Abstract
Waste glycerol is produced in excess by several industries, such as during biodiesel production. In this work, the metabolic versatility of anaerobic sludge was explored towards waste glycerol valorization. By applying different environmental (methanogenic and sulfate-reducing) conditions, three distinct microbial cultures were obtained from the same inoculum (anaerobic granular sludge), with high microbial specialization, within three different phyla (Thermodesulfobacteriota, Euryarchaeota and Pseudomonadota). The cultures are capable of glycerol conversion through different pathways: (i) glycerol conversion to methane by a bacterium closely related to Solidesulfovibrio alcoholivorans (99.8% 16S rRNA gene identity), in syntrophic relationship with Methanofollis liminatans (98.8% identity), (ii) fermentation to propionate by Propionivibrio pelophilus strain asp66 (98.6% identity), with a propionate yield of 0.88 mmol mmol−1 (0.71 mg mg−1) and a propionate purity of 80–97% and (iii) acetate production coupled to sulfate reduction by Desulfolutivibrio sulfoxidireducens (98.3% identity). In conclusion, starting from the same inoculum, we could drive the metabolic and functional potential of the microbiota towards the formation of several valuable products that can be used in industrial applications or as energy carriers.
Key points
-
Versatility of anaerobic cultures was explored for waste glycerol valorization
-
Different environmental conditions lead to metabolic specialization
-
Biocommodities such as propionate, acetate and methane were produced
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biodiesel production and ethanol production by yeast or the oleochemical industry generate glycerol as a by-product (Clomburg and Gonzalez 2013; Monteiro et al. 2018; Navarrete et al. 2014). Largely exceeding its demand, glycerol changed from a commodity chemical to a surplus by-product and even to a waste product, creating environmental and economic losses (Clomburg and Gonzalez 2013; Monteiro et al. 2018; Viana et al. 2012). Within this framework, anaerobic bioconversion of glycerol to valuable chemical compounds can be a sustainable treatment strategy, adding value to waste glycerol and to the biodiesel industry (Holm-Nielsen et al. 2009; Viana et al. 2012; Yazdani and Gonzalez 2007).
Under anaerobic conditions, the high reduction state of glycerol is an advantage, as glycerol fermentation results in the production of more reduced compounds than with sugars as glucose (Yazdani and Gonzalez 2007). Nevertheless, the high reduction state of glycerol also presents considerable challenges, since only a few fermentative bacteria are capable of easily disposing off the excess of reducing equivalents generated from glycerol. Other bacteria can oxidize glycerol coupled to the reduction of external electron acceptors, such as sulfate (Clomburg and Gonzalez 2013), or in syntrophy with hydrogenotrophic methanogens (Qatibi et al. 1991a, b). Syntrophic collaboration was even shown to accelerate glycerol degradation (Magalhães et al. 2020; Richter and Gescher 2014), possibly because it facilitates the maintenance of the proper intracellular redox balance. Zhang et al. (2015) suggested that the use of mixed cultures for glycerol degradation may present economic and process advantages.
The objective of this work was to drive the naturally occurring microbiome of anaerobic sludge towards glycerol consumption and valorization and to study the diversity and physiology of the obtained microorganisms and/or communities. Starting from the same inoculum (anaerobic sludge), three distinct specialized glycerol degrading cultures were obtained, and their physiology was studied. The obtained cultures are capable of metabolizing glycerol through different pathways, proving the metabolic versatility of using anaerobic mixed cultures, as well as proving the concept of the ability to shape mixed microbial communities towards specific needs (Oleskowicz-Popiel 2018).
Materials and methods
Enrichment of glycerol-degrading cultures
Enrichments were made in 120 mL serum bottles containing 50 mL of a bicarbonate-buffered mineral salt medium (basal medium, BM), prepared as previously described by Stams et al. (1993). The serum bottles were sealed with butyl rubber septa and aluminum crimp caps, and the headspace of the bottles was flushed and pressurized with N2/CO2 (80:20%, v/v) at a final pressure of 170 kPa. The medium was reduced with 1 mmol L−1 sodium sulfide and supplemented with salts and vitamins (Stams et al. 1993). Anaerobic granular sludge from a brewery wastewater treatment plant (Portugal) was used as inoculum. Glycerol (10 mmol L−1) was supplemented as a carbon and energy source. Enrichments were developed in the absence of any added external electron acceptor (methanogenic conditions), resulting in two different cultures coded as Gly-M and Gly-P or with 20 mmol L−1 sodium sulfate (sulfate-reducing conditions), coded as Gly-S. Successive transfers (10% v/v) were made to fresh medium after confirming glycerol consumption and microbial growth in all assays. Methane content in the bottles’ headspace and the concentration of soluble compounds, such as volatile fatty acids (VFA), lactate, aspartate, succinate, glycerol, ethanol, butanol, 1,3-propanediol (1,3-PDO) and 1,2-propanediol (1,2-PDO), were periodically measured. In the Gly-S set of experiments, sulfate reduction was assessed indirectly by the amount of sulfide produced (Eq. 1).
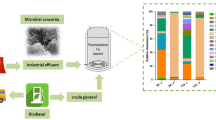
All inoculations and transfers were done aseptically. Incubations were performed at 37 °C, statically and in the dark. A schematic representation of the experiments is shown in Fig. 1 (“Results” section).
Physiological characterization of Gly-M-, Gly-P- and Gly-S-enriched cultures
Physiological characterization was done with the stable cultures of Gly-M, Gly-P and Gly-S after 15, 8 and 10 successive transfers, respectively. Unless otherwise stated, incubations were performed with 10 mmol L−1 glycerol as a carbon source. For Gly-S, sodium sulfate was used as the final electron acceptor at 20 mmol L−1. In the case of enrichment culture Gly-M, incubations with 2-bromoethanesulfonate (BrES), a specific inhibitor of the methanogens (20 mmol L−1), were also performed.
Purity check was done in Gly-P- and Gly-S-enriched cultures by microscopic examination after incubation with yeast extract (2 g L−1), glucose (10 mmol L−1), or pyruvate (10 mmol L−1). Cells from active cultures of Gly-P and Gly-S were Gram-strained and cell morphology was examined by phase contrast microscopy.
Physiological characterization of these two cultures was performed in the presence of different glycerol concentrations: 10, 30, 50, 100 and 200 mmol L−1. Supplemental Table S1 summarizes all the procedures applied.
For Gly-P-enriched culture, the ability of this culture to degrade ethanol, propanol or 1-butanol (10 mmol L−1), aspartate (20 mmol L−1) or succinate (20 mmol L−1) was tested as well. In regard to Gly-S culture, the capability to use ethanol, propanol and 1-butanol at different concentrations (10, 20, 30 and 40 mmol L−1) was also investigated. In the case of Gly-S, all incubations were done with (20 mmol L−1) or without sulfate as an electron acceptor. Additionally, using the obtained Gly-S culture, potential syntrophic growth with a methanogenic partner, in the absence of sulfate, was assessed. For that, Methanobacterium formicicum DSM 1535 T was acquired from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ, Braunschweig, Germany). The methanogen was pre-grown with H2/CO2 (80:20% v/v, at a final pressure of 170 kPa) in BM medium supplemented with 0.3 g L−1 sodium acetate, at 37 °C and 100 rpm. After the headspace was changed to N2/CO2 (80:20% v/v, 170 kPa) under sterile conditions, glycerol was added (10 mmol L−1) and well-grown Gly-S culture was transferred (10% v/v) to these bottles. The Gly-S culture was also used to inoculate (10% v/v) control bottles, containing fresh medium, glycerol (10 mmol L−1) and sulfate (20 mmol L−1). After verifying the growth and activity of the co-cultures (Gly-S + methanogenic partner), these were transferred again (10% v/v) to bottles containing pre-grown cultures of the methanogen (prepared as described before) and glycerol (10 mmol L−1).
Substrate consumption, liquid (soluble) and gaseous product formation, sulfate reduction (assessed indirectly by the sulfide produced) and cell growth were monitored over time, for all the experiments, as described in the “Analytical methods” section.
Cultures Gly-P and Gly-S were deposited in culture collections belonging to the World Data Centre for Microorganisms (WDCM), as detailed in the data availability statement. Regarding the enrichment culture Gly-M, it is accessible at the Laboratory of Environmental Technology from the Centre of Biological Engineering of the University of Minho (Braga, Portugal), with A.J. Cavaleiro.
Microbial communities’ composition
Microbial community composition of stable enriched cultures Gly-M, Gly-P and Gly-S was evaluated through 16S rRNA gene sequencing. Aliquots (15 mL) of well-homogenized stable enrichment cultures were collected from Gly-M, Gly-P and Gly-S, and immediately frozen at − 20 °C. Total genomic DNA was extracted using the FastDNA SPIN Kit for Soil (MP Biomedicals, Solon, OH) and purified by ethanol precipitation. Bacterial and archaeal 16S rRNA genes were amplified using a TaqDNA polymerase kit (Invitrogen, Carlsbad, CA) and the primers Bact27-F7/Uni1492-R and Arch109-F/Uni1492-R, respectively. PCR programs and reaction mixtures used were described elsewhere (Sousa et al. 2007). Cloning and Sanger sequencing of the obtained 16S rRNA genes were performed using the methodologies previously described by Sousa et al. (2007). Sanger sequencing method was performed by Macrogen Europe (Amsterdam, ND), and the obtained sequences were compared with the NCBI RefSeq_RNA database using the NCBI Nucleotide Blast tool (https://www.ncbi.nlm.nih.gov/nucleotide/). Nucleotide sequencing was submitted to the European Nucleotide Archive (ENA) under the study accession no. PRJEB72408.
Analytical methods
Phase contrast micrographs were obtained with an Olympus CX41 RF microscope (Tokyo, Japan) and an Olympus Altra 20 image acquisition system. The software used with this setup was the AnalySIS getIT (Olympus Soft Imaging Solutions GmbH). The Gram staining was performed as previously described by Halebian et al. (1981). Methane was quantified with a GC-2014 Shimadzu gas chromatograph equipped with a Porapak Q column and a flame ionization detector. N2 was used as carrier gas. Injection port, column and detector temperatures were 110 °C, 35 °C and 220 °C, respectively. VFA (formate, acetate, propionate, iso- and n-butyrate and valerate) and lactate, succinate and aspartate were analyzed by high-performance liquid chromatography (HPLC, Jasco, Tokyo, Japan), using an Agilent Hi-Plex H (300 × 7.7 mm) column at 60 °C and H2SO4 (2.5 mmol L−1) as mobile phase, at a flow rate of 0.6 mL min−1. Spectrophotometric ultraviolet (UV) detection was performed at 210 nm. Glycerol, ethanol, butanol, 1,3-PDO and 1,2-PDO were analyzed by HPLC using a Varian Aminex 87H (300 × 7.8 mm) column with a mobile phase of 5 mmol L−1 H2SO4 at a flow rate of 0.7 mL min−1, with the column temperature set at 60 °C and refractive index (RI) detection. Total dissolved sulfide was measured using cuvette tests and a DR 2800 spectrophotometer (Hach-Lange GmbH, Düsseldorf, Germany), as described by Alves et al. (2020). Total sulfide species were calculated from the measured dissolved sulfide values, considering the dissociation constants of the acid and pH = 7.5.
Results
Three different stable glycerol-degrading cultures, coded as Gly-M, Gly-P and Gly-S, were obtained after successive transfers under different environmental conditions, exhibiting distinct microbial composition and product profile (Fig. 1, Tables 1 and 2), producing mainly methane, propionate and acetate, respectively.
Methanogenic enrichment series, Gly-M, resulted in a co-culture of a bacterium closely related to Solidesulfovibrio alcoholivorans (99.8% 16S rRNA gene identity) and a hydrogenotrophic methanogen closely related to Methanofollis liminatans (98.8% 16S rRNA gene identity) (Table 1). Further transfers of the glycerol-degrading culture Gly-M led to a loss of the methane production ability in some of these cultures, and production of propionate was observed (Fig. 1). These enrichments were continued, and a highly specialized fermentative propionate-producing culture composed of a bacterium closely affiliated with the Propionivibrio pelophilus asp66 strain (98.6% 16S rRNA gene identity) was obtained (Fig. 1, Tables 1 and 2). This culture was designated Propionivibrio sp. strain Gly-P, or simply strain Gly-P or Gly-P culture. Additionally, microscopic observations of the Gly-P culture revealed that it was mainly composed of one morphotype (Fig. S1a), Gram (-) cells, which was in line with the results from the molecular characterization of this culture (Table 1). In the cultures in which sulfate was added to the anaerobic medium (Fig. 1), the microbial specialization resulted in a distinct culture, composed of only one morphotype (Fig. S1b), Gram (-) cells, closely related to Desulfolutivibrio sulfoxidireducens strain DSM 107105 (98.3% 16S rRNA gene identity) (Table 1). This culture was named Desulfolutivibrio sp. strain Gly-S or simply strain Gly-S or Gly-S culture.
The Gly-M co-culture was able to use glycerol in approximately 10 days and formed acetate and methane (Table 2, Fig. 2a). When the co-culture Gly-M was incubated with BrES, a specific inhibitor of the methanogens (DiMarco et al. 1990), glycerol was not degraded, as shown in Fig. 2b. Methane was not produced, and only a residual quantity of acetate could be detected (Fig. 2b).
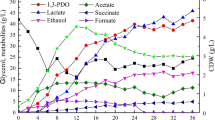
Propionivibrio sp. strain Gly-P was able to use aspartate (20 mmol L−1), with the production of acetate (~ 4 mmol L−1) and propionate (~ 9 mmol L−1). When grown with glycerol (10 mmol L−1), Gly-P produced mainly propionate (9.5 mmol L−1) and acetate (0.4 mmol L−1) in 12 days of fermentation (Fig. 3, Table 3). These values correspond to a propionate yield of 0.88 mmol propionate per mmol of glycerol consumed (0.71 mg mg−1) and a carbon recovery (considering the CO2 possibly produced concomitantly with acetate) of 92% (Table 3). It should be mentioned that biomass production was not included in this balance.
Propionivibrio sp. strain Gly-P was also able to ferment glycerol up to a concentration of approximately 200 mmol L−1. However, for glycerol concentrations of 100 mmol L−1 and 200 mmol L−1, only 36% and 15% of the glycerol added was consumed, respectively, after 12 days of fermentation (Table 3). Nevertheless, propionate yields remained similar for all the concentrations studied, with an average value of 0.82 ± 0.04 mmol mmol−1 (0.66 ± 0.03 mg mg−1).
Acetate yields increased with the increasing glycerol concentrations tested, accompanied by decreasing propionate/acetate molar ratios (from 23.8 to 8.9 for 10 mmol L−1 to 200 mmol L−1 glycerol) (Table 3). Succinate yields increased up to a glycerol concentration of 50 mmol L−1 and decreased thereafter (Table 3). Nevertheless, succinate and acetate were always produced in relatively low amounts, leading to a propionate purity in the medium between 80 and 97%. Glycerol consumption rate and propionate production rate increased for glycerol concentrations up to 50 mmol L−1 but decreased thereafter (data not shown).
Desulfolutivibrio sp. strain Gly-S was able to grow with glycerol, by oxidation to acetate coupled to sulfate reduction (Fig. 4, Table 4). When grown with an initial glycerol concentration of 10 mmol L−1, glycerol was converted to acetate in 14 days (Table 2, Fig. 4), with an acetate yield (mmol acetate per mmol of glycerol consumed) of 0.84 (Table 4). For higher glycerol concentrations, from 30 to 173 mmol L−1, the glycerol consumed by strain Gly-S was practically the same in all the cases, i.e., approximately 23 mmol L−1. Acetate (around 14–16 mmol L−1) and sulfide (approx. 16 mmol L−1) were formed at concentrations that were similar for all the assays (Table 4). Desulfolutivibrio sp. strain Gly-S was also able to grow with other alcohols, namely ethanol, propanol and 1-butanol, coupled to sulfate reduction, with acetate as the main product obtained in those situations (data not shown). In the absence of sulfate, no growth was observed, for all the different substrates tested. Glycerol was also degraded by Desulfolutivibrio sp. strain Gly-S in the absence of sulfate when incubated with Methanobacterium formicicum (Fig. 5).
Discussion
Although starting from the same inoculum, microbial specialization was evidenced for glycerol conversion, with organisms from three different phyla—Euryarchaeota, Thermodesulfobacteriota and Pseudomonadota—being found in the three distinct glycerol-degrading cultures. The use of anaerobic granular sludge proved to be an efficient microbial platform for the production of biocommodities, such as propionate, methane and/or acetate. The different products generated by the stable cultures suggest metabolic specialization, with glycerol being degraded through different pathways (Fig. S2).
In the Gly-M co-culture, glycerol was converted to acetate and H2 (Eq. 2), with subsequent conversion of hydrogen to methane by the hydrogenotrophic methanogen (Eq. 3). The conversion stoichiometry is shown in Eq. 4, and the possible metabolic pathway is illustrated in Fig. S2a. The absence of aceticlastic methanogens allows acetate production, for potential use as commodity chemical. The activity of the hydrogenotrophic methanogen mitigates the thermodynamic constraints associated with high hydrogen partial pressure, contributing to the redox balance by removing the excess reducing power (Fig. S2a).
Syntrophic relationships between fermentative bacteria (e.g., Thermoanaerobacter species, Escherichia coli) and methanogens were reported to facilitate glycerol fermentation (Magalhães et al. 2020; Richter and Gescher 2014; Zhang et al. 2015). However, co-culture Gly-M incubated with BrES was not able to degrade glycerol, nor to produce methane (Fig. 2b), highlighting that the presence of the methanogen is essential for glycerol conversion, and pointing to the occurrence of an obligatory syntrophic relationship.
Association between sulfate-reducing bacteria, such as Desulfovibrio species and methanogens, has been reported in the absence of sulfate, mostly regarding ethanol and lactate degradation (Rabus et al. 2006). Still, Solidesulfovibrio alcoholivorans, S. fructosovorans and S. carbinolicus were reported to degrade glycerol without sulfate in the presence of Methanospirillum hungatei (Qatibi et al. 1998; Qatibi et al. 1991a, b). From the known Solidesulfovibrio strains (former Desulfovibrio sp.), only S. fructosovorans DSM 3604 (Qatibi et al. 1998) and S. carbinolicus strain EDK82 (Nanning and Gottschal 1986) are able to perform glycerol fermentation. All the other strains that are known to degrade glycerol can only do it in the presence of sulfate as an external electron acceptor or in syntrophy with a methanogen.
After several transfers of Gly-M culture, propionate production was observed, leading to a new line of enrichments—Gly-P. Propionate production has attracted significant attention due to its importance as a chemical building block widely used in various industries, including feed and food preservatives, herbicides, cosmetics, plastics and pharmaceuticals (Ahmadi et al. 2017; Gonzalez-Garcia et al. 2017). Glycerol conversion to propionate results in higher production yields and less by-products compared to other substrates (Barbirato et al. 1997; Chen et al. 2016; Coral et al. 2008; Dishisha et al. 2015), mainly due to the high reduction state of glycerol. Moreover, this conversion is redox-neutral (Fig. S2b) and yields more energy (Barbirato et al. 1997).
The closest cultured relative of strain Gly-P, P. pelophilus strain asp66, was reported to be not able to degrade glycerol. The same is the case for all the other Propionivibrio species described (Brune et al. 2002; Hansen et al. 1990; Tanaka et al. 1990; Thrash et al. 2010). In fact, by analyzing the genome of P. pelophilus strain asp66 (DSM 12018 T), at the Integrated Microbial Genomes (IMG) (https://img.jgi.doe.gov/) and at The National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/) genomic platforms, it was possible to confirm that this bacterium lacks the genes encoding for the enzymes that are directly linked to glycerol utilization, such as glycerol dehydratase, glycerol dehydrogenase or glycerol kinase (Clomburg and Gonzalez 2013). This fact, together with a lower 16S rRNA gene identity than 98.7% (which is the threshold for the same species), points out that strain Gly-P could potentially represent a novel Propionivibrio species. Both Gly-P and Propionivibrio pelophilus strain asp66 (Hansen et al. 1990), its closest relative, were unable to grow with ethanol, propanol, butanol and succinate.
For all glycerol concentrations tested, propionate yield remained relatively constant (Table 3), corroborating the findings of Chen et al. (2016), who showed a minimal impact on propionate yield with increasing glycerol concentrations. Barbirato et al. (1997) also reported similar or lower propionate yields for Acidipropionibacterium acidipropionici (Nouioui et al. 2018), Cutibacterium acnes (Nouioui et al. 2018) and Anaerotignum propionicum (Ueki et al. 2017). Additionally, Zhang and Yang (2009) reported propionate yields from glycerol of 0.67–0.88 mmol mmol−1 (0.54–0.71 g g−1) by metabolically engineered Propionibacterium acidipropionici.
With the increase in glycerol concentrations, succinate and acetate yields started to increase (Table 3), most probably due to inhibition by propionate accumulation. Succinate is a precursor to propionate (Fig. S2b), and its accumulation in the assays (although at low concentrations) suggests inhibition of the succinate pathway of propionate formation. At the same time, the metabolic flux is also being redirected towards acetate production. End-product inhibition typically constrains propionic acid fermentation processes (Zhang and Yang 2009). Among volatile fatty acids, propionate concentrations can have the most significant inhibitory effect on glycerol degradation (Chen et al. 2016; Zhang et al. 2015), due to product-mediated inhibition on cell growth and metabolic activity (Blanc and Goma 1987).
Regarding to Gly-S culture (Fig. S2c), and considering the stoichiometry of the reaction shown in Eq. 5, electron recovery between 79.4 and 96.8% was calculated for the different glycerol concentrations studied (Table 4).
For glycerol concentrations higher than 30 mmol L−1, substrate inhibition strongly constrained the activity of this culture (Table 4). The capacity of its closest relative, Desulfolutivibrio sulfoxidireducens, to utilize glycerol was previously reported by Bak and Pfennig (1987), although showing very slow glycerol consumption and very poor growth. Chen et al. (2019) compared synthetic communities comprising a sulfate reducer (D. vulgaris strain Hildenborough) and two methanogens, assembled as syntrophic co- or tri-cultures. When the cultures were placed with sulfate, the methane production was highly diminished, which was attributed to a metabolic shift in bacteria towards respiration with sulfate, leading to a disruption in the methanogenic population (Chen et al. 2019). A similar biochemical conflict between the different metabolic processes (sulfate reduction and methanogenesis as biological electron acceptor) most probably shaped the microbial specialization observed in Gly-S culture.
When Gly-S culture was incubated without sulfate, no growth occurred, but when co-incubated with a methanogenic partner, such as Methanobacterium formicicum, glycerol was slowly converted to acetate and methane, showing that the methanogen was consuming the hydrogen/formate generated from glycerol and working as biological electron acceptor. It is worth recalling that Gly-S culture was enriched from a methanogenic granular sludge, which most probably influenced its metabolic traits.
In summary, this work explores the microbial diversity and metabolic specialization of anaerobic microorganisms involved in glycerol conversion and valorization. Three distinct stable cultures were developed, under different environmental conditions, using microbial mixed cultures as biocatalysts. These cultures have the ability to grow and convert glycerol into biocommodities that can be used in industrial applications or as energy carriers. Syntrophic (Gly-M), fermentative (Gly-P) and sulfate-reducing (Gly-S) cultures were obtained, allowing the sustainable treatment and valorization of glycerol. This work contributes to tackle the bottleneck of biodiesel production, caused by the surplus of glycerol. The specialization of cultures that was observed led to product diversification, also contributing to anaerobic process valorization. It was indicated that the top-down design of the microbiome is a promising strategy for not only utilization of troublesome waste but also suitable for dedicated platform chemical production (Lawson et al. 2019). The investment in biological methods of environmental-friendly nature is a demand for application at an industrial level and the development of novel bio-based technologies.
Data availability
Propionivibrio sp. strain Gly-P and Desulfolutivibrio sp. strain Gly-S were deposited in the China General Microbiological Culture Collection Center (CGMCC) under accession no. CGMCC 1.18109 and 1.18110, respectively. Nucleotide sequencing data have been submitted to the European Nucleotide Archive (ENA) under the study accession no. PRJEB72408. All other relevant data generated and analyzed during this study, which include experimental data, are included in this article and its supplementary information.
References
Ahmadi N, Khosravi-Darani K, Mortazavian AM (2017) An overview of biotechnological production of propionic acid: from upstream to downstream processes. Electron J Biotechnol 28:67–75. https://doi.org/10.1016/j.ejbt.2017.04.004
Alves JI, Salvador AF, Castro AR, Zheng Y, Nijsse B, Atashgahi S, Sousa DZ, Stams AJM, Alves MM, Cavaleiro AJ (2020) Long-chain fatty acids degradation by Desulfomonile species and proposal of “Candidatus desulfomonile palmitatoxidans”. Front Microbiol 11:539604. https://doi.org/10.3389/fmicb.2020.539604
Bak F, Pfennig N (1987) Chemolithotrophic growth of Desulfovibrio sulfodismutans sp. nov. by disproportionation of inorganic sulfur compounds. Arch Microbiol 147:184–189. https://doi.org/10.1007/BF00415282
Barbirato F, Chedaille D, Bories A (1997) Propionic acid fermentation from glycerol: comparison with conventional substrates. Appl Microbiol Biotechnol 47:441–446. https://doi.org/10.1007/s002530050953
Blanc P, Goma G (1987) Kinetics of inhibition in propionic acid fermentation. Bioprocess Eng 2:175–179. https://doi.org/10.1007/BF00387325
Brune A, Ludwig W, Schink B (2002) Propionivibrio limicula sp. nov., a fermentative bacterium specialized in the degradation of hydroaromatic compounds, reclassification of Propionibacter pelophilus as Propionivibrio pelophilus comb. nov. and amended description of the genus Propionivibrio. Int J Syst Evol Microbiol 52:441–444. https://doi.org/10.1099/00207713-52-2-441
Chen J, Wade MJ, Dolfing J, Soyer OS (2019) Increasing sulfate levels show a differential impact on synthetic communities comprising different methanogens and a sulfate reducer. J R Soc Interface 16. https://doi.org/10.1098/rsif.2019.0129
Chen Y, Wang T, Shen N, Zhang F, Zeng RJ (2016) High-purity propionate production from glycerol in mixed culture fermentation. Bioresour Technol 219:659–667. https://doi.org/10.1016/j.biortech.2016.08.026
Clomburg JM, Gonzalez R (2013) Anaerobic fermentation of glycerol: a platform for renewable fuels and chemicals. Trends Biotechnol 31:20–28. https://doi.org/10.1016/j.tibtech.2012.10.006
Coral J, Karp SG, De Souza P, Vandenberghe L, Parada JL, Pandey A, Soccol CR (2008) Batch fermentation model of propionic acid production by Propionibacterium acidipropionici in different carbon sources. Appl Biochem Biotechnol 151:333–341. https://doi.org/10.1007/s12010-008-8196-1
DiMarco AA, Bobik TA, Wolf RS (1990) Unusual coenzymes of methanogenesis. Annu Rev Biochem 59:355–394. https://doi.org/10.1146/annurev.bi.59.070190.002035
Dishisha T, Ibrahim MHA, Cavero VH, Alvarez MT, Hatti-Kaul R (2015) Improved propionic acid production from glycerol: combining cyclic batch and sequential batch fermentations with optimal nutrient composition. Bioresour Technol 176:80–87. https://doi.org/10.1016/j.biortech.2014.11.013
Gonzalez-Garcia RA, McCubbin T, Navone L, Stowers C, Nielsen LK, Marcellin E (2017) Microbial Propionic Acid Production Fermentation 3:1–20. https://doi.org/10.3390/fermentation3020021
Halebian S, Harris B, Finegold SM, Rolfe RD (1981) Rapid Method That Aids in Distinguishing Gram-Positive from Gram-Negative Anaerobic Bacteria 13:3. https://doi.org/10.1128/jcm.13.3.444-448.1981
Hansen TA, Nienhuis-Kuiper HE, Stams AJM (1990) A rod-shaped, gram-negative, propionigenic bacterium with a wide substrate range and the ability to fix molecular nitrogen. Arch Microbiol 155:42–45. https://doi.org/10.1007/BF00291272
Holm-Nielsen JB, Al Seadi T, Oleskowicz-Popiel P (2009) The future of anaerobic digestion and biogas utilization. Bioresour Technol 100:5478–5484. https://doi.org/10.1016/j.biortech.2008.12.046
Lawson CE, Harcombe WR, Hatzenpichler R, Lindemann SR, Löffler FE, O’Malley MA, Martín HG, Pfleger BF, Raskin L, Venturelli OS, Weissbrodt DG, Noguera DR, McMahon KD (2019) Common principles and best practices for engineering microbiomes. Nat Rev Microbiol 17:725–741. https://doi.org/10.1038/s41579-019-0255-9
Magalhães CP, Ribeiro JA, Guedes AP, Arantes AL, Sousa DZ, Stams AJM, Alves MM, Cavaleiro AJ (2020) Co-cultivation of Thermoanaerobacter strains with a methanogenic partner enhances glycerol conversion. Microb Biotechnol 13:962–973. https://doi.org/10.1111/1751-7915.13506
Monteiro MR, Kugelmeier CL, Pinheiro RS, Batalha MO, Silva César A (2018) Glycerol from biodiesel production: technological paths for sustainability. Renew Sustain Energy Rev 88:109–122. https://doi.org/10.1016/j.rser.2018.02.019
Nanninga HJ, Gottschal JC (1986) Isolation of a sulfate-reducing bacterium growing with methanol. FEMS Microbiol Ecol 38:125–130. https://doi.org/10.1111/j.1574-6968.1986.tb01959.x
Navarrete C, Nielsen J, Siewers V (2014) Enhanced ethanol production and reduced glycerol formation in fps1∆ mutants of Saccharomyces cerevisiae engineered for improved redox balancing. AMB Express 4:1–8. https://doi.org/10.1186/s13568-014-0086-z
Nouioui I, Carro L, García-López M, Meier-Kolthoff JP, Woyke T, Kyrpides NC, Pukall R, Klenk H-P, Goodfellow M, Göker M (2018) Genome-based taxonomic classification of the phylum Actinobacteria. Front Microbiol 9:2007. https://doi.org/10.3389/fmicb.2018.02007
Oleskowicz-Popiel P (2018) Designing reactor microbiomes for chemical production from organic waste. Trends Biotechnol 36:747–750. https://doi.org/10.1016/j.tibtech.2018.01.002
Qatibi AI, Bennisse R, Jana M, Garcia JL (1998) Anaerobic degradation of glycerol by Desulfovibrio fructosovorans and D. carbinolicus and evidence for glycerol-dependent utilization of 1,2- propanediol. Curr Microbiol 36:283–290. https://doi.org/10.1007/s002849900311
Qatibi AI, Bories A, Garcia JL (1991a) Sulfate reduction and anaerobic glycerol degradation by a mixed microbial culture. Curr Microbiol 22:47–52. https://doi.org/10.1007/BF02106212
Qatibi AI, Cayol JL, Garcia JL (1991b) Glycerol and propanediols degradation by Desulfovibrio alcoholovorans in pure culture in the presence of sulfate, or in syntrophic association with Methanospirillum hungatei. FEMS Microbiol Ecol 85:233–240. https://doi.org/10.1111/j.1574-6968.1991.tb04729.x
Rabus R, Hansen TA, Widdel F (2006) Dissimilatory sulfate- and sulfur-reducing Prokaryotes. In: The Prokaryotes. New York: Springer, 659–768. https://doi.org/10.1007/0-387-30742-7_22
Richter K, Gescher J (2014) Accelerated glycerol fermentation in Escherichia coli using methanogenic formate consumption. Bioresour Technol 162:389–391. https://doi.org/10.1016/j.biortech.2014.04.011
Sousa DZ, Pereira MA, Smidt H, Stams AJM, Alves MM (2007) Molecular assessment of complex microbial communities degrading long chain fatty acids in methanogenic bioreactors. FEMS Microbiol Ecol 60:252–265. https://doi.org/10.1111/j.1574-6941.2007.00291.x
Stams AJM, Van Dijk JB, Dijkema C, Plugge CM (1993) Growth of syntrophic propionate−oxidizing bacteria with fumarate in the absence of methanogenic bacteria. Appl Environ Microbiol 59:1114–1119. https://doi.org/10.1128/aem.59.4.1114-1119.1993
Tanaka K, Nakamura K, Mikami E (1990) Fermentation of maleate by a gram-negative strictly anaerobic non-spore-former, Propionivibrio dicarboxylicus gen. nov., sp. nov. Appl Microbiol 154:323–324. https://doi.org/10.1007/BF00276526
Thrash JC, Pollock J, Torok T, Coates JD (2010) Description of the novel perchlorate-reducing bacteria Dechlorobacter hydrogenophilus gen. nov., sp. nov. and Propionivibrio militaris, sp. nov. Appl Microbiol Biotechnol 86:335–343. https://doi.org/10.1007/s00253-009-2336-6
Ueki A, Goto K, Ohtaki Y, Kaku N, Uekiet N (2017) Description of Anaerotignum aminivorans gen. nov., sp. nov., a strictly anaerobic, amino-acid-decomposing bacterium isolated from a methanogenic reactor, and reclassification of Clostridium propionicum, Clostridium neopropionicum and Clostridium lactatifermentans as species of the genus Anaerotignum. Int J Syst Evol Microbiol 67:4146–4153. https://doi.org/10.1099/ijsem.0.002268
Viana MB, Freitas AV, Leitão RC, Pinto GAS, Santaella T (2012) Anaerobic digestion of crude glycerol: a review. Environ Technol Rev 1:81–92. https://doi.org/10.1080/09593330.2012.692723
Yazdani SS, Gonzalez, (2007) Anaerobic fermentation of glycerol: a path to economic viability for the biofuels industry. Curr Opin Biotechnol 18:213–219. https://doi.org/10.1016/j.copbio.2007.05.002
Zhang A, Yang ST (2009) Propionic acid production from glycerol by metabolically engineered Propionibacterium acidipropionici. Process Biochem 44:1346–1351. https://doi.org/10.1016/j.procbio.2009.07.013
Zhang F, Zhang Y, Chen Y, Dai K, van Loosdrecht MCM, Zeng RJ (2015) Simultaneous production of acetate and methane from glycerol by selective enrichment of hydrogenotrophic methanogens in extreme-thermophilic (70 °C) mixed culture fermentation. Appl Energy 148:326–333. https://doi.org/10.1016/j.apenergy.2015.03.104
Funding
Open access funding provided by FCT|FCCN (b-on). This study was supported by the Portuguese Foundation for Science and Technology (FCT) under the scope of the strategic funding of UIDB/04469/2020 unit, with https://doi.org/10.54499/UIDB/04469/2020. Work of POP and AD was partly financed from Poznan University of Technology structural funds (no. 0713/SBAD/0981).
Author information
Authors and Affiliations
Contributions
AJC, AJMS and CM contributed to the study conception and design. AJC provided guidance to CM and AD and streamlined communication between the different labs. Research was performed by CM and AD. CM and JA drafted the manuscript with the support of AJC. All authors participated in data interpretation and scientific discussion as well as revisions of the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Magalhães, C.P., Alves, J.I., Duber, A. et al. Metabolic versatility of anaerobic sludge towards platform chemical production from waste glycerol. Appl Microbiol Biotechnol 108, 419 (2024). https://doi.org/10.1007/s00253-024-13248-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-024-13248-6