Abstract
The escalating interest in Bacillus velezensis as a biocontrol agent arises from its demonstrated efficacy in inhibiting both phytopathogenic fungi and bacteria, positioning it as a promising candidate for biotechnological applications. This mini review aims to offer a comprehensive exploration of the multifaceted properties of B. velezensis, with particular focus on its beneficial interactions with plants and its potential for controlling phytopathogenic fungi. The molecular dialogues involving B. velezensis, plants, and phytopathogens are scrutinized to underscore the intricate mechanisms orchestrating these interactions. Additionally, the review elucidates the mode of action of B. velezensis, particularly through cyclic lipopeptides, highlighting their importance in biocontrol and promoting plant growth. The agricultural applications of B. velezensis are detailed, showcasing its role in enhancing crop health and productivity while reducing reliance on chemical pesticides. Furthermore, the review extends its purview in the industrial and environmental arenas, highlighting its versatility across various sectors. By addressing challenges such as formulation optimization and regulatory frameworks, the review aims to chart a course for the effective utilization of B. velezensis.
Key points
• B. velezensis fights phytopathogens, boosting biotech potential
• B. velezensis shapes agri-biotech future, offers sustainable solutions
• Explores plant-B. velezensis dialogue, lipopeptide potential showcased
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the face of mounting concerns regarding the ecological and health implications of chemical pesticides, there has been a notable shift toward exploring sustainable alternatives in agricultural and environmental research (Fan et al. 2018). The escalating interest in biocontrol agents has spurred to significant attention toward harnessing the antagonistic potential of Bacillus velezensis against a wide array of phytopathogenic fungi (Yang et al. 2020; Cheffi Azabou et al. 2020; Torres et al. 2020; Joly et al. 2021; El-Sersawy et al. 2021; Platel et al. 2021; Medhioub et al. 2022; Soliman et al. 2022). The surge in interest is underscored by the urgent need to develop eco-friendly and sustainable strategies for managing plant diseases while minimizing the use of synthetic chemicals.
Formerly known as B. amyloliquefaciens subsp. plantarum, B. velezensis is a Gram-positive, rod-shaped bacterium belonging to the B. subtilis group (Fan et al. 2018; Khan et al. 2020; Zaid et al. 2022). Widely distributed ecologically, B. velezensis has been isolated from various sources, including soil, rhizosphere, and plant surfaces (Fan et al. 2018; Khan et al. 2020). Demonstrating adaptability to diverse environmental conditions and interactions with a wide array of host plants, such as maize, soybean, tomato, and cotton (Yang et al. 2020), this bacterium holds promise for agricultural applications. In this study, we analyzed 47 meticulously selected complete genome sequences of B. velezensis sourced from the NCBI database, chosen based on stringent criteria to ensure comprehensive representation of the full genomes. Each genome corresponds to a distinct strain and its respective host, offering a detailed insight into the ecological associations of B. velezensis. To visually depict the relationship between strains and their respective plant hosts, we constructed a genome-derived phylogenetic tree using the resources provided by the Bacterial and Viral Bioinformatics Resource Center (BV-BRC) and visualized it using the Interactive Tree Of Life (ITOL) platform (https://itol.embl.de/) (Fig. 1).
Phylogenetic relationships among strains of B. velezensis and their respective hosts with Bacillus alcalophilus ATCC 27647 used as the outgroup. The analysis was conducted using 47 complete genome sequences, meticulously selected based on stringent criteria for full genome representation, sourced from the NCBI database. Each genome is linked to a unique strain and its associated host, providing a comprehensive overview of the ecological associations of B. velezensis. The phylogenetic tree was generated using the Bacterial and Viral Bioinformatics Resource Center (BV-BRC), with visualization facilitated by the Interactive Tree Of Life (ITOL) platform (https://itol.embl.de/)
Additionally, B. velezensis has been reported to thrive in diverse environments like compost piles, water bodies, and plant residues (Torres et al. 2020), showcasing its resilience across a spectrum of ecological niches. Its frequent isolation from rhizospheric soils and association with the plant root microbiome highlight its potential as a beneficial plant growth-promoting bacterium (Rabbee et al. 2019). The diverse isolation sources and host interactions of B. velezensis underscore its ecological significance and potential as a valuable asset in agricultural and environmental settings. The strain presents a compelling solution owing to its capacity to outcompete phytopathogens for nutrients and niche space, as well as its ability to produce an array of antimicrobial compounds such as cyclic lipopeptides and polyketides, and elicit systemic resistance in host plants (Rabbee et al. 2019; Fazle Rabbee and Baek 2020). Moreover, its compatibility with integrated pest management practices and its favorable environmental profile further accentuate its role as a pivotal player in sustainable agriculture (Sawant et al. 2023; Chen et al. 2020; Dutilloy et al. 2024). The growing interest in B. velezensis as a biocontrol agent is mirrored by a growing body of research aimed at unraveling the intricate mechanisms underpinning its antagonistic interactions with phytopathogenic fungi. This study paves the way for the development of effective and environmentally sustainable biocontrol strategies in agricultural and environmental contexts. This burgeoning interest not only underscores the potential of B. velezensis as a biocontrol agent but also highlights the need to explore and leverage the ecological and agricultural benefits of microbial-based solutions in the pursuit of sustainable and resilient agroecosystems.
The objective of this review is to provide a thorough examination of B. velezensis’ capabilities to combat phytopathogenic fungi, with a specific emphasis on its molecular interactions with plants and fungi, particularly through cyclic lipopeptides. Additionally, it endeavors to scrutinize the agricultural applications of B. velezensis in promoting crop health and reducing reliance on chemical pesticides. By addressing challenges like formulation optimization and regulatory considerations, the study intends to highlight the crucial role of B. velezensis as a multifaceted ally in contemporary agricultural and industrial practices.
The taxonomic evolution of Bacillus velezensis
Bacillus velezensis was initially characterized by Ruiz-García et al. (2005) following the discovery of novel lipopeptides in a screening of environmental isolates (Ruiz-García et al. 2005). Since its identification, the taxonomic classification of B. velezensis has been a subject of considerable debate and scrutiny within the scientific community. Originally identified as a distinct species within the B. subtilis group, the taxonomic status of B. velezensis has been a matter of ongoing re-evaluation and re-classification. Due to its close resemblance to B. amyloliquefaciens, it was subsequently deemed a later heterotypic synonym of B. amyloliquefaciens by Wang et al. (2008), based on multiple analyses including DNA sequence similarities and DNA–DNA hybridization experiments (Wang et al. 2008). Through genetic analysis, the authors found that the gyrB gene sequences of B. velezensis BCRC 17467 and B. amyloliquefaciens BCRC 14193 strains formed a phylogenetic grouping, indicating their close relationship. This finding was corroborated by phenotypic analyses, which demonstrated shared morphological, physiological, chemotaxonomic, and phylogenetic characteristics between the two taxa. Consequently, B. velezensis was affiliated with B. amyloliquefaciens. However, through comparison analysis process over decades, B. amyloliquefaciens species were then clustered into two “subspecies”: B. amyloliquefaciens subsp. plantarum and B. amyloliquefaciens subsp. amyloliquefaciens (Hossain et al. 2015). Bacillus amyloliquefaciens subsp. plantarum was reported as a distinct ecotype of plant-associated B. amyloliquefaciens strains which should be considered as a later heterotypic synonym of B. velezensis (Borriss et al. 2011; Dunlap et al. 2015; Fan et al. 2017). In their study, Borriss et al. (2011) highlighted that although the strains FZB42 and DSM7 shared similar phenotypic traits, they displayed differences in DNA sequences encoding genes such as 16S rRNA, gyrase subunit A (gyrA), and histidine kinase (cheA). Later, in 2016, Dunlap and colleagues proposed that B. methylotrophicus, B. amyloliquefaciens subsp. plantarum, and B. oryzicola could be regarded as heterotypic synonyms of B. velezensis, while B. amyloliquefaciens subsp. amyloliquefaciens remained distinct. Subsequently, B. amyloliquefaciens subsp. plantarum, B. methylotrophicus, and B. oryzicola were eventually reclassified as strains of B. velezensis (Dunlap et al. 2016).
Additionally, distinctions in their enzymatic and metabolic profiles have been noted (Chun et al. 2019). Bacillus velezensis, for instance, demonstrates a broader enzymatic repertoire, including cellulases, proteases, and lipases, thereby enhancing its metabolic adaptability. This enzymatic diversity holds significant implications for various industrial application, including biofuel production, waste remediation, and food processing (Deb et al. 2013; Ngalimat et al. 2021; Alenezi et al. 2021; Diabankana et al. 2022).
Genes associated with secondary metabolite biosynthesis are more prevalent in B. velezensis, while those related to energy metabolism show enrichment in B. amyloliquefaciens (Chun et al. 2019; Ngalimat et al. 2021). Moreover, B. velezensis exhibits heightened genetic content related to the synthesis of antimicrobial compounds and genes associated with D-galacturonate and D-fructuronate metabolism in contrast to B. amyloliquefaciens and B. siamensis (Ngalimat et al. 2021). Moreover, Rückert et al. (2011) pointed out the presence of an operon involved in xylan degradation (xylA, xynP, xynB, xylR) in B. subtilis and B. velezensis FZB42, contrasting with B. amyloliquefaciens DSM7. An analysis comparing the B. amyloliquefaciens Y2 genome with other B. amyloliquefaciens genomes identified 130 genes unique to subsp. plantarum, absent in subsp. amyloliquefaciens (Rückert et al. 2011).
Therefore, the ongoing reevaluation and reclassification of B. velezensis stem from its notable genetic and phenotypic resemblance to other closely related species, particularly B. amyloliquefaciens (Dunlap et al. 2016). The delineation of B. velezensis from related species is essential for accurate species identification, ecological studies, and the development of targeted applications in agriculture and biotechnology (Adeniji et al. 2019). Nonetheless, the current classification remains a significant limiting factor for comprehensively understanding Bacillus ecology, as many strains remain misclassified in databases.
Interactions at molecular level between Bacillus velezensis, plants, and phytopathogenic fungi
At the molecular level, B. velezensis engages in complex interactions with plants, where plant immunity plays a crucial role in facilitating root or rhizosphere colonization by the bacterium. Plants release nutrient-rich root exudates, which serve as chemoattractants, creating a conducive environment for beneficial microbes to thrive (Upadhyay et al. 2022; Hao et al. 2023). The response of rhizobacteria to these exudates involves complex chemotactic mechanisms, mediated by receptors such as chemotaxis receptors and quorum sensing systems (Aroney et al. 2021). Chemotaxis receptors enable B. velezensis to detect chemical gradients in the rhizosphere, guiding its movement toward nutrients and signaling molecules released by plant roots (Feng et al. 2021).
Quorum sensing systems further facilitate communication among B. velezensis and neighboring bacterial cells, allowing for coordinated responses to changes in the host environment, which are essential for its role as a plant growth-promoting rhizobacterium (Shao et al. 2023). Two-component signal transduction systems, consisting of sensor histidine kinases and response regulators, play a crucial role in perceiving signals from plants and adjusting gene expression accordingly (Fig. 2). Upon detection of specific stimuli, sensor histidine kinases undergo autophosphorylation, initiating a cascade of intracellular signaling events that modulate gene expression.
Sequential events that take place in the rhizosphere following the application of biocontrol agent B. velezensis. The process initiates with the root colonization of B. velezensis facilitated by (i) chemotaxis, (ii) root surface attachment, and subsequent (iii) biofilm formation. Root exudates serve as signals, nutrients, and antimicrobial compounds during the colonization process. Environmental cues trigger the autophosphorylation of the histidine kinase (HK) on a histidine residue, activating a two-component signal transduction pathway. Subsequently, the phosphate group from the histidine is transferred to an aspartate residue on a response regulator (RR), leading to the phosphorylated RR acting as a transcription factor to induce the expression of genes responsive to the stimuli. These molecular reactions commonly result in the activation of a downstream MAP kinase cascade, which, in turn, stimulates the expression of transcription factors regulating genes involved in cellular responses to environmental changes. The antimicrobial compounds produced by B. velezensis exhibit antagonistic effects on pathogenic microbes within the rhizosphere (iv) while simultaneously eliciting plant growth promotion and enhancing defense responses in plants (v)
In the context of tripartite interactions, fungal hyphae release compounds that elicit chemotactic responses from rhizobacteria, contributing to the establishment of an underground “fungal highway” around plant roots. These interactions underscore the complexity of molecular communications between B. velezensis, plants, and phytopathogenic fungi, highlighting the importance of understanding these mechanisms for agricultural and biotechnological applications. After B. velezensis establishes interaction with the plant, the plant’s pattern-recognition receptors (PRRs) identify specific molecules known as microbe-associated molecular patterns (MAMPs) produced by the rhizobacteria (Boller and Felix 2009). This recognition initiates a cascade of biochemical and genetic responses in the plant, triggering the activation of defense pathways and ultimately limiting pathogen proliferation (Fan et al. 2018; Rabbee et al. 2019; Akintayo et al. 2023).
Bacillus velezensis strains have been shown to regulate pathogen proliferation by balancing plant hormone levels, as demonstrated by B. velezensis FZB42. The colonization of plant roots by this strain prevents the entry of foliar pathogens such as Nicotiana benthamiana through stomata by activating the abscisic acid (ABA) and salicylic acid (SA)-regulated pathways, which induce stomatal closure post-pathogen infection (Wu et al. 2018). By modulating plant hormones, beneficial strains can improve plant growth, particularly by stimulating auxin, cytokinin, or gibberellin production while inhibiting ethylene production through 1-aminocyclopropane-1-carboxylate deaminase (ACC deaminase) activity (Santner et al. 2009). For instance, B. velezensis BACO3 was found to increase radish root and leaf weights, likely due to its production of indole-3-acetic acid and ammonia, as well as its ACC deaminase activity (Meng et al. 2016). Another example is B. velezensis FZB42, which has been demonstrated to produce IAA (Idris et al. 2007). Moreover, in the interactions between B. velezensis, plants, and phytopathogenic fungi, a multifaceted communication network exists at the molecular level. This communication involves the secretion of cyclic lipopeptides (CLiPs) by B. velezensis, serving as signaling molecules that can trigger plant defenses, acting as antagonists against phytopathogens, and modulating various biological processes (Lam et al. 2021; Anckaert et al. 2021; Platel et al. 2022).
Bacillus velezensis bioactive metabolites: unveiling the role of polyketides and cyclic lipopeptides
Bacillus velezensis is known for its capacity to produce a diverse array of bioactive metabolites, with approximately 5–8% of its genome dedicated to synthesizing antimicrobial compounds such as volatile organic compounds (VOCs), bacteriocins, hydrolase enzymes, polyketides (PKs), and cyclic lipopeptides (CLiPs) (Chen et al. 2007; Ongena and Jacques 2008; Chun et al. 2019; Anckaert et al. 2021). Several studies have already highlighted the vital role of the VOCs in both stimulating plant growth and exerting anti-pathogenic effects. For instance, B. velezensis G341 could inhibit the mycelial growth of various phytopathogenic fungi by producing dimethyl sulfoxide, 1-butanol, and acetoin, while B. velezensis BAC03 enhances the growth of several crops through the production of acetoin and 2,3-butanediol (Lim et al. 2017; Meng et al. 2016).
Bacillus velezensis exerts its plant protection effect through the production of polyketides (pKs) and cyclic lipopeptides (CLiPs). These CLiPs are amphiphilic molecules featuring a hydrophilic peptide part and a lipophilic lipid part, which contribute to their antifungal properties. Both PKs and CLiPs share the common characteristic of not being produced by ribosomes but assembled by enzymatic complexes known as polyketide synthases (PKS) and nonribosomal peptide synthetases (NRPSs), respectively.
NRPSs are megaenzymes organized into modules composed of adenylation (A), thiolation (T), and condensation (C) domains, with each module responsible for recognizing, activating, and connecting specific amino acid to produce the final peptide (Gao et al. 2018; Challis and Naismith 2004; Weber and Marahiel 2001). These basic domains can be extended by substrate-modifying domains, including domains for substrate epimerization, β hydroxylation, N methylation, and heterocyclic ring formation. Finally, the last module includes the thioesterase (Te) domain responsible for removing the fully assembled peptide from the T domain in the ultimate module (Challis and Naismith 2004). In some circumstances, the Te domain can participate in an intramolecular reaction that results in the formation of a cyclic or partially cyclic peptide.
Compared to polypeptides produced by ribosomal peptide biosynthesis, the primary structure of NRPs is mainly partially or totally cyclic, and the biodiversity of monomers is not limited to the 20 proteogenic amino acids residues. These monomers can include modified versions such as 2-aminoisobutyric acid, hydroxyphenylglycine, and 2,3-dihydroxybenzoic acid (Caboche et al. 2010).
Polyketide biosynthesis, on the other hand, requires at least four domains that can be organized into modules like to those in NRPSs. These domains include acyltransferase (AT), ketosynthase (KS), and thioesterase (Te), with additional domains such as ketoreductase (KR), dehydratase (DH), and enol reductase (ER) modifying the structure (Hertweck 2009; Esmaeel et al. 2016). Bacillus species are also capable of producing NRPS/PKS products synthesized via hybrid gene clusters containing gene encoding both NRPSs and PKs module-containing proteins, like locillomycin and bacillaene metabolites.
Among PKs, bacillaene, difficidin, and macrolactin are the main types with antibacterial properties, effective against various rice pathogens such as Xanthomonas oryzae pv. oryzae and X. oryzae pv. Oryzicola (Anckaert et al. 2021; Rabbee et al. 2019). The production of macrolactin can inhibit cell division and downregulating the transcription of chvB and chvE in Agrobacterium tumefaciens C58 (Chen et al. 2021). Additionally, other studies have also shown that bacillaene exhibits antibiotic properties and regulates biofilm formation, thereby enhancing the bacterium’s defense mechanisms (Li et al. 2021; Erega et al. 2021) (Table 1).
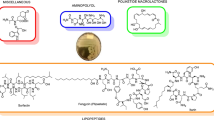
The three main cyclic-lipopeptides characterized in B. velezensis are the families of iturin, fengycin, and surfactin. Notable members within the iturin family include bacillomycin, iturin, and mycosubtilin. Iturins disturb the cytoplasmic membrane, resulting in the leakage of K + ions and other cellular constituents, ultimately leading to cell death (Park et al. 2022). Fengycin compounds, on the other hand, demonstrate efficacy against filamentous fungi by interacting with sterols and phospholipids present in the fungal membrane. Deleu et al. (2005) proposed two mechanisms of action for fengycin, depending on its concentration: at low concentration, fengycin aggregates to form pores, leading to permeability changes in the membrane, while at high concentration, it acts as a detergent, solubilizing the membrane (Deleu et al. 2005). Regarding surfactin, although it may not independently exhibit a potent antifungal impact, it demonstrates synergistic action with the iturin and fengycin families, amplifying the suppression of fungal growth (Mejri et al. 2018) (Table 2).
Lipopeptides are not only powerful antifungal compounds through direct action on the pathogen membrane but are also effective in stimulating induced systemic resistance (ISR) in plants. The complete mechanism of interaction between lipopeptides and the plant cells remains unclear; however, some studies have demonstrated that the recognition is not protein receptor dependent but rather involves a process based on the interaction and penetration of lipopeptides into the lipid bilayer fraction of plant membranes (Crouzet et al. 2020). Consequently, numerous strains of B. velezensis can promote plant defense reaction by stimulating the expression of plant defense genes. For instance, B. velezensis strain BE2 was able to reduce the incidence of Zymoseptoria tritici on wheat and Pyrenophora teres on barley when applied at root level (Dutilloy et al. 2024). Application of the strain BE2 before the infection at the leaf level induced an upregulation of several genes implicated in plant protection. Using ULPC-MS analyses, the authors detected the production of seven metabolite families, including the three CLiPs (Dutilloy et al. 2024). Other studies have established the role of CLiPs in inducing ISR in plant. The mutant AK3, which produces only surfactin, induced systemic resistance in ryegrass, enhancing hydrogen peroxide (H2O2) development, elevating cell wall/apoplastic peroxidase activity, and promoting the deposition of callose and phenolic/polyphenolic compounds (Fan et al. 2018). Additionally, fengycin and iturin produced by B. velezensis were found to be necessary for inducing ISR against rice blast in potting soil and acid sulfate soil conditions (Lam et al. 2021). During this study, it was noted that plants react differently to lipopeptides, likely due to differences in cell membrane composition. Peptide composition also plays a key role in biological activity. For example, lichenysin exhibits higher surfactant power than surfactin, despite the main structural difference being the presence of a glutaminyl residue in position 1 of the peptide sequence in place of glutamic acid in surfactin (Grangemard et al. 2001).
Therefore, B. velezensis strains play a pivotal role within the Bacillus species. Their multifaceted mechanisms for combating the most deleterious pathogens for crops have rendered them a very promising alternative to pesticides for the foreseeable future. Among these mechanisms, the use of cyclic lipopeptides has become a key area of research, with numerous studies focused on optimizing their application in agricultural settings.
Agricultural applications of B. velezensis
As previously discussed, extensive research supports the use of B. velezensis in the agricultural sector as a sustainable alternative to synthetic fertilizers and chemical pesticides. This beneficial bacterium is employed for its plant growth-promoting properties, including the production of antimicrobial compounds, biocontrol of plant pathogens, and enhancement of nutrient uptake, and stress tolerance in diverse crops. Its versatility and effectiveness make it as a promising candidate for sustainable agricultural practices (Table 3).
Bacillus velezensis strains exhibit remarkable antifungal capacity against a wide range of pathogens affecting various plant hosts. As indicated in Fig. 1, which reflects only a subset of characterized strains compared to those registered in the NCBI, B. velezensis interacts with cereals, vegetables, grapevines, and trees, effectively reducing the impact and incidence of necrotrophic (Sawant et al. 2023), biotrophic (Chen et al. 2020), or hemibiotrophic (Dutilloy et al. 2024) pathogens. These strains are particularly studied for their application in farming, as they show efficacy against major pathogens such as Fusarium graminearum, Botrytis cinerea, Plasmodiophora brassicae, or Z. tritici (Zhu et al. 2020; Asaturova et al. 2022; Li et al. 2023).
For instance, B. velezensis strain FZB42 has been shown to produce a range of secondary metabolites, such as cyclic lipopeptides previously described, which exhibit strong antifungal and antibacterial activities (Chowdhury et al. 2015). These antimicrobial compounds not only directly suppress the growth of pathogens but also induce systemic resistance in plants, enhancing their defense mechanisms against diseases (Ongena and Jacques 2008). The underlying mechanism for the inhibition was then investigated where it was shown that bacilysin played a pivotal role in inhibiting the pathogen by damaging its hyphal structures and suppressing relevant gene expression. In addition, the strain FZB42 significantly inhibited the expression of Phytophthora sojae genes related to growth, macromolecule biosynthesis, pathogenicity, and ribosomes. Among them, the genes for pectate lyase were the most significantly downregulated (Han et al. 2021). Moreover, B. velezensis FZB42 significantly inhibited Fusarium graminearum growth by producing bacillomycin D, causing structural damage to fungal hyphae and conidia (Gu et al. 2017). In this context, B. velezensis 83 synthesizes bacillomycin D in vitro, resulting in detrimental effects on the cell membranes of Colletotrichum gloeosporioides, consequently impacting its survivability (Luna-Bulbarela et al., 2018). Originally isolated from the phyllosphere of mango trees in Mexico, this particular strain exhibits efficacy similar to that of chemical treatments such as Captan 50 PH™ or Cupravit hidro™ in the efficient control of anthracnose in Kent mangoes (Balderas-Ruíz et al. 2020). Furthermore, a study highlighted that CLiPs produced by the KB21 strain serve as competent biocontrol agents against plant fungal pathogens, especially pepper anthracnose, due to the production of iturin (Park et al. 2022). Additionally, fluorescent-labeled endophytic B. velezensis CC09 demonstrated disease control efficacy of 66.67% and 21.68% against take-all disease caused by Gaeumannomyces graminis var. tritici and spot blotches of wheat leaves caused by Bipolaris sorokiniana, respectively (Kang et al. 2018). This study suggested that the antifungal activity of iturin A may result from the indirect effect of systemic resistance generated by B. velezensis CC09 in wheat plants (Kang et al. 2018). To reduce the incidence of wheat powdery mildew, Cai et al. (2017) conducted a field trial investigating the efficacy of a bioactive metabolite extract derived from B. velezensis CC09. The application of these metabolites resulted in a substantial 86.12% reduction in the severity of the mildew disease, surpassing the performance of the commercial fungicide triazolone, which achieved a 50.39% reduction when utilized as pretreatment samples (Cai et al. 2017).
Moreover, B. velezensis B5 has demonstrated significant efficacy in inhibiting cabbage fusarium wilt (CFW) caused by Fusarium oxysporum f. sp. conglutinans (Li et al. 2022). Additionally, strain YB-130, through the production of fungal cell wall degrading enzymes, markedly inhibited the growth and hyphal development of Fusarium graminearum PH-1, the causative agent of wheat scab. Furthermore, strain YB-130 exhibited a reduction in deoxynivalenol production by Fusarium graminearum PH-1 through the suppression of key genes such as tri5, tri3, and tri8, essential for deoxynivalenol production (Xu et al. 2020). Likewise, Bacillus sp. MEP2 18, a soil bacterium known for its rich reservoir of bioactive molecules, predominantly synthesizes C16–C17 fengycin and other CLiPs when cultivated under optimized conditions (Medeot et al. 2023).
Previous studies have reported that the inoculation of MEP2 18 strain significantly increased the growth of maize seedlings under normal and saline conditions. Moreover, the cell-free supernatant of MEP218 exhibits potent antifungal properties, effectively suppressing the growth of Fusarium spp. and Sclerotinia spp. This antifungal activity was attributed to the production of the iturin A C15 (Medeot et al. 2017). As for HN_Q_8 strain, it exhibits considerable potential in controlling potato common scab caused by Streptomyces scabies through the production of bacteriocin (Zhao et al. 2022). Another study highlighted its antagonistic effect on Alternaria solani, the causal agent of potato early blight, achieved through the modulation of defensive enzyme activities and the induction of the JA/ET pathway. Additionally, it promotes plant growth by regulating the contents of IAA, GA3, and ABA, enhancing the chlorophyll content, and stimulating root activity (Bai et al. 2023). Husna et al. (2023) reported the strain’s biocontrol efficacy against pathogenic contamination in lettuce hydroponics through surfactin production. Moreover, some studies have shown that endophytes can enhance host resistance to diseases. Hamaoka et al. (2021) found that the endophyte B. velezensis KOF112 induced grapevine defense responses through both salicylic acid- and jasmonic acid-dependent defense pathways. This strain had antagonistic activities against gray mold caused by Botrytis cinerea, anthracnose caused by Colletotrichum gloeosporioides, and downy mildew caused by Plasmopara viticola. Similarly, B. velezensis S3-1, isolated from the rhizosphere soil of cucumber, demonstrated inhibition of plant pathogens, promotion of plant growth, and efficient colonization of rhizosphere soils. The strain produced 13 kinds of CLiPs, including surfactin, iturin, and fengycin families (Jin et al. 2017). A recent study revealed its biocontrol potential against pepper wilt caused by Fusarium sp. F1T, achieved by inhibiting spore germination and colony growth of pathogenic fungus, enhancing the expression levels of defense enzymes genes in pepper, thereby enhancing its disease resistance (Fan et al. 2023). Furthermore, B. velezensis YYC significantly reduced the incidence of bacterial wilt in tomato plants caused by Pseudomonas solanacearum by enhancing plant basal immunity through the increased activity of defense-related genes such as PAL, POD, and SOD (Yan et al. 2022). Regarding verticillium wilt of cotton, B. velezensis AL7 efficiently inhibited the growth of Verticillium dahliae Kleb through fengycin production (Liu et al. 2021). In addition, B. velezensis GUMT319 exhibited a significant effect on grape yields (Chen et al. 2022) and remarkable biocontrol activity against tobacco black shank disease caused by Phytophthora nicotianae (Ding and Mo 2021). The strain ZY1, isolated from pepper leaves, recently demonstrated strong biocontrol efficacy against bacterial fruit blotch (BFB) of Cucurbitaceae (Ji et al. 2023). Lim et al. (2017) reported that B. velezensis strain G341, isolated from Korean ginseng roots, produces diffusible and volatile antifungal compounds effective against various phytopathogenic fungi, including those responsible for rice sheath blight, tomato gray mold, tomato late blight, wheat leaf rust, barley powdery mildew, and red pepper anthracnose (Lim et al. 2017). Additionally, Liang et al. (2021) highlighted the antimicrobial activities of B. velezensis ATR2 strain against ginger rhizome rot disease caused by Bacillus pumilus GR8. Genome analysis revealed that B. velezensis ATR2 harbors a series of genes closely related to promoting plant growth and triggering plant immunity (Liang et al. 2021).
Overall, B. velezensis offers a myriad of agricultural benefits, serving as a biocontrol agent against plant pathogens, enhancing nutrient uptake, promoting plant growth, and improving plant stress tolerance. Its effectiveness and versatility position make it as a promising candidate for sustainable agriculture practices aimed at reducing reliance on chemical inputs and enhancing crop productivity. However, further research and field trials are necessary to fully explore and harness the potential of this bacterium in agricultural systems.
Industrial and environmental applications of B. velezensis
Different strains of B. velezensis have demonstrated potential applications in the degradation of diverse toxic and hazardous industrial byproducts. One of the notable industrial applications of B. velezensis is its ability to produce enzymes of industrial interest. For instance, B. velezensis strain QST713 has been found to produce a range of enzymes, including amylases, proteases, cellulases, and lipases, which have been utilized in various industrial processes (Ngalimat et al. 2021). These enzymes have applications in the food industry for improving the processing of starch, proteins, and lipids, as well as in the production of biofuels, detergents, and other biotechnological products.
Furthermore, B. velezensis has shown promise in the field of bioremediation. This bacterium has the ability to degrade a wide range of organic pollutants, including hydrocarbons, pesticides, and aromatic compounds. For example, B. velezensis has been reported to effectively degrade polycyclic aromatic hydrocarbons (PAHs) and promote the remediation of PAH-contaminated soil (Safitri et al. 2019; Lu et al. 2020; Sultana et al. 2021). Similarly, B. velezensis strain MHNK1 has demonstrated the capability to degrade the herbicide atrazine and has potential applications in the cleanup of atrazine-contaminated environments (Jakinala et al. 2019).
Overall, the industrial and environmental applications of B. velezensis encompass enzyme production, bioremediation, production of bioactive compounds, and promotion of plant growth and soil health. The versatility and beneficial characteristics of B. velezensis make it a promising candidate for various industrial sectors, including biotechnology, environmental remediation, and agriculture. Further research and development are essential to fully exploit the potential of this bacterium and translate it into practical applications that contribute to sustainable industrial processes and environmental management.
Challenges and future perspectives
The widespread adoption of B. velezensis as a biocontrol agent faces several challenges and limitations that must be addressed for successful implementation. One key challenge lies in formulating B. velezensis-based products, similar to most bacterial-based formulations. Although several biocontrol products based on Bacillus strains, including B. velezensis, are already available on the market, ensuring the stability and viability of these products during storage and application is crucial for their effectiveness. Additionally, their efficacy can be negatively impacted by inconsistent environmental conditions such as humidity and temperature, as well as competition with native soil and plant microbiomes, leading to variable performance. Hence, developing efficient and cost-effective formulations that ensure high survival rates and prolonged shelf life remains a significant challenge (Alenezi et al. 2021; Diabankana et al. 2022).
Moreover, regulatory considerations pose hurdles to the widespread adoption. The registration and approval processes for biocontrol agents involve rigorous testing, safety assessments, and compliance with regulatory standards, which can be time-consuming and costly, impeding commercialization and accessibility (Baker et al. 2020; Sundh and Eilenberg 2021; Stenberg et al. 2021; Lahlali et al. 2022; Maral-Gül and Eltem 2024).
To overcome these challenges, future research directions and technological advancements can focus on several aspects. Firstly, optimizing the formulation of B. velezensis-based products to improve shelf life, stability, and delivery methods is imperative. Exploring encapsulation techniques, protective additives, and innovative delivery systems can enhance the survival and efficacy during application. Additionally, further studies are needed to understand the mechanisms underlying the interactions between B. velezensis, target pathogens, and host plants.
Another limitation lies in the efficacy of B. velezensis as a biocontrol agent (Thanh Tam et al. 2023). Although B. velezensis strains produce a diverse array of bioactive metabolites with significant agricultural and biotechnological applications, their production in very small quantities limits their scalability and commercial viability. Addressing this challenge requires innovative approaches such as heterologous expression and other genetic strategies. Introducing biosynthetic gene clusters responsible for metabolite production from B. velezensis into genetically tractable host can overcome limitations in native production. Notably, B. velezensis strain FZB42 has found industrial application as a biocontrol agent in various pesticides (Fan et al. 2018; Put et al. 2024).
Effective collaboration among researchers, industry stakeholders, and regulatory agencies is crucial to streamline regulatory processes. Establishing clear guidelines and standardized protocols for registration and approval of B. velezensis-based products can facilitate their commercialization and market acceptance. Overall, addressing these challenges comprehensively will enhance the biocontrol efficacy of B. velezensis and enable tailored approaches for different agricultural systems and target pathogens.
In conclusion, B. velezensis emerges as a promising candidate for sustainable agricultural practices and biotechnological applications. Its multifaceted interactions with plants and phytopathogenic fungi, coupled with its biocontrol mechanisms mediated by cyclic lipopeptides, highlight its potential as a biocontrol agent and plant growth promoter. The agricultural benefits of using B. velezensis in enhancing crop health and yield, combined with its industrial and environmental applications, highlight its versatility and economic potential. Despite a broad issue existing challenges in deployment, such as formulation complexities and regulatory hurdles, the future outlook for B. velezensis remains promising. Continued research and innovation in harnessing the full potential of this bacterium are crucial for maximizing its impact in sustainable agriculture and environmental management. Bacillus velezensis stands ready to play a pivotal role in shaping the future of agriculture and biotechnology, offering sustainable solutions to complex challenges in modern farming practices.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Adeniji AA, Aremu OS, Babalola OO (2019) Selecting lipopeptide-producing, Fusarium-suppressing Bacillus spp.: metabolomic and genomic probing of Bacillus velezensis NWUMFkBS10.5. MicrobiologyOpen 8:00742. https://doi.org/10.1002/mbo3.742
Akintayo SO, Hosseini B, Vahidinasab M, Messmer M, Pfannstiel J, Bertsche U, Hubel P, Henkel M, Hausmann R, Voegele RT, Lilge L (2023) Characterization of antifungal properties of lipopeptide-producing Bacillus velezensis strains and their proteome-based response to the phytopathogens. Diaporthe Spp Front Bioeng Biotechnol 11:1228386. https://doi.org/10.3389/fbioe.2023.1228386
Alenezi F, Ben Slama H, Chenari Bouket A, Cherif-Silini H, Silini A, Luptakova L, Nowakowska J, Oszako T, Lassaad B (2021) Bacillus velezensis: a treasure house of bioactive compounds of medicinal, biocontrol and environmental importance. Forests 12:1714. https://doi.org/10.3390/f12121714
Anckaert A, Arias AA, Hoff G, Calonne-Salmon M, Declerck S, Ongena M (2021) The use of Bacillus spp. as bacterial biocontrol agents to control plant diseases. Microbial bioprotectants for plant disease management. Burleigh Dodds Series in Agricultural Science. Burleigh Dodds Science Publishing Limited, pp 247–300. https://doi.org/10.19103/as.2021.0093.10
Arima K, Kakinuma A, Tamura G (1968) Surfactin, a crystalline peptidelipid surfactant produced by Bacillus subtilis: isolation, characterization and its inhibition of fibrin clot formation. Biochem Biophys Res Commun 31:488–494. https://doi.org/10.1016/0006-291X(68)90503-2
Aroney STN, Poole PS, Sánchez-Cañizares C (2021) Rhizobial chemotaxis and motility systems at work in the soil. Front Plant Sci 12:725338. https://doi.org/10.3389/fpls.2021.725338
Asaturova AM, Zhevnova NA, Tomashevich NS, Sidorova TM, Homyak AI, Dubyaga VM, Nadykta VD, Zharikov AP, Kostyukevich YI, Tupertsev BS (2022) Evaluation of Bacillus velezensis biocontrol potential against Fusarium fungi on winter wheat. Agronomy 12:1956. https://doi.org/10.3390/agronomy12081956
Bai X, Li Q, Zhang D, Zhao Y, Zhao D, Pan Y, Wang J, Yang Z, Zhu J (2023) Bacillus velezensis strain HN-Q-8 induced resistance to Alternaria solani and stimulated growth of potato plant. Biology 12:856. https://doi.org/10.3390/biology12060856
Baker BP, Green TA, Loker AJ (2020) Biological control and integrated pest management in organic and conventional systems. Biol Control 140:104095. https://doi.org/10.1016/j.biocontrol.2019.104095
Balderas-Ruíz KA, Bustos P, Santamaria RI, González V, Cristiano-Fajardo SA, Barrera-Ortíz S, Mezo-Villalobos M, Aranda-Ocampo S, Guevara-García ÁA, Galindo E, Serrano-Carreón L (2020) Bacillus velezensis 83 a bacterial strain from mango phyllosphere, useful for biological control and plant growth promotion. AMB Express 10:163. https://doi.org/10.1186/s13568-020-01101-8
Besson F, Hourdou M-L, Michel G (1990) Studies on the biosynthesis of iturin, an antibiotic of Bacillus subtilis, and alipopeptide containing β-hydroxy fatty acids. Biochim Biophys Acta BBA - Gen Subj 1036:101–106. https://doi.org/10.1016/0304-4165(90)90020-W
Boller T, Felix G (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60:379–406. https://doi.org/10.1146/annurev.arplant.57.032905.105346
Borriss R, Chen X-H, Rueckert C, Blom J, Becker A, Baumgarth B, Fan B, Pukall R, Schumann P, Spröer C, Junge H, Vater J, Pühler A, Klenk H-P (2011) Relationship of Bacillus amyloliquefaciens clades associated with strains DSM 7T and FZB42T: a proposal for Bacillus amyloliquefaciens subsp. amyloliquefaciens subsp. nov. and Bacillus amyloliquefaciens subsp. plantarum subsp. nov. based on complete genome sequence comparisons. Int J Syst Evol Microbiol 61:1786–1801. https://doi.org/10.1099/ijs.0.023267-0
Caboche S, Leclère V, Pupin M, Kucherov G, Jacques P (2010) Diversity of monomers in nonribosomal peptides: towards the prediction of origin and biological activity. J Bacteriol 192:5143. https://doi.org/10.1128/JB.00315-10
Cai X-C, Liu C-H, Wang B-T, Xue Y-R (2017) Genomic and metabolic traits endow Bacillus velezensis CC09 with a potential biocontrol agent in control of wheat powdery mildew disease. Microbiol Res 196:89–94. https://doi.org/10.1016/j.micres.2016.12.007
Challis GL, Naismith JH (2004) Structural aspects of non-ribosomal peptide biosynthesis. Curr Opin Struct Biol 14:748–756. https://doi.org/10.1016/j.sbi.2004.10.005
Cheffi Azabou M, Gharbi Y, Medhioub I, Ennouri K, Barham H, Tounsi S, Triki MA (2020) The endophytic strain Bacillus velezensis OEE1: an efficient biocontrol agent against Verticillium wilt of olive and a potential plant growth promoting bacteria. Biol Control 142:104168. https://doi.org/10.1016/j.biocontrol.2019.104168
Chen XH, Koumoutsi A, Scholz R, Eisenreich A, Schneider K, Heinemeyer I, Morgenstern B, Voss B, Hess WR, Reva O, Junge H, Voigt B, Jungblut PR, Vater J, Süssmuth R, Liesegang H, Strittmatter A, Gottschalk G, Borriss R (2007) Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat Biotechnol 25:1007–1014. https://doi.org/10.1038/nbt1325
Chen L, Wang X, Ma Q, Bian L, Liu X, Xu Y, Zhang H, Shao J, Liu Y (2020) Bacillus velezensis CLA178-induced systemic resistance of Rosa multiflora against crown gall disease. Front Microbiol 11:587667. https://doi.org/10.3389/fmicb.2020.587667
Chen L, Wang X, Liu Y (2021) Contribution of macrolactin in Bacillus velezensis CLA178 to the antagonistic activities against Agrobacterium tumefaciens C58. Arch Microbiol 203:1743–1752. https://doi.org/10.1007/s00203-020-02141-1
Chen X, Yang F, Bai C, Shi Q, Hu S, Tang X, Peng L, Ding H (2022) Bacillus velezensis strain GUMT319 reshapes soil microbiome biodiversity and increases grape yields. Biology 11:1486. https://doi.org/10.3390/biology11101486
Chen Z, Wang Z, Xu W (2024) Bacillus velezensis WB induces systemic resistance in watermelon against Fusarium wilt. Pest Manag Sci 80:1423–1434. https://doi.org/10.1002/ps.7873
Chowdhury SP, Hartmann A, Gao X, Borriss R (2015) Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42 – a review. Front Microbiol 6:137829. https://doi.org/10.3389/fmicb.2015.00780
Chun BH, Kim KH, Jeong SE, Jeon CO (2019) Genomic and metabolic features of the Bacillus amyloliquefaciens group– B. amyloliquefaciens, B. velezensis, and B. siamensis– revealed by pan-genome analysis. Food Microbiol 77:146–157. https://doi.org/10.1016/j.fm.2018.09.001
Crouzet J, Arguelles-Arias A, Dhondt-Cordelier S, Cordelier S, Pršić J, Hoff G, Mazeyrat-Gourbeyre F, Baillieul F, Clément C, Ongena M, Dorey S (2020) Biosurfactants in plant protection against diseases: rhamnolipids and lipopeptides case study. Front Bioeng Biotechnol 8:1014. https://doi.org/10.3389/fbioe.2020.01014
Deb P, Talukdar SA, Mohsina K, Sarker PK, Sayem SA (2013) Production and partial characterization of extracellular amylase enzyme from Bacillus amyloliquefaciens P-001. Springerplus 2:154. https://doi.org/10.1186/2193-1801-2-154
Deleu M, Paquot M, Nylander T (2005) Fengycin interaction with lipid monolayers at the air–aqueous interface—implications for the effect of fengycin on biological membranes. J Colloid Interface Sci 283:358–365. https://doi.org/10.1016/j.jcis.2004.09.036
Diabankana RGC, Shulga EU, Validov SZ, Afordoanyi DM (2022) Genetic characteristics and enzymatic activities of Bacillus velezensis KS04AU as a stable biocontrol agent against phytopathogens. Int J Plant Biol 13:201–222. https://doi.org/10.3390/ijpb13030018
Ding H, Mo W (2021) Whole genome sequence of Bacillus velezensis strain GUMT319: a potential biocontrol agent against tobacco black shank disease. Front Microbiol 12. https://doi.org/10.3389/fmicb.2021.658113
Duitman EH, Hamoen LW, Rembold M, Venema G, Seitz H, Saenger W, Bernhard F, Reinhardt R, Schmidt M, Ullrich C, Leenders F, Vater J (1999) The mycosubtilin synthetase of Bacillus subtilis ATCC6633: a multifunctional hybrid between a peptide synthetase, an amino transferase, and a fatty acid synthase. Proc Natl Acad Sci U S A 96:13294–13299. https://doi.org/10.1073/pnas.96.23.13294
Dunlap CA, Kim S-J, Kwon S-W, Rooney AP (2015) Phylogenomic analysis shows that Bacillus amyloliquefaciens subsp. plantarum is a later heterotypic synonym of Bacillus methylotrophicus. Int J Syst Evol Microbiol 65:2104–2109. https://doi.org/10.1099/ijs.0.000226
Dunlap CA, Kim S-J, Kwon S-W, Rooney AP (2016) Bacillus velezensis is not a later heterotypic synonym of Bacillus amyloliquefaciens; Bacillus methylotrophicus, Bacillus amyloliquefaciens subsp. plantarum and “Bacillus oryzicola” are later heterotypic synonyms of Bacillus velezensis based on phylogenomics. Int J Syst Evol Microbiol 66:1212–1217. https://doi.org/10.1099/ijsem.0.000858
Dutilloy E, Arguelles Arias A, Richet N, Guise J-F, Duban M, Leclère V, Selim S, Jacques P, Jacquard C, Clément C, Ait Barka E, Esmaeel Q (2024) Bacillus velezensis BE2 controls wheat and barley diseases by direct antagonism and induced systemic resistance. Appl Microbiol Biotechnol 108:1–24. https://doi.org/10.1007/s00253-023-12864-y
El-Sersawy MM, Hassan SE-D, El-Ghamry AA, El-Gwad AMA, Fouda A (2021) Implication of plant growth-promoting rhizobacteria of Bacillus spp. as biocontrol agents against wilt disease caused by Fusarium oxysporum Schlecht. in Vicia faba L. Biomol Concepts 12:197–214. https://doi.org/10.1515/bmc-2021-0020
Erega A, Stefanic P, Dogsa I, Danevčič T, Simunovic K, Klančnik A, Smole Možina S, Mandic Mulec I (2021) Bacillaene mediates the inhibitory effect of Bacillus subtilis on Campylobacter jejuni biofilms. Appl Environ Microbiol 87:e0295520. https://doi.org/10.1128/AEM.02955-20
Esmaeel Q, Pupin M, Kieu NP, Chataigné G, Béchet M, Deravel J, Krier F, Höfte M, Jacques P, Leclère V (2016) Burkholderia genome mining for nonribosomal peptide synthetases reveals a great potential for novel siderophores and lipopeptides synthesis. MicrobiologyOpen 5:512–526. https://doi.org/10.1002/mbo3.347
Fan B, Blom J, Klenk H-P, Borriss R (2017) Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis form an “operational group B. amyloliquefaciens” within the B. subtilis species complex. Front Microbiol 8:22. https://doi.org/10.3389/fmicb.2017.00022
Fan B, Wang C, Song X, Ding X, Wu L, Wu H, Gao X, Borriss R (2018) Bacillus velezensis FZB42 in 2018: the Gram-positive model strain for plant growth promotion and biocontrol. Front Microbiol 9:2491. https://doi.org/10.3389/fmicb.2018.02491
Fan Y, He X, Dai J, Yang N, Jiang Q, Xu Z, Tang X, Yu Y, Xiao M (2023) Induced resistance mechanism of Bacillus velezensis S3–1 against pepper wilt. Curr Microbiol 80:367. https://doi.org/10.1007/s00284-023-03470-2
Fazle Rabbee M, Baek K-H (2020) Antimicrobial activities of lipopeptides and polyketides of Bacillus velezensis for agricultural applications. Molecules 25:4973. https://doi.org/10.3390/molecules25214973
Feng H, Fu R, Hou X, Lv Y, Zhang N, Liu Y, Xu Z, Miao Y, Krell T, Shen Q, Zhang R (2021) Chemotaxis of beneficial rhizobacteria to root exudates: the first step towards root–microbe rhizosphere interactions. Int J Mol Sci 22:6655. https://doi.org/10.3390/ijms22136655
Gao L, Guo J, Fan Y, Ma Z, Lu Z, Zhang C, Zhao H, Bie X (2018) Module and individual domain deletions of NRPS to produce plipastatin derivatives in Bacillus subtilis. Microb Cell Factories 17:84. https://doi.org/10.1186/s12934-018-0929-4
Grangemard I, Wallach J, Maget-Dana R, Peypoux F (2001) Lichenysin: a more efficient cation chelator than surfactin. Appl Biochem Biotechnol 90:199–210. https://doi.org/10.1385/abab:90:3:199
Gu Q, Yang Y, Yuan Q, Shi G, Wu L, Lou Z, Huo R, Wu H, Borriss R, Gao X (2017) Bacillomycin D produced by Bacillus amyloliquefaciens is involved in the antagonistic interaction with the plant-pathogenic fungus Fusarium graminearum. Appl Environ Microbiol 83:e01075-e1117. https://doi.org/10.1128/AEM.01075-17
Hamaoka K, Suzuki S (2021) Complete genome sequence of Bacillus velezensis KOF112, an antifungal endophytic isolate from shoot xylem of the indigenous japanese wine grape Vitis sp. cv. Koshu. Microbiol Resour Announc 10:e0042221. https://doi.org/10.1128/MRA.00422-21
Hamaoka K, Aoki Y, Suzuki S (2021) Isolation and characterization of endophyte Bacillus velezensis KOF112 from grapevine shoot xylem as biological control agent for fungal diseases. Plants 10:1815. https://doi.org/10.3390/plants10091815
Han X, Shen D, Xiong Q, Bao B, Zhang W, Dai T, Zhao Y, Borriss R, Fan B (2021) The plant-beneficial rhizobacterium Bacillus velezensis FZB42 controls the soybean pathogen Phytophthora sojae due to bacilysin production. Appl Environ Microbiol 87:e0160121. https://doi.org/10.1128/AEM.01601-21
Hao Y, Zhang J, Sun C, Chen X, Wang Y, Lu H, Chen J, Shi Z, Zhang L, Yang L, Huang S (2023) Thymol induces cell death of Fusarium oxysporum f. sp. niveum via triggering superoxide radical accumulation and oxidative injury in vitro. Agronomy 13:189. https://doi.org/10.3390/agronomy13010189
Hertweck C (2009) The biosynthetic logic of polyketide diversity. Angew Chem Int Ed Engl 48:4688–4716. https://doi.org/10.1002/anie.200806121
Hossain MJ, Ran C, Liu K, Ryu C-M, Rasmussen-Ivey CR, Williams MA, Hassan MK, Choi S-K, Jeong H, Newman M, Kloepper JW, Liles MR (2015) Deciphering the conserved genetic loci implicated in plant disease control through comparative genomics of Bacillus amyloliquefaciens subsp. plantarum. Front Plant Sci 6:631. https://doi.org/10.3389/fpls.2015.00631
Husna K-E, Won M-H, Jeong M-I, Oh K-K, Park DS (2023) Characterization and genomic insight of surfactin-producing Bacillus velezensis and its biocontrol potential against pathogenic contamination in lettuce hydroponics. Environ Sci Pollut Res Int 30:121487–121500. https://doi.org/10.1007/s11356-023-30871-4
Idris EE, Iglesias DJ, Talon M, Borriss R (2007) Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol Plant-Microbe Interactions 20:619–626. https://doi.org/10.1094/MPMI-20-6-0619
Jakinala P, Lingampally N, Kyama A, Hameeda B (2019) Enhancement of atrazine biodegradation by marine isolate Bacillus velezensis MHNK1 in presence of surfactin lipopeptide. Ecotoxicol Environ Saf 182:109372. https://doi.org/10.1016/j.ecoenv.2019.109372
Ji W, Yang Y, Liu D, Wang C, Yu C, Guan W, Zhao T (2023) Complete genome sequence of Bacillus velezensis strain ZY1, a potential biological control agent for bacterial fruit blotch. PhytoFrontiers 3:734–737. https://doi.org/10.1094/PHYTOFR-03-23-0030-A
Jin Q, Jiang Q, Zhao L, Su C, Li S, Si F, Li S, Zhou C, Mu Y, Xiao M (2017) Complete genome sequence of Bacillus velezensis S3–1, a potential biological pesticide with plant pathogen inhibiting and plant promoting capabilities. J Biotechnol 259:199–203. https://doi.org/10.1016/j.jbiotec.2017.07.011
Joly P, Calteau A, Wauquier A, Dumas R, Beuvin M, Vallenet D, Crovadore J, Cochard B, Lefort F, Berthon J-Y (2021) From strain characterization to field authorization: highlights on Bacillus velezensis strain B25 beneficial properties for plants and its activities on phytopathogenic fungi. Microorganisms 9:1924. https://doi.org/10.3390/microorganisms9091924
Kang X, Zhang W, Cai X, Zhu T, Xue Y, Liu C (2018) Bacillus velezensis CC09: a potential ‘vaccine’ for controlling wheat diseases. Mol Plant-Microbe Interactions 31:623–632. https://doi.org/10.1094/MPMI-09-17-0227-R
Khan MS, Gao J, Chen X, Zhang M, Yang F, Du Y, Moe TS, Munir I, Xue J, Zhang X (2020) The endophytic bacteria Bacillus velezensis Lle-9, isolated from Lilium leucanthum, harbors antifungal activity and plant growth-promoting effects. J Microbiol Biotechnol 30:668–680. https://doi.org/10.4014/jmb.1910.10021
Kim E-N, Gao M, Choi H, Jeong G-S (2020) Marine microorganism-derived macrolactins inhibit inflammatory mediator effects in lps-induced macrophage and microglial cells by regulating BACH1 and HO-1/Nrf2 signals through inhibition of TLR4 Activation. Molecules 25:656. https://doi.org/10.3390/molecules25030656
Lahlali R, Ezrari S, Radouane N, Kenfaoui J, Esmaeel Q, El Hamss H, Belabess Z, Barka EA (2022) Biological control of plant pathogens: a global perspective. Microorganisms 10:596. https://doi.org/10.3390/microorganisms10030596
Lam VB, Meyer T, Arias AA, Ongena M, Oni FE, Höfte M (2021) Bacillus cyclic lipopeptides iturin and fengycin control rice blast caused by Pyricularia oryzae in potting and acid sulfate soils by direct antagonism and induced systemic resistance. Microorganisms 9:1441. https://doi.org/10.3390/microorganisms9071441
Li H, Han X, Dong Y, Xu S, Chen C, Feng Y, Cui Q, Li W (2021) Bacillaenes: decomposition trigger point and biofilm enhancement in Bacillus. ACS Omega 6:1093–1098. https://doi.org/10.1021/acsomega.0c03389
Li E, Li Y, Dai X, Yan W, Wang G (2022) Identification of two Bacillus strains with antimicrobial activity and preliminary evaluation of their biocontrol efficiency. Horticulturae 8:744. https://doi.org/10.3390/horticulturae8080744
Li Z, Li J, Yu M, Quandahor P, Tian T, Shen T (2023) Bacillus velezensis FX-6 suppresses the infection of Botrytis cinerea and increases the biomass of tomato plants. PLoS ONE 18:e0286971. https://doi.org/10.1371/journal.pone.0286971
Liang L, Fu Y, Deng S, Wu Y, Gao M (2021) Genomic, antimicrobial, and aphicidal traits of Bacillus velezensis ATR2, and its biocontrol potential against ginger rhizome rot disease caused by Bacillus pumilus. Microorganisms 10:63. https://doi.org/10.3390/microorganisms10010063
Lim SM, Yoon M-Y, Choi GJ, Choi YH, Jang KS, Shin TS, Park HW, Yu NH, Kim YH, Kim J-C (2017) Diffusible and volatile antifungal compounds produced by an antagonistic Bacillus velezensis G341 against various phytopathogenic fungi. Plant Pathol J 33:488–498. https://doi.org/10.5423/PPJ.OA.04.2017.0073
Liu H, Zeng Q, Yalimaimaiti N, Wang W, Zhang R, Yao J (2021) Comprehensive genomic analysis of Bacillus velezensis AL7 reveals its biocontrol potential against Verticillium wilt of cotton. Mol Genet Genomics 296:1287–1298. https://doi.org/10.1007/s00438-021-01816-8
Lu K, Jin Q, Lin Y, Lu W, Li S, Zhou C, Jin J, Jiang Q, Ling L, Xiao M (2020) Cell-free fermentation broth of Bacillus velezensis strain S3–1 improves pak choi nutritional quality and changes the bacterial community structure of the rhizosphere soil. Front Microbiol 11:2043. https://doi.org/10.3389/fmicb.2020.02043
Maral-Gül D, Eltem R (2024) Evaluation of Bacillus isolates as a biological control agents against soilborne phytopathogenic fungi. Int Microbiol Off J Span Soc Microbiol. 1–15. https://doi.org/10.1007/s10123-024-00490-1
Medeot DB, Bertorello-Cuenca M, Liaudat JP, Alvarez F, Flores-Cáceres ML, Jofré E (2017) Improvement of biomass and cyclic lipopeptides production in Bacillus amyloliquefaciens MEP218 by modifying carbon and nitrogen sources and ratios of the culture media. Biol Control 115:119–128. https://doi.org/10.1016/j.biocontrol.2017.10.002
Medeot D, Sannazzaro A, Estrella MJ, Torres Tejerizo G, Contreras-Moreira B, Pistorio M, Jofré E (2023) Unraveling the genome of Bacillus velezensis MEP218, a strain producing fengycin homologs with broad antibacterial activity: comprehensive comparative genome analysis. Sci Rep 13:22168. https://doi.org/10.1038/s41598-023-49194-y
Medhioub I, Cheffi M, Tounsi S, Triki MA (2022) Study of Bacillus velezensis OEE1 potentialities in the biocontrol against Erwinia amylovora, causal agent of fire blight disease of rosaceous plants. Biol Control 167:104842. https://doi.org/10.1016/j.biocontrol.2022.104842
Mejri S, Siah A, Coutte F, Magnin-Robert M, Randoux B, Tisserant B, Krier F, Jacques P, Reignault P, Halama P (2018) Biocontrol of the wheat pathogen Zymoseptoria tritici using cyclic lipopeptides from Bacillus subtilis. Environ Sci Pollut Res Int 25:29822–29833. https://doi.org/10.1007/s11356-017-9241-9
Meng Q, Jiang H, Hao JJ (2016) Effects of Bacillus velezensis strain BAC03 in promoting plant growth. Biol Control 98:18–26. https://doi.org/10.1016/j.biocontrol.2016.03.010
Ngalimat MS, Yahaya RSR, Baharudin MMA, Yaminudin SM, Karim M, Ahmad SA, Sabri S (2021) A review on the biotechnological applications of the operational group Bacillus amyloliquefaciens. Microorganism 9:614. https://doi.org/10.3390/microorganisms9030614
Ongena M, Jacques P (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:115–125. https://doi.org/10.1016/j.tim.2007.12.009
Park JK, Hasumi K, Endo A (1995) Inhibition of the binding of oxidized low density lipoprotein to the macrophages by iturin orelated compounds. J Antibiot 48:226–232. https://doi.org/10.7164/antibiotics.48.226
Park JS, Ryu GR, Kang BR (2022) Target mechanism of iturinic lipopeptide on differential expression patterns of defense-related genes against Colletotrichum acutatum in pepper. Plants 11:1267. https://doi.org/10.3390/plants11091267
Patel PS, Huang S, Fisher S, Pirnik D, Aklonis C, Dean L, Meyers E, Fernandes P, Mayerl F (1995) Bacillaene, a novel inhibitor of procaryotic protein synthesis produced by Bacillus subtilis: production, taxonomy, isolation, physico-chemical characterization and biological activity. J Antibiot 48:997–1003. https://doi.org/10.7164/antibiotics.48.997
Peypoux F, Pommier M-T, Das BC, Besson F, Delcambe L, Michel G (1984) Structures of bacillomycin d and bacillomycin l peptidolipid antibiotics from Bacillus subtilis. J Antibiot 37:1600–1604. https://doi.org/10.7164/antibiotics.37.1600
Platel R, Sawicki M, Esmaeel Q, Randoux B, Trapet P, El Guilli M, Chtaina N, Arnauld S, Bricout A, Rochex A, Facon N, Halama P, Jacquard C, Ait Barka E, Reignault P, Magnin-Robert M, Siah A (2021) Isolation and identification of lipopeptide-producing Bacillus velezensis strains from wheat phyllosphere with antifungal activity against the wheat pathogen Zymoseptoria tritici. Agronomy 12:95. https://doi.org/10.3390/agronomy12010095
Platel R, Lucau-Danila A, Baltenweck R, Maia-Grondard A, Chaveriat L, Magnin-Robert M, Randoux B, Trapet P, Halama P, Martin P, Hilbert J-L, Höfte M, Hugueney P, Reignault P, Siah A (2022) Bioinspired rhamnolipid protects wheat against Zymoseptoria tritici through mainly direct antifungal activity and without major impact on leaf physiology. Front Plant Sci 13:1530. https://doi.org/10.3389/fpls.2022.878272
Príncipe A, Alvarez F, Castro MG, Zacchi LF, Fischer SE, Mori GB, Jofré E (2007) Biocontrol and PGPR features in native strains isolated from saline soils of Argentina. Curr Microbiol 55:314–322. https://doi.org/10.1007/s00284-006-0654-9
Put H, Gerstmans H, Vande Capelle H, Fauvart M, Michiels J, Masschelein J (2024) Bacillus subtilis as a host for natural product discovery and engineering of biosynthetic gene clusters. Nat Prod Rep. https://doi.org/10.1039/d3np00065f
Rabbee MF, Ali MS, Choi J, Hwang BS, Jeong SC, Baek K (2019) Bacillus velezensis: a valuable member of bioactive molecules within plant microbiomes. Molecules 24:1046. https://doi.org/10.3390/molecules24061046
Romero-Tabarez M, Jansen R, Sylla M, Lünsdorf H, Häußler S, Santosa DA, Timmis KN, Molinari G (2006) 7-O-malonyl macrolactin A, a new macrolactin antibiotic from Bacillus subtilis active against methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococci, and a small-colony variant of Burkholderia cepacia. Antimicrob Agents Chemother 50:1701–1709. https://doi.org/10.1128/AAC.50.5.1701-1709.2006
Rückert C, Blom J, Chen X, Reva O, Borriss R (2011) Genome sequence of B. amyloliquefaciens type strain DSM7(T) reveals differences to plant-associated B. amyloliquefaciens FZB42. J Biotechnol 155:78–85. https://doi.org/10.1016/j.jbiotec.2011.01.006
Ruiz-García C, Béjar V, Martínez-Checa F, Llamas I, Quesada E (2005) Bacillus velezensis sp. nov., a surfactant-producing bacterium isolated from the river Vélez in Málaga, southern Spain. Int J Syst Evol Microbiol 55:191–195. https://doi.org/10.1099/ijs.0.63310-0
Safitri R, Handayani S, Surono W, Astika H, Damayanti R, Kusmaya FD, Rukiah BRL (2019) Biodegradation of phenol, anthracene and acenaphthene singly and consortium culture of indigenous microorganism isolates from underground coal gasification area. IOP Conf Ser Earth Environ Sci 306:012026. https://doi.org/10.1088/1755-1315/306/1/012026
Santner A, Calderon-Villalobos LIA, Estelle M (2009) Plant hormones are versatile chemical regulators of plant growth. Nat Chem Biol 5:301–307. https://doi.org/10.1038/nchembio.165
Sawant SS, Song J, Seo H-J (2023) Study of new biocontrol bacterial agent Bacillus velezensis S41L against Rosellinia necatrix. Biol Control 185:105305. https://doi.org/10.1016/j.biocontrol.2023.105305
Shao J, Xun W, Shen Q, Miao Y, Zhang N, Zhang R, Feng H, Xu Z, Liu Y (2023) Chemical communication in plant–microbe beneficial interactions: a toolbox for precise management of beneficial microbes. Curr Opin Microbiol 72:102269. https://doi.org/10.1016/j.mib.2023.102269
Soliman SA, Khaleil MM, Metwally RA (2022) Evaluation of the antifungal activity of Bacillus amyloliquefaciens and B. velezensis and characterization of the bioactive secondary metabolites produced against plant pathogenic fungi. Biology 11:1390. https://doi.org/10.3390/biology11101390
Stenberg JA, Sundh I, Becher PG, Björkman C, Dubey M, Egan PA, Friberg H, Gil JF, Jensen DF, Jonsson M, Karlsson M, Khalil S, Ninkovic V, Rehermann G, Vetukuri RR, Viketoft M (2021) When is it biological control? A framework of definitions, mechanisms, and classifications. J Pest Sci 94:665–676. https://doi.org/10.1007/s10340-021-01354-7
Sultana OF, Lee S, Seo H, Mahmud HA, Kim S, Seo A, Kim M, Song H-Y (2021) Biodegradation and removal of PAHs by Bacillus velezensis isolated from fermented food. J Microbiol Biotechnol 31:999–1010. https://doi.org/10.4014/jmb.2104.04023
Sundh I, Eilenberg J (2021) Why has the authorization of microbial biological control agents been slower in the EU than in comparable jurisdictions? Pest Manag Sci 77:2170–2178. https://doi.org/10.1002/ps.6177
Thanh Tam LT, Jähne J, Luong PT, Phuong Thao LT, Nhat LM, Blumenscheit C, Schneider A, Blom J, Kim Chung LT, Anh Minh PL, Thanh HM, Hoat TX, Hoat PC, Son TC, Weinmann M, Herfort S, Vater J, Van Liem N, Schweder T, Lasch P, Borriss R (2023) Two plant-associated Bacillus velezensis strains selected after genome analysis, metabolite profiling, and with proved biocontrol potential, were enhancing harvest yield of coffee and black pepper in large field trials. Front Plant Sci 14:1194887. https://doi.org/10.3389/fpls.2023.1194887
Torres M, Llamas I, Torres B, Toral L, Sampedro I, Béjar V (2020) Growth promotion on horticultural crops and antifungal activity of Bacillus velezensis XT1. Appl Soil Ecol 150:103453. https://doi.org/10.1016/j.apsoil.2019.103453
Upadhyay SK, Srivastava AK, Rajput VD, Chauhan PK, Bhojiya AA, Jain D, Chaubey G, Dwivedi P, Sharma B, Minkina T (2022) Root exudates: mechanistic insight of plant growth promoting rhizobacteria for sustainable crop production. Front Microbiol 13:916488. https://doi.org/10.3389/fmicb.2022.916488
Vanittanakom N, Loeffler W, Koch U, Jung G (1986) Fengycin-a novel antifungal lipopeptide antibiotic produced by Bacillus subtilis F-29-3. J Antibiot 39:888–901. https://doi.org/10.7164/antibiotics.39.888
Wang L-T, Lee F-L, Tai C-J, Kuo H-P (2008) Bacillus velezensis is a later heterotypic synonym of Bacillus amyloliquefaciens. Int J Syst Evol Microbiol 58:671–675. https://doi.org/10.1099/ijs.0.65191-0
Weber T, Marahiel MA (2001) Exploring the domain structure of modular nonribosomal peptide synthetases. Structure 9:R3–R9. https://doi.org/10.1016/S0969-2126(00)00560-8
Wilson KE, Flor JE, Schwartz RE, Joshua H, Smith JL, Pelak BA, Liesch JM, Hensens OD (1987) Difficidin and oxydifficidin: novel broad spectrum antibacterial antibiotics produced by Bacillus subtilis. II. Isolation and Physico-Chemical Characterization J Antibiot 40:1682–1691. https://doi.org/10.7164/antibiotics.40.1682
Wu L, Huang Z, Li X, Ma L, Gu Q, Wu H, Liu J, Borriss R, Wu Z, Gao X (2018) Stomatal closure and SA-, JA/ET-signaling pathways are essential for Bacillus amyloliquefaciens FZB42 to restrict leaf disease caused by Phytophthora nicotianae in Nicotiana benthamiana. Front Microbiol 9:847. https://doi.org/10.3389/fmicb.2018.00847
Xu W, Zhang L, Zhang J, Wang Q, Yang L (2020) Isolation, identification, and complete genome assembly of an endophytic Bacillus velezensis YB-130, potential biocontrol agent against Fusarium graminearum. Front Microbiol 11:598285. https://doi.org/10.3389/fmicb.2020.598285
Yan Y, Xu W, Hu Y, Tian R, Wang Z (2022) Bacillus velezensis YYC promotes tomato growth and induces resistance against bacterial wilt. Biol Control 172:104977. https://doi.org/10.1016/j.biocontrol.2022.104977
Yang Y-L, Xu Y, Straight P, Dorrestein PC (2009) Translating metabolic exchange with imaging mass spectrometry. Nat Chem Biol 5:885–887. https://doi.org/10.1038/nchembio.252
Yang F, Zhang R, Wu X, Xu T, Ahmad S, Zhang X, Zhao J, Liu Y (2020) An endophytic strain of the genus Bacillus isolated from the seeds of maize (Zea mays L.) has antagonistic activity against maize pathogenic strains. Microb Pathog 142:104074. https://doi.org/10.1016/j.micpath.2020.104074
Zaid DS, Cai S, Hu C, Li Z, Li Y (2022) Comparative genome analysis reveals phylogenetic identity of Bacillus velezensis HNA3 and genomic insights into its plant growth promotion and biocontrol effects. Microbiol Spectr 10:e0216921. https://doi.org/10.1128/spectrum.02169-21
Zhang P, Xie G, Wang L, Xing Y (2022) Bacillus velezensis BY6 promotes growth of poplar and improves resistance contributing to the biocontrol of Armillaria solidipes. Microorganisms 10:2472. https://doi.org/10.3390/microorganisms10122472
Zhao J, Zhou Z, Zhang L (2022) A novel of new class II bacteriocin from Bacillus velezensis HN-Q-8 and its antibacterial activity on Streptomyces scabies. Front Microbiol 13:943232. https://doi.org/10.3389/fmicb.2022.943232
Zhu M, He Y, Li Y, Ren T, Liu H, Huang J, Jiang D, Hsiang T, Zheng L (2020) Two new biocontrol agents against clubroot caused by Plasmodiophora brassicae. Front Microbiol 10:3099. https://doi.org/10.3389/fmicb.2019.03099
Acknowledgements
This work was supported by the University of Reims Champagne-Ardenne and the region Grand Est, France, GrapeSynCom project in the frame of the allocation post-doctoral region grand Est.
Funding
We gratefully acknowledge the financial support provided by the University of Reims Champagne-Ardenne and the region Grand Est, France GrapeSynCom project in the frame of the allocation post-doctoral region grand Est.
Author information
Authors and Affiliations
Contributions
JK, ED, SB, and QE conceptualized, wrote the manuscript, and made figures and tables. JK, ED, SB, RL, EAB, and QE revised and approved the final version of the manuscript. All authors contributed to the manuscript structure and discussions.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jihane Kenfaoui and Emma Dutilloy are co-first authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kenfaoui, J., Dutilloy, E., Benchlih, S. et al. Bacillus velezensis: a versatile ally in the battle against phytopathogens—insights and prospects. Appl Microbiol Biotechnol 108, 439 (2024). https://doi.org/10.1007/s00253-024-13255-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-024-13255-7