of metastatic lesions in the proximal femur: a mini review. Medicine (Baltimore). 2016;95(28):e3892.
Yao WJ, Hoh CK, Hawkins RA, Glaspy JA, Weil JA, Lee SJ, et al. Quantitative PET imaging of bone marrow glucose metabolic response to hematopoietic cytokines. J Nucl Med. 1995;36(5):794–9.
Vande Berg BC, Lecouvet FE, Galant C, Simoni P, Malghem J. Normal variants of the bone marrow at MR imaging of the spine. Semin Musculoskelet Radiol. 2009;13(2):87–96.
Hernando D, Haldar JP, Sutton BP, Ma J, Kellman P, Liang ZP. Joint estimation of water/fat images and field inhomogeneity map. Magn Reson Med. 2008;59(3):571–80.
Rosenkrantz AB, Friedman K, Chandarana H, Melsaether A, Moy L, Ding YS, et al. Current status of hybrid PET/MRI in oncologic imaging. AJR Am J Roentgenol. 2016;206(1):162–72.
Moore SG, Dawson KL. Red and yellow marrow in the femur: age-related changes in appearance at MR imaging. Radiology. 1990;175(1):219–23.
Sambuceti G, Brignone M, Marini C, Massollo M, Fiz F, Morbelli S, et al. Estimating the whole bone-marrow asset in humans by a computational approach to integrated PET/CT imaging. Eur J Nucl Med Mol Imaging. 2012;39(8):1326–38.
Vande Berg BC, Malghem J, Lecouvet FE, Maldague B. Magnetic resonance imaging of the normal bone marrow. Skelet Radiol. 1998;27(9):471–83.
Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8(2):98–101.
Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–8.
Wehrli FW, Ford JC, Haddad JG. Osteoporosis: clinical assessment with quantitative MR imaging in diagnosis. Radiology. 1995;196(3):631–41.
Baum T, Yap SP, Karampinos DC, Nardo L, Kuo D, Burghardt AJ, et al. Does vertebral bone marrow fat content correlate with abdominal adipose tissue, lumbar spine bone mineral density, and blood biomarkers in women with type 2 diabetes mellitus? J Magn Reson Imaging. 2012;35(1):117–24.
Song HK, Wehrli FW, Ma J. Field strength and angle dependence of trabecular bone marrow transverse relaxation in the calcaneus. J Magn Reson Imaging. 1997;7(2):382–8.
Hofmann M, Bezrukov I, Mantlik F, Aschoff P, Steinke F, Beyer T, et al. MRI-based attenuation correction for whole-body PET/MRI: quantitative evaluation of segmentation- and atlas-based methods. J Nucl Med. 2011;52(9):1392–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Fukuda, T., Huang, M., Janardhanan, A. et al. Correlation of bone marrow cellularity and metabolic activity in healthy volunteers with simultaneous PET/MR imaging. Skeletal Radiol 48, 527–534 (2019). https://doi.org/10.1007/s00256-018-3058-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-018-3058-6