Abstract
The lincoamide antibiotic lincomycin, derived from Streptomyces lincolnensis, is widely used for the treatment of infections caused by gram-positive bacteria. As a common global regulatory factor of GntR family, DasR usually exists as a regulatory factor that negatively regulates antibiotic synthesis in Streptomyces. However, the regulatory effect of DasR on lincomycin biosynthesis in S. lincolnensis has not been thoroughly investigated. The present study demonstrates that DasR functions as a positive regulator of lincomycin biosynthesis in S. lincolnensis, and its overexpression strain OdasR exhibits a remarkable 7.97-fold increase in lincomycin production compared to the wild-type strain. The effects of DasR overexpression could be attenuated by the addition of GlcNAc in the medium in S. lincolnensis. Combined with transcriptome sequencing and RT-qPCR results, it was found that most structural genes in GlcNAc metabolism and central carbon metabolism were up-regulated, but the lincomycin biosynthetic gene cluster (lmb) were down-regulated after dasR knock-out. However, DasR binding were detected with the DasR responsive elements (dre) of genes involved in GlcNAc metabolism pathway through electrophoretic mobility shift assay, while they were not observed in the lmb. These findings will provide novel insights for the genetic manipulation of S. lincolnensis to enhance lincomycin production.
Key points
• DasR is a positive regulator that promotes lincomycin synthesis and does not affect spore production
• DasR promotes lincomycin production through indirect regulation
• DasR correlates with nutrient perception in S. lincolnensis
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lincomycin is an important lincoamide antibiotic produced by S. lincolnensis. It is commonly used in the clinical treatment of infections caused by gram-positive bacteria, such as respiratory tract, urinary system, skin and soft tissue infections and other diseases, such as otitis media and eye diseases (Zhang et al. 1992). In the past decades of production practice, the industrial strains of lincomycin are mainly mutated by physical or chemical methods. However, these approaches are confronted with challenges of high reaction demands and prolonged reaction times. Therefore, there is an urgent need for a genetically engineered strain capable of producing lincomycin via fermentation to meet the demands of industrial production. The biosynthesis process of lincomycin has been extensively studied and its synthetic pathway has become increasingly clear. The widely recognized synthesis of lincomycin can be divided into three parts (Neusser et al. 1998; Spizek and Rezanka 2017; Wang et al. 2020): the synthesis of Methylthiolincosamide (MTL), the synthesis of 4-propyl-L-proline (PPL), and the subsequent condensation and modification processes. However, the current understanding of the regulatory mechanism underlying lincomycin biosynthesis in S. lincolnensis remains limited, impeding its potential for industrial production.
DasR belongs to the GntR family of transcriptional suppressor proteins. As a global transcriptional regulatory protein, DasR binds to the DasR responsive element (dre) in the gene promoter region of Streptomyces species to exert transcriptional regulation (Colson et al. 2007; Rigali et al. 2008). These binding sites are widely distributed, covering genes related to a number of primary and secondary metabolisms such as phosphotransferase system (PTS), aerial mycelium growth, chitin degradation, antibiotic synthesis, etc. (Colson et al. 2007; Nazari et al. 2013; Rigali et al. 2006), suggesting an important global regulatory role played by DasR. dasR-deficient S. griseus showed abnormalities in both aerial mycelial growth and spore production when glucose was used as a carbon source. In S. griseus, the gene dasA has been identified alongside the gene dasR, which is located adjacent to it but transcribed in the opposite direction. Mutation or overexpression of both genes has been reported to affect the morphology of S. griseus strains (Seo et al. 2002).
DasR also has an important regulatory role in antibiotic synthesis. In most cases, DasR exerts a negative regulatory effect on antibiotic synthesis (Huang et al. 2022). In the dasR deficient S. coelicolor BAP29, actinomycetin production was significantly increased (Rigali et al. 2004). In S. verticillus, though there is no dre sequence in the bleomycin biosynthesis gene cluster, DasR exerts an inhibitory effect on the production of bleomycin through indirect regulation (Chen et al. 2020).
Streptomyces, as a microorganism widely living in soil, usually takes the hydrolysates of chitin as its food source. Chitin is digested into N-acetylglucosamine (GlcNAc) and dimer (GlcNAc)2 and released into the environment to be re-taken by new cells (Colson et al. 2008). GlcNAc is an important signaling molecule (Rigali et al. 2006), which is transferred to the intracellular by phosphotransferase system and then phosphorylated to generate GlcNAc-6P, and then deacetylated by NagA to generate GlcN-6P (Nothaft et al. 2010). Both GlcNAc-6P and GlcN-6P specifically inhibit the binding of DasR to the promoter of the gene, releasing the original DNA target, which is an important effector molecule for DasR regulation (Rigali et al. 2006), and then catalyzed by NagB to generate Fru-6P, which can enter the glycolytic pathway (Chen et al. 2019). DasR binding sites were also generally found in the existing upstream of many GlcNAc-related genes, which are the targets of DasR, including the genes of the chitinolytic system, as well as the transport and metabolism genes of GlcNAc and its polymers. The activity of DasR and its response to GlcN-6P and GlcNAc-6P levels depend on environmental conditions: adding high concentrations of GlcNAc in nutrient-poor conditions (e.g., on minimal medium) enhances antibiotic production; however, GlcNAc inhibited antibiotic production in enriched medium (Van Wezel et al. 2009). These results indicate that the regulation of DasR-GlcNAc is influenced by the nutrient level of bacteria.
The structural genes, resistance genes, and regulatory genes within the lincomycin biosynthetic gene cluster have been relatively well studied. The findings suggest that the regulatory role of DasR in Streptomyces growth, differentiation, and antibiotic production is intricate and significant. However, the regulatory mechanism of global factor DasR on the biosynthesis of lincomycin by S. lincolnensis remains elusive. The compound lincomycin serves as the prototypical member of the lincoamide antibiotics and elucidating the role of DasR on regulating lincomycin production in S. lincolnensis holds significant implications for enhancing the synthesis of lincoamide antibiotics. Therefore, we investigated the regulatory mechanism of the global factor DasR on the biosynthesis of lincomycin in S. lincolnensis by engineering bacterial construction and fermentation, transcriptome sequencing, and in vitro binding assay of DasR protein and gene.
In this study, the wild-type S. lincolnensis NBRC_13054 was used as the original starting strain to study the regulation effect of DasR on lincomycin biosynthesis. DasR is proved to be a positive regulator for lincomycin biosynthesis, and the effects of DasR on regulating lincomycin production in S. lincolnensis were analyzed by transcriptome sequencing, RT-qPCR and electrophoretic mobility shift assay. It was discovered that DasR potentially exerts a global impact by directly regulating the carbon metabolism, thereby influencing lincomycin production.
Materials and methods
Bacteria, plasmids, and media
All plasmids and strains used or constructed in this study are listed in Table 1. Escherichia coli strains were cultivated in LB (Luria-Bertani) medium at 37 °C. S. lincolnesis strains were cultured on MS (mannitol soya flour) medium at 28 °C for spore preparation and phenotypic observation, in YEME (yeast extract-malt extract) medium at 28 °C for cellular growth, or in ISP-2 media at 28 °C for lincomycin fermentation.
Strain construction
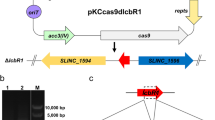
A CRISPR/Cas9-mediated genetic editing method (Huang et al. 2015) was used for the deletion of dasR from S. lincolnensis NBRC_13054 (wild type, WT). The construction process is shown in Fig. S1a. The recognition site GGG of S. lincolnensis dasR gene and its first 20 bp guide sequence GTCAGCAGTGCGGAGAACGA were selected through the website (https://zlab.squarespace.com/guide-design-resourcesforecast). Then, PCR amplification was performed to obtain the guide sequence and homologous arms of dasR gene with an upstream and downstream length of 1000 bp, using pKCcas9dO plasmid and S. lincolnensis NBRC_13054 genome as templates respectively. A recombinant fragment of about 2100 bp in length was obtained by fusion PCR (Fig. S1). Then, the recombinant fragment was ligated between to the SpeI and HindIII sites of pKCcas9dO plasmid through seamless cloning resulting in pKCcas9dO-dasR. The primers used in this experiment are shown in Table S1. The pKCcas9dO-dasR plasmid was transformed into E. coli ET12567 (pUZ8002), and conjugative transfer experiments were carried out according to the method reported (Hou et al. 2018). The correct clone, namely DdasR, was confirmed through PCR and sequencing analysis as depicted in Fig. S1b, c.
The CDS region of dasR gene was amplified by PCR using the genome of S. lincolnensis NBRC_13054 as templates and was then inserted to the NdeI and EcoRI sites of pIB139 by the same seamless cloning method resulting in pIB139-dasR. The primers used in this experiment are shown in Table S2. The plasmid was conjugated and transferred to S. lincolnensis NBRC_13054, getting strain OdasR. The construction process is shown in Fig. S2.
Fermentation and lincomycin bioassay detection
S. lincolnensis NBRC_13054 and its derivatives were coated on spore-producing solid medium at 28 °C for 5–7 days, then 1–2 cm2 fresh S. lincolnensis moss were shoveled from the solid medium with a sterile cell spatula, inoculated into 25 mL of Fermentation Primary Medium (250 mL shaker), and fermented in the shaker (220 rpm) for 2 days at 28 °C. According to the 10% inoculum volume, the bacterial sap was transferred to 25 mL of fermentation secondary medium (250 mL shaker) and incubated at 28 °C, 220 rpm for 7 days.
One milliliter of fermentation broth at the end of fermentation was taken, and the supernatant was centrifuged and detected by high-performance liquid chromatography (HPLC). Detection conditions: column: C18 (SinoChrom ODS-BP, 4.6 × 250 mm, 5 μm, Dalian Elite Analytical Instruments Co., Ltd.); mobile phase: 50 mM ammonium acetate solution: methanol = 4:6; flow rate: 0.6 mL/min; injection volume: 20 µL; detection wavelength: 210 nm.
Dry weight was used as the index of biomass detection. After shaking the fermentation broth, 4 mL of the fermentation broth was taken into an EP tube, centrifuged and washed for sedimentation, the EP tube in which the bacterial sediment was collected was dried in an electric blower drying oven with the lid open until the mass no longer changed, and the data were recorded; the dry weight was recorded.
RNA-Seq transcriptomic analysis
The initial strain NBRC_13054 and the knockout strain DdasR were selected for subsequent transcriptome sequencing analysis on days 2, 4, and 7 of fermentation, which represent the rapid growth stage of the bacterium in the pre-fermentation period, the stabilization stage in the mid-fermentation period, and the end of fermentation, respectively. Transcriptome sequencing was based on the Illumina sequencing platform, and gene expression was calculated using the FPKM (Fragments Per Kilo bases per Million reads) (Mortazavi et al. 2008) method; and NBRC_13054 (GeneBank ID: CP016438.1) was used as the control group, which was screened according to the significance of difference criterion, i.e., the differential gene expression change was more than 2-fold (|log2(Fold Change)|>1) and q-value ≤ 0.05, gene significance differential expression up- and downregulation was counted. The transcriptome raw data of WT and DdasR were deposited in the NCBI Sequence Read Archive (SRA) under accession no. PRJNA1077293.
RNA extraction and quantitative real-time PCR (RT-qPCR) analyses
Total RNA was extracted using the RNAprep pure Cell/Bacteria Kit from Tengen Biotech Co., Ltd. Reverse transcription experiments were performed using the SPARK script II RT Plus Kit (With gDNA Eraser) for first-strand cDNA synthesis from Shandong Sparkjade Biotechnology Co. The enhancer 1,2,4-triazole can be added to the system optionally to improve the amplification specificity of the high GC template. The PCR primers used in the experiments are shown in Table S2, and the amplified products were best 100–150 bp long. The relative expression of the gene was calculated by 2−ΔΔCt.
Construction and purification of DasR protein expression vector
The expression of soluble protein DasR by E. coli requires the construction of pCold II vector containing dasR gene. The construction process is shown in Fig. S3.
After PCR amplification of the pCold II linearized vector and dasR gene using primers are shown in Table S3, the target fragment was connected to the vector plasmid by seamless cloning. The insertion position of dasR gene was between NedI and XhoI sites after the 6×His label. After construction, it was transformed into E. coli receptor state BL21 (DE3), then the correctly sequenced plasmid was stored in E. coli at low temperature, and the DasR expression strain containing pCold II-dasR vector was constructed. After determining the optimal imidazole elution concentration, the eluate containing the target protein was concentrated in a MILLIPORE protein ultrafiltration tube, and all the eluate was concentrated to about 1.5 mL.
Protein preparation and electrophoretic mobility shift assays (EMSAs)
EMSA was performed according to the kit instructions. Probes were prepared using Cy5 fluorescent labeling for two rounds of PCR amplification. Configure 20 µL of gel migration assay system. A 6% non-denaturing polyacrylamide gel was prepared under light-avoiding conditions, electrophoresed, and the results were recorded using a fluorescent imaging system. First PCR primers for the promoter regions of the target genes are shown in Table S4.
Result
DasR positively regulates lincomycin production in S. lincolnensis
In order to explore the effect of DasR on the lincomycin production and growth of S. lincolnensis, we constructed knockout (DdasR) and overexpression (OdasR) strains of the DasR gene. As shown in Fig. 1a, the dry cell weight (DCW) changes of DdasR strain and the wild-type strain (WT) are similar: both strains undergo logarithmic growth two days prior to fermentation, followed by a gradual transition to a stable stage and subsequent decline. These findings indicated that the knockout of dasR gene does not affect the growth of S. lincolnensis. However, the OdasR strain grew rapidly on the first day of fermentation, and its DCW dropped significantly from the second day. The overexpression of the DasR gene is speculated to enhance the transcriptional inhibitory role of DasR, thereby impacting strain growth and driving it towards a decline phase. The lincomycin production of strains in the fermentation process was significantly weakened by the deletion of the DasR gene, as depicted in Fig. 1b. Conversely, overexpression of the DasR gene led to a significant increase in lincomycin yield. However, the lincomycin titer appears to have decreased following the DCW of OdasR. The lincomycin titer was 27.25 mg/L, 46.63 mg/L, and 371.69 mg/L after 7 days of fermentation for WT, DdasR, and OdasR strains, respectively. Moreover, the highest lincomycin titer reached 499.86 mg/L after 2 days fermentation of OdasR, exhibiting a remarkable increase of 7.97 times compared to the wild-type strain. According to the results, DasR plays a positive role in lincomycin production but severely impacts the growth of S. lincolnensis. Therefore, phenotypic differences of the engineered strains were evaluated on the spore-producing plate (Fig. 1c). The sporulation of DdasR and OdasR strains was observed to be significantly delayed compared to that in the WT strain. Moreover, the spores produced by OdasR exhibited slightly light gray coloration compared to the other two strains, suggesting that DasR gene may have an effect on the morphological differentiation of S. lincolnensis.
GlcNAc addition weakened the positive regulatory effect of DasR on lincomycin production
As an important signaling molecule of Streptomyces, GlcNAc can relieve the inhibition of global transcription factor DasR on GlcNAc metabolism, sugar transport, antibiotic synthesis, and development. To investigate its effect on lincomycin production, GlcNAc was added to the fermentation medium at final concentrations of 50 mM and 100 mM. GlcNAc was added to the fermentation medium; the production of lincomycin could not be detected for WT and DdasR strains. However, the addition of either 50 mM or 100 mM GlcNAc exhibited negligible impact on the production of lincomycin with OdasR. Therefore, 200 mM GlcNAc was then added, and the lincomycin production decreased from 371.69 to 220.00 mg/L with strain OdasR. These results indicate that GlcNAc or its intermediate metabolites can inhibit lincomycin production in S. lincolnensis under eutrophic conditions, and the inhibiting effects could be partially released by DasR.
The addition of GlcNAc also resulted in an increase in the DCW of the three strains after fermentation, as depicted in Fig. 2a since exogenous GlcNAc could provide more carbon sources for S. lincolnensis. These results were consistent with the increase in DCW after the addition of GlcNAc in Streptomyces tsukubaensis culture medium (Ordonez-Robles et al. 2018). The fermentation broth of WT and DdasR exhibited a deep brown color as the exogenous GlcNAc concentration increased, while the color of OdasR fermentation broth remained largely unchanged (Fig. 2b). This observation suggests that the regulatory network governed by DasR genes may also be involved in pigment synthesis. The addition of GlcNAc to the nutritive spore plate resulted in a significant inhibition of spore production for all three strains, particularly in the case of DdasR where no spores were observed on the 100 mM GlcNAc plate after 6 days of cultivation.
When GlcNAc was added for fermentation, it was also observed that the color of the fermentation broth was different from that of the fermentation broth without GlcNAc. Figure 2b shows the color comparison of the three strains. The fermentation broths of wild bacteria and DdasR deepened with increasing concentrations of exogenous GlcNAc, and the color of the fermentation broths of OdasR was essentially unchanged with the addition of GlcNAc, suggesting that the regulation between the GlcNAc and dasR genes may also involve the synthesis of other secondary metabolites, such as pigments.
Effect of different concentrations of GlcNAc on WT, DdasR, and OdasR strains. a The effect of different concentrations of GlcNAc on dry cell weight of WT, DdasR, and OdasR strains. b Effect of different concentrations of GlcNAc on the color of WT, DdasR, and OdasR fermentation broth. c Effect of 50 mM GlcNAc on sporulation of WT, DdasR, and O dasR. d Effect of 100 mM GlcNAc on sporulation of WT, DdasR, and O dasR
The transcriptome and RT-qRCR analysis of DasR-engineered strains
To provide a comprehensive understanding of the global regulation mediated by DasR, the transcriptome sequencing analysis was conducted on both the wild-type strain NBRC_13054 and the DasR knock-out strain DdasR at 48 h, 96 h, and 168 h, respectively. We focused on finding whether they contained genes within the lincomycin biosynthesis cluster and found that reduced transcript levels of cluster genes were detected in the knockout bacteria compared to the wild type in the first two periods, except on day 7. These genes covered the vast majority of structural, regulatory, and resistance genes within lmb, suggesting that the reduced transcript levels of the cluster genes due to the deletion of the dasR gene are directly responsible for the reduced lincomycin production (Fig. S4). Therefore, we generated a comprehensive map of differentially expressed genes (DEGs) associated with lincomycin biosynthesis based on the transcriptome sequencing results at 48 h, as shown in Fig. 3. After DasR gene knockout, all structural genes involved in lincomycin biosynthesis gene cluster were downregulated in the process of synthesis of LSM and PPL and their condensation. However, in the part of primary metabolism, some genes in glycolysis and pentose phosphate pathway are basically transcriptionally upregulated due to the deletion of dasR. Moreover, genes related to the metabolism of signaling molecules GlcNAc, such as dasA, nagK, nagA, and nagB, are also transcriptionally upregulated due to the deletion of dasR (Fig. S4). These results indicated that DasR may play a certain regulatory relationship with GlcNAc metabolism, central carbon metabolism, and secondary metabolism.
The purple part is the biosynthesis of LSM, the red part is the biosynthesis of PPL, the green part is the condensation of the two, and the yellow part is the basic metabolic pathway, including some key processes of glycolysis, pentose phosphate pathway, L-tyrosine synthesis of branching acids, and GlcNAc transport from extracellular to intracellular metabolism. The red triangle upward indicates that the knockout bacteria has upregulated transcription compared to the original bacteria, and the blue triangle downward indicates that the knockout bacteria has downregulated transcription compared to the original bacteria. 1 indicates 4-(3-carboxy-2-methylpropyl)-2,3-dihydro-1 H-pyrrole-2carboxylic acid. 2 indicates (E)-4-(prop-1-en-l-yl)-3,4-dihydro-2 H-pyrrole-2-carboxylic acid. 3 indicates (E)-4-propylidene-3,4-dihydro-2 H-pyrrole-2-carboxylic acid. 4 indicates S-(3,4,5-trihydroxy-6-(2-hydroxy-1-(1-methy1-4-propylpyrolidine-2-carboxamido)propyl)tetrahydro-2 H-pyran-2-yl)cysteine.
The DEGs related to central carbon metabolism at 48 h, 96 h, and 168 h of fermentation were then collected and exhibited in Fig. 4. The overall trend was that the transcription level of the majority of genes in central carbon metabolism of DdasR was significantly upregulated at 48 and 96 h, whereas there were no significant differences in other genes at 168 h except slinc_7065, which encodes transketolase (TKT) and showed a significant downregulation compared to the wild type (WT). These results further suggests that the regulation of central carbon metabolism by DasR is closely related to fermentation time.
In order to verify the accuracy of transcriptome sequencing, genes concerning lincomycin biosynthesis and GlcNAc metabolism were selected for RT-qPCR analysis (Fig. 5a, b). As shown in Fig. 5a, the differentially expressed genes analyzed by RT-qPCR were consistent with the transcriptome sequencing results. The transcription of lmb genes in OdasR was also analyzed by RT-qPCR (Fig. 5b), compared with WT strains; the transcription levels of structural genes and resistance genes in the lmb cluster are significantly improved, which explains the higher lincomycin production in OdasR strain.
Analysis of DasR transcriptional regulatory targets in S. lincolnensis by EMSA
It has been reported that DasR can specifically bind to a 16 bp sequence of gene promoter region, ASTGGTCTAGACCAST, to exert transcriptional regulation. Therefore, this sequence was uploaded to MEME/MAST (https://meme-suite.org/meme/doc/mast.html) to predict potential DasR binding sites in S. lincolnensis NBRC_13054. Relevant attention was directed towards the genes within the Lincomycin biosynthetic gene cluster and GlcNAc metabolism. Figure S5 shows the Weblogo of the dre sequence and the upstream region of genes predicted by MEME that may be bound by DasR. A total of seven binding sites were predicted, including three genes (lmrA, lmbR, and lmbU) located in the Lincomycin biosynthetic gene cluster, three genes (dasA, nagB, and nagK) in GlcNAc metabolism and dasR itself. Previous studies have shown that the dasABCD gene, involved in GlcNAc metabolism in S. coelicolor and S. verticillus, is located within the same transcriptional unit and transcribed in the opposite direction to the upstream adjacent dasR gene, as well as downstream adjacent nagB, nagK, and nagA genes present within the same transcriptional unit in their respective genomes (Chen et al. 2019). There were 90.12%, 83.52%, 70.25%, and 68.78% amino acid sequence similarities between DasR, NagB, NagK, and NagA in S. lincolnensis and their homologs in S. verticillus, respectively. Based on these findings, as well as the prediction results from MEME and NCBI’s analysis of the whole genome sequence of wild-type S. lincolnensis NBRC_13054, it is speculated that the arrangement of these genes of S. lincolnensis used in this study is the same as that of S. coelicolor and S. verticillus. Subsequently, EMSA was conducted to validate the binding affinity of DasR towards these target genes, and the corresponding results are presented in Fig. 6. Although MEME predicted that there were dre sequences in the upstream regions of lmrA, lmbR, and lmbU of the three genes in the lincomycin biosynthesis gene, the EMSA results found that DasR did not bind to these locations. Combined with the transcriptome results, it could be inferred that the upregulation of the in-cluster gene transcription by DasR might be carried out through other indirect regulatory modes. The EMSA results for dasA, nagB, and nagK genes indicated a direct regulation mode to GlcNAc metabolism by DasR. As a regulatory protein, DasR also binds to the promoter region of its own gene dasR to exert negative feedback on its own transcription, and this auto-regulatory mechanism has been previously reported in literature (Xu et al. 2020). Additionally, in vitro assays were conducted to evaluate the activity of several negative regulators of lincomycin production, namely SLINC_3938, SLINC_4481, SLINC_4906, and SLINC_6156. This investigation was prompted by the observation that DasR exhibited binding affinity towards certain sites in S. coelicolor that lacked obvious similarity to dre (Swiatek-Polatynska et al. 2015). However, no direct binding was observed in our experiment.
EMSAs of DasR protein and 50 ng/µL target gene probe. The fifth lane of each group in the Fig. 6 is the system with additional 10 mg/mL salmon sperm
Discussion
As a common global regulator of GntR family, DasR, has been found to affect the growth, antibiotic synthesis, and other metabolic processes in S. coelicolor (Engel et al. 2020) and Saccharopolyspora erythraea (You et al. 2018) in recent years. The DdasR knockout strain and OdasR overexpressed strain were initially constructed in this study, followed by a comparison of lincomycin production and growth phenotypes between the engineered strains and the wild-type bacteria. Additionally, the effects of GlcNAc on the growth and fermentation of all three strains were investigated.
It was found that the dasR gene did not affect the spore production, but the wild type produced spores faster, and the two engineered strains produced spores with a slight delay. The spores produced by OdasR were slightly light gray, unlike the other two strains, which were pure white. The overexpression strain OdasR showed a 7.97-fold increase in lincomycin yield over the wild type. In S. coelicolor, the dasR-deficient BAP29 rarely produced spores on SFM solid medium, with a spore count about three orders of magnitude lower than that of the parental M145 and did not produce spores on R2YE medium (Rigali et al. 2008). In S. roseosporus, the effect of DasR on its morphology contrasted with S. griseus and S. coelicolor, where the dasR-deficient bacteria not only produced white spores but also spore formation at an earlier time, whereas the spore formation time of backfilled and dasR overexpressing strains did not differ significantly from that of the wild type (Chen et al. 2022). In addition, the pigment production of the dasR deletion strain was much lower than that of the wild type, but the difference between the back-complemented and overexpression strains was not significant, which also proved that DasR was associated with pigment production. Comparing the changes in lincomycin production, it is intuitively obvious that knockdown of the dasR gene significantly impairs the ability of the strain to produce lincomycin, which is contrary to the negative regulatory effects of most of the dasR genes. In rare cases, DasR positively regulates the production of antibiotics, such as the monensin production in S. cinnamonensis (Zhang et al. 2016). Overexpression, knockdown, and antisense repression of the dasR gene in S. cinnamonensis revealed that DasR binds to the promoter regions of the monE, monT, monAIX, and monRII genes within the monensin BGCs, upregulates their transcription, and facilitates the production of monensin, but the regulatory effects are related to environmental factors.
The DCW of the knockout strain DdasR was comparable to that of the wild-type strain, while the lincomycin production exhibited a decrease. Reduced and elevated expression of the lmb gene was directly responsible for the significant reduction and elevation of lincomycin production in DdasR and OdasR, and it was experimentally found that overexpression of the lmbB1 gene effectively increased the production of lincomycin A, inhibited melanin production, and reduced the production of the by-product lincomycin B (Yang et al. 2020). Combined with the results of transcriptome analysis, most of the gene expression levels were downregulated after dasR knockdown, but most of the genes of the central carbon metabolism pathway were upregulated, and the number of differentially expressed genes decreased dramatically with the prolongation of the fermentation time, and the number of upregulated genes and downregulated genes gradually converged to the same number, which may imply that dasR regulation is closely related to the nutrient metabolism, especially the metabolism of the carbon source. As early as 2015, studies showed that the binding of DasR and dre targets in S. coelicolor was time-specific, such as the binding of DasR with primary metabolism-related genes during the vegetative growth phase (Swiatek-Polatynska et al. 2015). Considering the biomass of DdasR and WT at 48 h in this study, it is speculated that S. lincolnensis was still in the vegetative growth stage at this time, and DasR might have a negative regulatory relationship with these genes, showing a time-specific effect on these genes in the later stage. In addition, the upregulation of key genes related to central carbon metabolism after dasR gene knockout in S. lincolnensis was similar to the dasR deletion mutant of S. cerulean which upregulated the glycolysis, TCA cycle, gluconeogenesis and pyruvate metabolism, as well as the biosynthesis pathway of amino acids, nucleotides, and fatty acids metabolism. However, knockdown of dasR resulted in a significant decrease in lincomycin production, and the expression levels of all relevant genes in the lincomycin biosynthesis pathway showed downregulation.
It has been found that in Streptomyces azureus DasR also binds to a number of sites that have no obvious similarity to dre (Swiatek-Polatynska et al. 2015), and similarly, the non-coding RNA scr5239 was found to control carbon source metabolism in Streptomyces, whereas the expression of scr5239 is directly regulated by the global regulator protein DasR (Engel et al. 2020). Therefore, amplification of the relevant promoter region is also necessary to verify this in wet experiments. Since in vitro experiments did not identify dasR binding sites in the lincomycin synthesis gene cluster, it is hypothesized that dasR may control the synthesis of lincomycin, or even other secondary metabolites, through a more complex regulatory network. Similar phenomena were also found in SLINC_1596 (renamed as LcbR1) (Wang et al. 2023), inspired by these cases, DasR may regulate lincomycin biosynthesis via unknown transporter substance(s) or dasR homologs. In S. verticillus, there is no dre sequence within the bleomycin biosynthesis gene cluster, and DasR, although it does not directly regulate bleomycin synthesis, acts as an inhibitor of bleomycin production through indirect regulation (Chen et al. 2020). When overexpressing dasR, the dry weight of the knockout bacteria grew rapidly on the first day of fermentation and dropped significantly from the second day. However, lincomycin production peaked the next day. The higher expression of lincomycin biosynthesis genes was also observed. It was speculated that the overexpression of dasR gene might exert a stronger transcriptional inhibitory effect on central carbon metabolism or primary metabolism, thereby promoting an earlier activation of secondary metabolism in S. lincolnensis. In subsequent studies, it might be more beneficial to the industrial production of lincomycin by regulating the expression intensity or the expression time sequence of dasR in order to balance the synthesis of the antibiotic and the growth of the bacterium.
The dry weight of all three strains increased at the end of fermentation due to the addition of GlcNAc, and it is hypothesized that it may be due to the fact that the exogenous GlcNAc provided S. lincolnensis with more carbon sources, which promoted basal metabolism such as cell growth and development. Such results are consistent with previous studies showing an increase in cell dry weight after the addition of GlcNAc to the medium of S. tsukubaensis and verifying the use of the transcriptome that the addition of GlcNAc stimulated the transcription of genes related to glycolysis, pyruvate metabolism, and fatty acid biosynthesis (Ordonez-Robles et al. 2018).
Meanwhile, it was found that the addition of GlcNAc significantly inhibited the spore formation of S. lincolnensis, and the inhibition effect became more and more significant with the increase of the addition amount, and the addition of 100 mM of GlcNAc caused DdasR to almost lose the ability of spore formation, suggesting that dasR gene may have an effect on the morphological differentiation of S. lincolnensis. GlcNAc is the basic building block of many important polysaccharides in biological cells and is also an important component of bacterial cell wall peptidoglycan (Saito et al. 2007). GlcNAc was found to act as a signaling molecule regulating morphological differentiation and antibiotic synthesis in cells of S. coelicolor, and its metabolites inhibit the DNA-binding ability of the transcription factor DasR, thereby promoting the synthesis of secondary metabolites (Tenconi et al. 2015). In this study, we found that the addition of GlcNAc significantly reduced lincomycin production and significantly attenuated the promotion of lincomycin synthesis by dasR, suggesting that in S. lincolnensis, intracellular GlcNAc levels are an important component in the regulatory network of the global transcription factor DasR. In vitro EMSA experiments revealed that DasR inhibits the transcription of dasA, nagK, and nagB genes associated with GlcNAc metabolism by directly binding at the dre sequence position in the promoter region of these genes, a self-feedback regulation that may play an important role in maintaining the balance between bacterial growth and secondary metabolism. Exogenous addition of GlcNAc inhibits sporulation and lincomycin production in S. lincolnensis under enriched nutrient conditions, and the inhibition of the growth of engineered S. lincolnensis sporulation is particularly more pronounced, suggesting that DasR is associated with S. lincolnensis nutrient sensing (Rigali et al. 2006, 2008; Van Wezel et al. 2009).
In conclusion (Fig. 7), DasR indirectly regulates lincomycin production by facilitating the intracellular transport of GlcNAc polymer through the ABC transporter system composed of DasABC. This process is catalyzed by DasD to convert GlcNAc polymer into GlcNAc monomer, which is further converted into GlcNAc-6P and GlcN-6P catalyzed by NagK and NagA enzymes (Nothaft et al. 2010). Both GlcNAc-6P and GlcN-6P specifically inhibit the binding of DasR to the promoter of the gene, releasing the original DNA target (Rigali et al. 2006), and then catalyzed by NagB to generate Fru-6P, which can enter the glycolytic pathway (Chen et al. 2019). The aforementioned process holds the potential to further optimize GlcNAc metabolism, thereby promoting mycelium vegetative growth while impeding antibiotic synthesis and spore formation. The exogenous addition of GlcNAc exerted inhibitory effects on sporulation and lincomycin production in S. lincolnensis under eutrophic conditions, thus proved this assumption. In addition, it has been reported that DasR also acts as a regulatory protein that binds to the promoter region of its own gene, dasR, and represses its own transcription (Xu et al. 2020). However, as a transcription suppressor, DasR exerts a positive influence on lincomycin production, suggesting its involvement in a complex regulatory mechanism that may encompass unidentified negative regulators of lincomycin biosynthesis. Therefore, many efforts should be down to excavate the hidden floor between DasR and lincomycin production.
Regulation system of DasR involved in antibiotic production and GlcNAc metabolism. Pointed arrows represent activation. Flat-headed arrows represent repression. Solid lines represent direct regulation. Dashed line represents indirect regulation. Gray shadow: represents the speculation based on the references and the experimental results, and there is currently no direct data to confirm it
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Chen H, Wang J, Cui J, Wang C, Liang S, Liu H, Wen J (2019) Negative regulation of bleomycins biosynthesis by ArsR/SmtB family repressor BlmR in Streptomyces verticillus. Appl Microbiol Biotechnol 103(16):6629–6644. https://doi.org/10.1007/s00253-019-09923-8
Chen H, Cui J, Wang P, Wang X, Wen J (2020) Enhancement of bleomycin production in Streptomyces verticillus through global metabolic regulation of N-acetylglucosamine and assisted metabolic profiling analysis. Microb Cell Fact 19(1). https://doi.org/10.1186/s12934-020-01301-8
Chen Q, Zhu J, Li X, Wen Y (2022) Transcriptional Regulator DasR represses Daptomycin Production through both Direct and Cascade Mechanisms in Streptomyces roseosporus. Antibiotics-Basel 11(8). https://doi.org/10.3390/antibiotics11081065
Colson S, Stephan J, Hertrich T, Saito A, van Wezel GP, Titgemeyer F, Rigali S (2007) Conserved cis-acting elements upstream of genes composing the chitinolytic system of streptomycetes are DasR-responsive elements. J Mol Microbiol Biotechnol 12(1–2):60–66. https://doi.org/10.1159/000096460
Colson S, van Wezel GP, Craig M, Noens EEE, Nothaft H, Mommaas AM, Titgemeyer F, Joris B, Rigali S (2008) The chitoblose-binding protein, DasA, acts as a link between chitin utilization and morphogenesis in Streptomyces coelicolor. Microbiology-Sgm 154:373–382. https://doi.org/10.1099/mic.0.2007/011940-0
Engel F, Ossipova E, Jakobsson PJ, Vockenhuber MP, Suess B (2020) sRNA scr5239 involved in feedback loop regulation of Streptomyces coelicolor central metabolism. Front Microbiol 10. https://doi.org/10.3389/fmicb.2019.03121
Hou B, Tao L, Zhu X, Wu W, Guo M, Ye J, Wu H, Zhang H (2018) Global regulator BldA regulates morphological differentiation and lincomycin production in Streptomyces lincolnensis. Appl Microbiol Biotechnol 102(9):4101–4115. https://doi.org/10.1007/s00253-018-8900-1
Huang H, Zheng G, Jiang W, Hu H, Lu Y (2015) One-step high-efficiency CRISPR/Cas9-mediated genome editing in Streptomyces. Acta Biochim Biophys Sin 47(4). https://doi.org/10.1093/abbs/gmv007
Huang X, Guo W, Geng M, Zhuang Z, Bai L (2022) Global regulatory protein DasR in Streptomyces. Acta Microbiol Sinica 62(4):1260–1269. https://doi.org/10.13343/j.cnki.wsxb.20210477
Lin YB, Wang XY, Fang H, Ma YN, Tang J, Tang M, Wei GH (2012) Streptomyces shaanxiensis Sp nov., a blue pigment-producing streptomycete from sewage irrigation soil. Int J Syst Evol Microbiol 62:1725–1730. https://doi.org/10.1099/ijs.0.029959-0
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5(7):621–628. https://doi.org/10.1038/nmeth.1226
Nazari B, Kobayashi M, Saito A, Hassaninasab A, Miyashita K, Fujii T (2013) Chitin-induced gene expression in secondary metabolic pathways of Streptomyces coelicolor A3(2) grown in soil. Appl Environ Microbiol 79(2):707–713. https://doi.org/10.1128/aem.02217-12
Neusser D, Schmidt H, Spizèk J, Novotnà J, Peschke U, Kaschabeck S, Tichy P, Piepersberg W (1998) The genes lmbB1 and lmbB2 of Streptomyces lincolnensis encode enzymes involved in the conversion of L-tyrosine to propylproline during the biosynthesis of the antibiotic lincomycin A. Arch Microbiol 169(4):322–332. https://doi.org/10.1007/s002030050578
Nothaft H, Rigali S, Boomsma B, Swiatek M, McDowall KJ, van Wezel GP, Titgemeyer F (2010) The permease gene nagE2 is the key to N-acetylglucosamine sensing and utilization in Streptomyces coelicolor and is subject to multi-level control. Mol Microbiol 75(5):1133–1144. https://doi.org/10.1111/j.1365-2958.2009.07020.x
Ordonez-Robles M, Rodriguez-Garcia A, Martin JF (2018) Genome-wide transcriptome response of Streptomyces tsukubaensis to N-acetylglucosamine: effect on tacrolimus biosynthesis. Microbiol Res 217:14–22. https://doi.org/10.1016/j.micres.2018.08.014
Rigali S, Schlicht M, Hoskisson P, Nothaft H, Merzbacher M, Joris B, Titgemeyer F (2004) Extending the classification of bacterial transcription factors beyond the helix-turn-helix motif as an alternative approach to discover new cis/trans relationships. Nucleic Acids Res 32(11):3418–3426. https://doi.org/10.1093/nar/gkh673
Rigali S, Nothaft H, Noens EEE, Schlicht M, Colson S, Mueller M, Joris B, Koerten HK, Hopwood DA, Titgemeyer F, van Wezel GP (2006) The sugar phosphotransferase system of Streptomyces coelicolor is regulated by the GntR-family regulator DasR and links N-acetylglucosamine metabolism to the control of development. Mol Microbiol 61(5):1237–1251. https://doi.org/10.1111/j.1365-2958.2006.05319.x
Rigali S, Titgemeyer F, Barends S, Mulder S, Thomae AW, Hopwood DA, van Wezel GP (2008) Feast or famine: the global regulator DasR links nutrient stress to antibiotic production by Streptomyces. EMBO Rep 9(7):670–675. https://doi.org/10.1038/embor.2008.83
Saito A, Shinya T, Miyamoto K, Yokoyama T, Kaku H, Minami E, Shibuya N, Tsujibo H, Nagata Y, Ando A, Fujii T, Miyashita K (2007) The dasABC gene cluster, adjacent to dasR, encodes a novel ABC transporter for the uptake of N,N’-diacetylchitobiose in Streptomyces coelicolor A3(2). Appl Environ Microbiol 73(9):3000–3008. https://doi.org/10.1128/aem.02612-06
Seo JW, Ohnishi Y, Hirata A, Horinouchi S (2002) ATP-binding cassette transport system involved in regulation of morphological differentiation in response to glucose in Streptomyces griseus. J Bacteriol 184(1):91–103. https://doi.org/10.1128/jb.184.1.91-103.2002
Spizek J, Rezanka T (2017) Lincosamides: chemical structure, biosynthesis, mechanism of action, resistance, and applications. Biochem Pharmacol 133:20–28. https://doi.org/10.1016/j.bcp.2016.12.001
Swiatek-Polatynska MA, Bucca G, Laing E, Gubbens J, Titgemeyer F, Smith CP, Rigali S, van Wezel GP (2015) Genome-wide analysis of in vivo binding of the master regulator DasR in Streptomyces coelicolor identifies novel non-canonical targets. PLoS ONE 10(4). https://doi.org/10.1371/journal.pone.0122479
Tenconi E, Urem M, Swiatek-Polatynska MA, Titgemeyer F, Muller YA, van Wezel GP, Rigali S (2015) Multiple allosteric effectors control the affinity of DasR for its target sites. Biochem Biophys Res Commun 464(1):324–329. https://doi.org/10.1016/j.bbrc.2015.06.152
Van Wezel GP, McKenzie NL, Nodwell JR (2009) Applying the genetics of secondary metabolism in model actinomycetes to the discovery of new antibiotics. 458(issue): 117-+. https://doi.org/10.1016/s0076-6879(09)04805-8
Wang X, Zhang C, Wang M, Lu W (2014) Genome-scale metabolic network reconstruction of Saccharopolyspora spinosa for Spinosad Production improvement. Microb Cell Fact 13. https://doi.org/10.1186/1475-2859-13-41
Wang SA, Lin CI, Zhang J, Ushimaru R, Sasaki E, Liu HW (2020) Studies of lincosamide formation complete the biosynthetic pathway for lincomycin A. Proc Natl Acad Sci USA 117(40):24794–24801. https://doi.org/10.1073/pnas.2009306117
Wang R, Zhao J, Chen L, Ye J, Wu H, Zhang H (2023) LcbR1, a newly identified GntR family regulator, represses lincomycin biosynthesis in Streptomyces lincolnensis. Appl Microbiol Biotechnol 107(24):7501–7514. https://doi.org/10.1007/s00253-023-12756-1
Wilkinson CJ, Hughes-Thomas ZA, Martin CJ, Böhm I, Mironenko T, Deacon M, Wheatcroft M, Wirtz G, Staunton J, Leadlay PF (2002) Increasing the efficiency of heterologous promoters in actinomycetes. J Mol Microbiol Biotechnol 4(4):417–426
Xu Y, Tang Y, Wang N, Liu J, Cai X, Cai H, Li J, Tan G, Liu R, Bai L, Zhang L, Wu H, Zhang B (2020) Transcriptional regulation of a leucine-responsive regulatory protein for directly controlling lincomycin biosynthesis in Streptomyces lincolnensis. Appl Microbiol Biotechnol 104(6):2575–2587. https://doi.org/10.1007/s00253-020-10381-w
Yang J, Ye R, Zhang H, Liu Y (2020) Amplification of lmbB1 gene in Streptomyces lincolnensis improves quantity and quality of lincomycin A fermentation. Prep Biochem Biotechnol 50(6):529–537. https://doi.org/10.1080/10826068.2019.1710714
You D, Zhang B-Q, Ye B-C (2018) GntR family regulator DasR controls acetate assimilation by directly pepressing the acsA gene in Saccharopolyspora erythraea. J Bacteriol 200(13). https://doi.org/10.1128/jb.00685-17
Zhang HZ, Schmidt H, Piepersberg W (1992) Molecular cloning and characterization of two lincomycin-resistance genes, lmrA and lmrB, from Streptomyces lincolnensis 78-11. Mol Microbiol 6(15):2147–2157. https://doi.org/10.1111/j.1365-2958.1992.tb01388.x
Zhang Y, Lin C, Li X, Tang Z, Qiao J, Zhao G (2016) DasR positively controls monensin production at two-level regulation in Streptomyces cinnamonensis. J Ind Microbiol Biotechnol 43(12):1681–1692. https://doi.org/10.1007/s10295-016-1845-4
Acknowledgements
Thanks for the support of the Key Laboratory of the Ministry of Education for Conservation and Utilization of Special Biological Resources in the Western.
Funding
Ningxia Hui Autonomous Region Key R&D Program (No. 2021BEG02003) and National Key R&D Program of China (No. 2023YFF0713805).
Author information
Authors and Affiliations
Contributions
HP and YL conducted the experiments, analyzed data, and wrote the manuscript. CZ provided assistance in data analysis and manuscript revision. JS and WL designed and supervised the project strictly. HP and YL contribute equally to this article. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pai, H., Liu, Y., Zhang, C. et al. Effects of the pleiotropic regulator DasR on lincomycin production in Streptomyces lincolnensis. Appl Microbiol Biotechnol 108, 373 (2024). https://doi.org/10.1007/s00253-024-13201-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-024-13201-7