Abstract
Aims
The aim of this study was to introduce a new concept for accurate measurement of the global metabolic activity of the red marrow by combining segmented volumetric data from structural imaging techniques such as magnetic resonance imaging (MRI) and quantitative metabolic information provided by functional modalities such as positron emission tomography (PET).
Materials and Methods
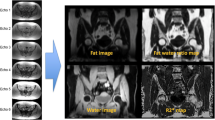
Imaging studies from five subjects who had undergone both MRI and 2-deoxy-2-[F-18]fluoro-d-glucose(FDG)-PET were selected for this analysis to test the feasibility of this approach. In none of the subjects, there were any marrow abnormalities as determined either by the MRI or by FDG-PET studies. The mean blood glucose level was 96 ± 25 mg/dl. The first step was to calculate vertebral volume at L1, L3, and L5 from the available MRI studies. The red and yellow marrows were then segmented within the lumbar vertebrae using the 3DVIEWNIX software system from which the respective volumes were also calculated for each. This also allowed calculating the bone volume in each of the vertebral bodies examined. By employing the standard techniques, the mean of the maximum standardized uptake values (mean SUVmax) for the bone marrow were calculated in L1, L3 and L5 of the lumbar spine, and then global red marrow activity was calculated using the following approach: (1) Whole vertebral metabolic activity (WVMA) = vertebral volume × mean SUVmax of the entire marrow, (2) whole vertebral metabolic activity for yellow marrow (WVMAYM) = yellow marrow volume × mean SUVmax of fat (obtained from measurements of subcutaneous fat), (3) whole vertebral metabolic activity for red marrow (WVMARM) = WVMA − WVMAYM; and finally, (4) SUVmax for pure red marrow = whole vertebral metabolic activity for red marrow (WVMARM)/red marrow volume (obtained from the segmentation data).
Results
The mean volume of the lumbar vertebral body was 15.6 ± 1.4 cm3, the bone marrow mean SUVmax was 1.5 ± 0.3, and the MVP for the lumbar vertebral body was 23.4 ± 5.9. The mean volume of the yellow marrow in the lumbar vertebral body was 7.7 ± 1.1 cm3, the yellow marrow mean SUVmax was estimated to be 0.38 ± 0.1 and the MVP for the yellow marrow in the lumbar vertebral body was 2.9 ± 0.9. The mean volume of the red marrow in lumbar vertebral body was 7.9 ± 1.1 cm3, the red marrow mean SUVmax was estimated to be 2.6 ± 0.6, and the MVP for the red marrow in the lumbar vertebral body was 20.5 ± 5.9.
Conclusion
Estimation of the individual component of the bone marrow is plausible using medical image segmentation with combined structure–function approach. This can have potential research and clinical applications concerning the study of global metabolic activity of the individual component and diagnosis of benign and malignant bone marrow disorders.



Similar content being viewed by others
References
Zaidi H (2006) Medical image segmentation: Quo Vadis. Comput Meth Prog Biomed 84:63–67
Olabarriaga SD, Smeulders AW (2006) Interaction in the segmentation of medical images: A survey. Med Image Anal 5:127–142, 2001 H. Zaidi (ed.), Quantitative analysis in nuclear medicine imaging. Springer: New York
Duncan JS, Ayache N (2000) Medical image analysis: progress over two decades and the challenges ahead. IEEE Trans Patt Anal Mach Intell 22:85–106
Basu S, Zaidi H, Houseni M, Bural G, Udupa J, Acton P, Torigian DA, Alavi A (2007) Novel quantitative techniques for assessing regional and global function and structure based on modern imaging modalities: implications for normal variation, aging and diseased states. Semin Nucl Med 37:223–39 (May)
Montgomery DW, Amira A, Zaidi H (2007) Fully automated segmentation of oncological PET volumes using a combined multiscale and statistical model. Med Phys. 34:722–736 (Feb)
Behloul F, Lelieveldt BP, Boudraa A, et al. (2001) Neuro-fuzzy systems for computer-aided myocardial viability assessment. IEEE Trans Med Imaging 20:1302–1313
Wong K-P, Dagan F, Meikle SR, et al. (2002) Segmentation of dynamic PET images using cluster analysis. IEEE Trans Nucl Sci 49:200–207
Boudraa A, Cexus J-C, Zaidi H (2006) Functional segmentation of dynamic nuclear medicine images by cross-PsiB energy operator. Comput Meth Prog Biomed 84:148–154
Baete K, Nuyts J, Laere KV, et al. (2004) Evaluation of anatomy based reconstruction for partial volume correction in brain FDG-PET. NeuroImage 23:305–317
Ricci C, Cova M, Kang YS, et al. (1990) Normal age-related patterns of cellular and fatty bone marrow distribution in the axial skeleton: MR imaging study. Radiology 177:83–88
Alavi A, Newberg AB, Souder E, et al. (1993) Quantitative analysis of PET and MRI data in normal aging and Alzheimer’s disease: atrophy weighted total brain metabolism and absolute whole brain metabolism as reliable discriminators. J Nucl Med 34:1681–1687
Udupa JK, Odhner D, Samarasekera S, et al. (1994) 3DVIEWNIX: an open, transportable, multidimensional, multimodality, multiparametric imaging software system. In: Kim Y (ed): Medical imaging 1994: image capture, formatting, and display. Vol 2164. Newport Beach, CA, SPIE, pp 58–73
Kricum ME (1985) Red-yellow marrow conversion: its effect on the location of some solitary bone lesions. Skeletal Radiol 14(1):10–19
Bonner H (1988) The blood and the lymphoid organs. In E Rubin, Farber JL, Rubin E, Farber JL: Pathology, Philadelphia, Lipincott
Draffin JE, McFarlane S, Hill A, et al. (2004) CD44 potentiates the adherence of metastatic prostate and breast cancer cells to bone marrow endothelial cells. Cancer Res 64:5702–5711
Acknowledgement
This work was supported in part by the International Union against Cancer (UICC), Geneva, Switzerland, under the ACSBI fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Basu, S., Houseni, M., Bural, G. et al. Magnetic Resonance Imaging Based Bone Marrow Segmentation for Quantitative Calculation of Pure Red Marrow Metabolism Using 2-Deoxy-2-[F-18]fluoro-d-glucose- Positron Emission Tomography: a Novel Application with Significant Implications for Combined Structure–Function Approach. Mol Imaging Biol 9, 361–365 (2007). https://doi.org/10.1007/s11307-007-0112-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-007-0112-5