Abstract
Bacterial biofilms commonly cause chronic and persistent infections in humans. Bacterial biofilms consist of an inner layer of bacteria and an autocrine extracellular polymeric substance (EPS). Biofilm dispersants (abbreviated as dispersants) have proven effective in removing the bacterial physical protection barrier EPS. Dispersants are generally weak or have no bactericidal effect. Bacteria dispersed from within biofilms (abbreviated as dispersed bacteria) may be more invasive, adhesive, and motile than planktonic bacteria, characteristics that increase the probability that dispersed bacteria will recolonize and cause reinfection. The dispersants should be combined with antimicrobials to avoid the risk of severe reinfection. Dispersant-based nanoparticles have the advantage of specific release and intense penetration, providing the prerequisite for further antibacterial agent efficacy and achieving the eradication of biofilms. Dispersant-based nanoparticles delivered antimicrobial agents for the treatment of diseases associated with bacterial biofilm infections are expected to be an effective measure to prevent reinfection caused by dispersed bacteria.
Key points
• Dispersed bacteria harm and the dispersant’s dispersion mechanisms are discussed.
• The advantages of dispersant-based nanoparticles in bacteria biofilms are discussed.
• Dispersant-based nanoparticles for cutting off reinfection in vivo are highlighted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
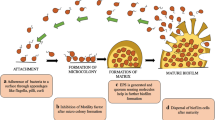
It is well documented that persistent infections caused by bacteria, including periodontitis, urethritis, and pulmonary cystic fibrosis, are usually associated with biofilm formation (Davies 2003; Green and Jones 2022; Ma et al. 2022; Tang et al. 2022). Biofilms are three-dimensional (3D) structures of bacterial communities encapsulated by autocrine extracellular polymeric substances (EPS), also known as “microbial cities,” with tight physical barriers and extensive transport and communication networks (Fang et al. 2020; Karygianni et al. 2020). EPS mainly comprises extracellular polysaccharides, proteins, and extracellular DNA (eDNA) (Karygianni et al. 2020). Physical barrier EPS-protected bacteria require antimicrobials 10 ~ 1000 times higher than planktonic bacteria (Davies 2003; Ji et al. 2022). In addition, EPS can help bacteria evade the body's immune system (Alhede et al. 2014; Scherr et al. 2014; Ramírez-Larrota and Eckhard 2022). All these reasons contribute to the refractory and complex nature of bacterial biofilm infections.
Currently, biofilm dispersants (abbreviated as dispersants) have proven to be widely used as a standard means of removing the physical protective barrier of bacteria, Such as nitric oxide (NO), D-amino acids (D-AA), enzymes, and surfactants(Fleming and Rumbaugh 2017; Verderosa et al. 2019; Jiang et al. 2020). Dispersion is part of the life cycle of Biofilms, and no studies have yet shown that bacteria are resistant to dispersants (Tian et al. 2021). Dispersants usually have weak or no bactericidal effect. Bacteria that escape the biofilm (abbreviated as dispersed bacteria) can cause recolonization and serious reinfection if not removed promptly (Pettigrew et al. 2014; Fleming and Rumbaugh 2018). For example, in a mouse trauma model, enzyme-catalyzed induction of Pseudomonas aeruginosa (P. aeruginosa) biofilm dispersion leads to massive bacterial entry into the circulation, causing fatal sepsis (Fleming and Rumbaugh 2018), Undispersed Staphylococcus epidermidis (S. epidermidis) did not cause serious reinfection (Wang et al. 2011). In addition, bacteria colonizing the interior of the biofilm respond to deteriorating conditions (e.g., high cell density, nutrient depletion, toxic waste accumulation, and signal conditioning), triggering dispersion of the biofilm and leading to reinfection (Bridges and Bassler 2019; Rumbaugh and Sauer 2020; Andersen et al. 2021). Most dispersants are often in combination with antimicrobial agents as a promising means of addressing biofilm reinfection. The insoluble nature of both and their sensitivity to the host immune system make it difficult for them to exert synergistic effects at the site of infection, thus limiting their clinical application (Tian et al. 2021). The desired result is achieved by increasing the corresponding dose, but high doses often lead to side effects (e.g., neurotoxicity, allergic reactions, and liver toxicity) (Bangert and Hasbun 2019). With the continuous development of nano-delivery technology, it has been widely evaluated in improving the solubility of refractory drugs, protecting and hiding Unsteady able medicines, and enhancing drug targeting (Li et al. 2021; Wang et al. 2021; Thorn et al. 2021). So far, there are no detailed reports on the role of dispersants and antimicrobial agents in eradicating bacterial biofilm infections and avoiding reinfection via nano-drug delivery systems (NDDS). This article aims to introduce the causes of harm from dispersed bacteria; the Mechanism of dispersant-mediated biofilm dispersion; the advantages of dispersant-based nanoparticles; clinical applications of dispersion-based nanoparticle-delivered antimicrobial agents in diseases associated with bacterial biofilm infections. It provides an effective means of eradicating bacterial biofilms and avoiding reinfection.
Causes of reinfection from dispersed bacteria
Dispersed bacteria are a distinct group of bacteria, different from bacteria settled in biofilms or planktonic bacteria. Compared to planktonic bacteria, dispersed bacteria are more virulent, mainly due to increased adhesion, motility, and resistance. It is also more motile and has easier nutrient access than bacteria that settle inside biofilms. These characteristics of dispersed bacteria can subsequently mean more acute severe infections for the host (Fig. 1).
Enhanced virulence
Genes encoding virulence-related genes upregulate when dispersed bacteria escape from the biofilm (Fig. 1). Gene transcript expression profiles showed dispersed P. aeruginosa virulence-related genes (e.g., secB) upregulate (Chua et al. 2014). In addition, dispersed Candida albicans (C. albicans) virulence-associated genes (e.g., SAP) upregulate and are not expressed in planktonic bacteria (Uppuluri et al. 2018). SAP has multiple pathogenic functions, such as high adhesion to tissues, invasion, and evasion of the host immune system (Hube and Naglik 2001). The corresponding virulence of dispersed Klebsiella pneumoniae (K. pneumoniae) is also enhanced and resistant to the phagocytosis of immune cells (Guilhen et al. 2019). Biofilm dispersion is also associated with flagellar formation. For example, P. aeruginosa encodes flagellum-associated genes that are upregulated and have enhanced motility compared to biofilm bacteria (Cai et al. 2020). In addition, the study showed that dispersed Streptococcus mutants (S. mutants) also showed increased virulence, mainly in the form of increased adherence, which was 4 times higher than that of planktonic bacteria (Liu et al. 2013). In summary, dispersed bacteria are generally present with increased invasiveness, motility, and adhesion, increasing the chances of further transmission and reinfection.
Enhanced resistance
There may be a corresponding increase in the resistance of dispersed bacteria compared to planktonic ones (Fig. 1). Dispersed bacteria are involved in the upregulation of acid-regulated gene (atpD) expression compared to planktonic S. mutants, which may enhance acid resistance by participating in intracellular proton pumping (Liu et al. 2013). In addition, dispersed bacteria showed more drug resistance compared to planktonic bacteria. Dispersed bacteria are less sensitive to chlorhexidine (CHX) (Liu et al. 2013). Dispersed bacteria may also have a more robust detoxification capacity than planktonic bacteria. The cusCFBA manipulator in the CH1034 genome overexpressed in dispersed K. pneumoniae (Guilhen et al. 2016). The study showed that the cusCFBA manipulator encodes the efflux pumps associated with copper and silver ion detoxification in Escherichia coli (E. coli) (Chacón et al. 2014). As a result, dispersed bacteria are usually more resistant than planktonic bacteria, making treatment more difficult.
Enhanced metabolic capacity
Dispersed bacteria have different metabolic capabilities compared to bacteria settled within biofilms and planktonic bacteria (Fig. 1). Genes acquiring zinc, iron, and amino acids in the same nutrient-rich environment significantly upregulate in dispersed C. albicans (Uppuluri et al. 2018). In addition, an increase in the activity of the dispersed S. mutants phosphotransferase system PTS. It utilizes carbohydrates, such as sucrose, glucose, and mannose, more efficiently than planktonic and bacteria settled within biofilms (Liu et al. 2013). Similar characteristics were demonstrated in influenza A virus (IAV)-induced K. pneumoniae dispersion (Pettigrew et al. 2014). These suggest that dispersed bacteria have a high capacity to acquire nutrients and facilitate adaptation to harsh environments.
Dispersion mechanisms of different types of dispersants
Although biofilms provide a natural defense barrier for bacteria, preventing the penetration of antimicrobial agents and evading the body’s immune defenses, bacteria inhabiting different locations within the biofilm structure can respond to deteriorating conditions, leading to the dispersal of biofilm bacteria (Rumbaugh and Sauer 2020). In addition, the physical barrier to bacteria can be removed clinically by dispersants, such as dispersing signal molecules, EPS removal molecules, and chelating molecules, which promote the dispersion of biofilms (Table 1).
Regulation of c-di-GMP levels
Nitric oxide (NO)
NO in nature is considered an essential dispersal signaling molecule in biological systems. Because of the short half-life of NO and the difficulty in controlling the release of NO, NO donors such as sodium nitroprusside (SNP), SNO, s-nitrosothiols (rnos), and n-diazobenzenedicarboxylic acid (NONOates) have been investigated (Wang et al. 2002; Dong et al. 2019; Kulbir et al. 2021). According to the study, NO can mediate bactericidal and EPS decomposition through reactive nitrogen oxides (e.g., ONOO-) (Yang et al. 2018). In addition, At lower levels, NO can induce biofilm diffusion by activating phosphodiesterase (PDE) to reduce c-di-GMP levels (Barraud et al. 2009). For example, SNP causes biofilm diffusion in P. aeruginosa by decreasing c-di-GMP levels (Barraud et al. 2006, 2009; Cai and Webb 2020). The study showed that NO is also involved in bacterial motility and regulates the dispersal of bacterial biofilms. For example, endogenous NO production by Vibrio cholerae also stimulates the retraction of MSHA hairs to mediate biofilm dispersion (Hughes et al. 2022). NO, as an essential dispersion signal, has been widely used (Table 1).
Fatty acids
Initially, fatty acid dispersion signaling molecules (DSF) was identified in Xanthomonas campestris as regulating bacterial motility, biofilm formation, and dispersion (Ryan and Dow 2011; Zhou et al. 2017). Cis-2-decanoic acid (cis-DA) from the DSF family is a signaling molecule produced by P. aeruginosa that induces the dispersion of various microbial biofilms (Davies and Marques 2009). Cis-DA-induced dispersion was associated with regulating c-di-GMP levels, a finding confirmed in biofilms of wild Xanthomonas campestris (Table 1) (Dow et al. 2003; Tao et al. 2010). The cis-DA trans isomer 2 heptylcyclopropane-1-carboxylic acid (2CP) prevents the conversion of 2CP to the active low trans conformation T2DA due to the presence of cyclopropanation bonds. The ability to disperse Staphylococcus aureus (S. aureus) and P. aeruginosa biofilms were significantly more robust than cis-DA and T2DA (Harrison et al. 2021). DSF is expected to become a common means of dispersing biofilms.
Rhamnolipid (RHL)
RHL is a biosurfactant found in the biofilm of P. aeruginosa with functions of maintaining water channels, transporting nutrients and metabolic wastes, and dispersing biofilms, etc. (Boles et al. 2005; Irie et al. 2005; Silva et al. 2017). For example, large amounts of RHL can disperse the biofilm of P. aeruginosa and form a central cavity structure (Boles et al. 2005). The study showed that RHL-mediated biofilm dispersion of P. aeruginosa might be associated with c-di-GMP regulation (Harmsen et al. 2010). In addition, RHL plays a crucial role in regulating the response of E. coli biofilms to the dispersion signal N-(3-oxo-dodecanoyl) homoserine lactone (Bhattacharjee et al. 2016). RHL will be an effective reagent for dispersing biofilms (Table 1).
Effect on target EPS structure
EPS is the main component of biofilms and has various functions, such as preventing drug penetration, evading the immune system, promoting adhesion and aggregation, etc. We are promoting biofilm dispersion by targeting the significant components that make up EPS (e.g., extracellular polysaccharides, proteins, and eDNA) is a commonly used method today.
Biological enzymes
Biological enzymes have been shown to disperse biofilms by targeting extracellular polysaccharides, proteins, and eDNA in EPS (Thallinger et al. 2013; Thorn et al. 2021). Extracellular polysaccharides are long-chain polymers linked by glycosidic bonds and play an essential role in maintaining the structure of bacterial biofilms (Fleming and Rumbaugh 2017; Jiang et al. 2020). Glycoside hydrolases (GHs) targeting extracellular polysaccharides in biofilms have become a research hotspot for dispersed bacterial biofilms (Redman et al. 2020). In cystic fibrosis (CF) patients with high alginate content, alginate catabolic enzymes can alleviate lung infections by breaking down alginate (Blanco-Cabra et al. 2020). α-Amylase also achieved similar effects, significantly promoting wound healing (Fleming et al. 2017). Extracellular proteins are the main components that maintain the physical barrier structure (Fleming and Rumbaugh 2017; Jiang et al. 2020). Proteases promote biofilm dispersion by breaking down proteins in EPS and may also disrupt intercellular communication by solubilizing type I signal peptidases. Protease is considered to be an effective method for EPS removal (Schallenberger et al. 2012; Thallinger et al. 2013). For example, serine proteases produced by S. epidermidis can eradicate pre-formed biofilms (Martínez-García et al. 2018; Kumari and Sarkar 2018; Weldrick et al. 2019). The eDNA with glue effect integrates bacteria and other components into a 3D framework, which is beneficial in helping to promote bacterial adhesion and gene transfer (Jakubovics et al. 2013; Okshevsky and Meyer 2015; Fleming and Rumbaugh 2017). Deoxyribonuclease (DNase) can specifically target eDNA to promote biofilm dispersion (Thallinger et al. 2013; Jiang et al. 2020). DNase, a marketed mucolytic agent, has been reported to reduce lung infections in CF patients by degrading DNA in sticky sputum (Delfino et al. 2021). The advantage of low off-target probability and high activity of biological enzymes is usually a standard means of causing large-scale dispersion of biofilms. However, biological enzymes are easily deactivated under the influence of pH, ionic strength, or temperature, leading to the rapid development of various nano enzymes.
Nano enzymes
In recent years, nanomaterials with enzymatic activity (called nano enzymes) will become environmentally friendly reagents due to their stable structure, low cost, high catalytic activity, and good antibacterial properties (Chen et al. 2018b). Currently, nano enzymes with peroxidase (POD) activity, such as iron oxide, V2O5, and graphite nitride (g-C3N423), are reported to catalyze the conversion of H2O2 to more toxic highly reactive oxygen species (hROS), such as superoxide anion (-O2−), hydroxyl radical (-OH) and singlet oxygen (1O2) (Vatansever et al. 2013; Ji et al. 2016; Wang et al. 2017). Inactivates bacteria and breaks down EPS by irreversibly damaging DNA and degrading polysaccharides and proteins (Gao et al. 2014; Ji et al. 2016). In addition, nano enzymes with DNase-like activity, such as cerium (Ce), have been shown to promote DNA hydrolysis (Chen et al. 2016; Hu et al. 2022). Therefore, nano enzymes are expected to be widely used for the catalytic treatment of diseases associated with bacterial biofilm infections (Table 1).
Chelating molecules
Chelating molecules trigger biofilm dissipation by binding certain substances within the biofilm, such as c-di-GMP, magnesium, calcium, and iron, etc. (Jiang et al. 2020). BdcA was identified as a regulator of dispersed biofilms, mediating the dispersion of E. coli, P. aeruginosa, and Pseudomonas fluorescens (P. fluorescens) by binding to c-di-GMP (Ma et al. 2011a, 2011b). Large amounts of cations, such as magnesium, calcium, and iron, are essential for maintaining the 3D structure of biofilms (Banin et al. 2006; Wang et al. 2019). Lactoferrin (LF) is an antimicrobial agent from the host immune system that has been shown to trigger biofilm dispersion by chelating iron in the iron carrier system and has anti-inflammatory properties (Disease 2009; Alves et al. 2013; Ammons and Copié 2013). LF has been shown to disperse bacterial biofilms, including P. aeruginosa, E. coli, Enterococcus faecalis (E. faecalis), and S. aureus (Alves et al. 2013; Ammons and Copié 2013). EDTA, 5-nitro-8-hydroxyquinoline, and halogenated phenols (HPs) have also been reported to induce biofilm dispersion by chelating metal ions (Banin et al. 2006; Sobke et al. 2012; Naclerio and Sintim 2021). Therefore, substances required to maintain bacterial biofilm structure through binding are also commonly used to trigger biofilm dispersion.
Effect on bacterial cell wall synthesis
D-AA is the enantiomer of natural L-amino acids such as D-leucine, D-cysteine, D-tyrosine, etc. D-AA plays an essential role in the formation and dispersion of bacterial biofilms (Kolodkin-Gal et al. 2010; Leiman et al. 2013). D-AA has been shown to have the effect of dispersing biofilms of S. aureus and P. aeruginosa (Sanchez et al. 2013; Chang et al. 2022). According to the data, exogenous D-AA can integrate into the cell wall by replacing the original peptidoglycan (PG) structural unit. For example, De Pedro et al. found that the original peptidoglycan structural unit is present in the periplasm of E. coli and that exogenous D-cysteine integrates the peptidoglycan structure (De Pedro et al. 2003). Pidgeon, S. E. also found similar results (Pidgeon and Pires 2017). The ability of D-AA to inhibit biofilm formation and promote biofilm dispersion is associated with interference with the cross-linking of peptidoglycan chains. D-AAs have significant potential in treating biofilm infections (Table 1).
Electrostatic interactions
The study showed that cationic substances usually exert their antibacterial and biofilm-damaging abilities through electrostatic interactions (Table 1) (Strempel et al. 2014; Tamara et al. 2018; Khan et al. 2020). Chitosan(CS) is a cationic amino polysaccharide with excellent biocompatibility and biodegradability and is considered an effective antimicrobial and anti-biofilm compound (Khan et al. 2020). The unique antibacterial and antibacterial biofilm properties of CS are often thought to be associated with amines. Positively charged amines cause leakage of cytoplasmic contents and disruption of bacterial biofilm structure through electrostatic counteraction (Tamara et al. 2018; Khan et al. 2020). In addition, most antimicrobial peptides (AMPs) typically exhibit a positive net charge and have similar antibacterial and anti-biofilm mechanisms (Strempel et al. 2014; Yasir et al. 2018). Three bacteriocins, nisin A, lacticin Q, and nukacin ISK-1 can disrupt the membrane potential of Methicillin-resistant Staphylococcus aureus (MRSA) within biological membranes, resulting in ATP efflux from bacteria (Okuda et al. 2013). These cationic substances usually exhibit a high degree of permeability. For example, chitosan can disrupt the EPS of many microorganisms to facilitate drug penetration (Orgaz et al. 2011; Ng et al. 2013; Mu et al. 2014). CSA-13 penetrates rapidly into the interior of EPS and penetrates bacteria within P. aeruginosa biofilms (Nagant et al. 2013). Therefore, such substances often achieve the ability to penetrate EPS through electrostatic interactions to facilitate the profound delivery of antimicrobial agents to biofilms.
Advantages of dispersant-based nanoparticles
Some dispersant-based nanoparticles respond to microenvironmental conditions (e.g., low pH, enzyme overexpression, high glutathione content) and exogenous stimuli (e.g., light) to achieve specific release upon reaching the site of bacterial infection. Dispersants further improve the efficacy of antibacterial drugs by breaking the dense physical barrier and exposing the bacteria inside. In addition, positively charged substances with permeability are often used as carriers or modified on the surface of nanoparticles to deliver antimicrobial agents to biofilms’ interiors effectively. Dispersant-based nanoparticles with specific release and penetration capabilities provide the prerequisites for further improving the antimicrobial effect and avoiding new infections caused by dispersed bacteria (Fig. 2).
Specific activations
Endogenous response
Although the dense EPS provides a rigid physical barrier to bacteria, the bacteria inside the EPS often accumulate large amounts of metabolites in an oxygen-deprived acidic environment (Wang et al. 2013; Fulaz et al. 2019). In addition, the acidic metabolites produced during the inflammatory response increase the acidity of the infected microenvironment. Therefore, acidic microenvironments are often used to design a series of smart responding nanoparticles to trigger the release of dispersants (Fig. 2). pH-sensitive cis-aconitine-D-tyrosine (CA-Tyr) prodrugs bound to polymeric micelle surfaces via electrostatic interactions (Chen et al. 2019; Fan et al. 2021). When this micelle reaches the site of infection, the pH-sensitive cis-aconitine bond breaks, releasing D-Tyr and exposing the positively charged micelle to deliver azithromycin (AZM) efficiently.
A unique high expression of enzymes characterizes the microenvironment of bacterial infection compared to normal tissue (Chen et al. 2018a; Ding et al. 2022). Hyaluronidase (HAS) is a virulence factor highly expressed by Gram-positive bacteria (Hynes and Walton 2000). Hyaluronic acid (HA) is often used as a capping agent to respond to HAS, releasing the protected dispersant (Fig. 2). Qu et al. constructed a smart nanoparticle (AA@GS@HA-MNPs) by coating HA on the sandwich-like graphene-mesoporous silica (GS) loaded with ascorbic acid AA (Ji et al. 2016). HA will be degraded by the HAS secreted by bacteria, releasing H2O2 prodrug AA, catalyzed by MNPs to produce •OH. Compared with GS@HA-MNPs, AA@GS@HA-MNPs significantly dispersed approximately 80% of the biofilm and nearly killed the bacteria embedded in the biofilm. In the presence of HAS, the cumulative release of AA exceeded 73%in 12 h. Successfully established an “on-demand” drug delivery platform. Liu et al. also constructed drug delivery systems that respond to HAS to release dispersants to achieve physical barrier removal and further effective antibacterial (Liu et al. 2019).
Glutathione (GSH) is also present in biofilms, where it is present in concentrations up to 10.0 mM and can protect bacteria from oxidative stress, toxins, and acidity (Gales et al. 2008; Chen et al. 2013; Ding et al. 2022). Nanoparticles that release dispersants in response to this specific microenvironment have been designed (Fig. 2). Alpha-cyclodextrin (α-CD) is conjugated with NO donor through GSH-sensitive bonding to form NO prodrug (α-CD-NO), which then binds to polyethylene glycol (PEG) block peptide copolymer (PEG-(KLAKLAK)2-DA) through host–guest interaction to form smart nanocarriers α-CD-Ce6-NO-DA (Hu et al. 2020). In simulated MRSA biofilms, nanoparticles incubated for 2 h at a concentration of 8 mM GSH released significant amounts of NO but were relatively stable in healthy tissue. The nanoparticles that penetrate the biofilm respond to the overexpression of GSH in the biofilm, leading to a rapid release of NO.
H2O2 levels at the site of biofilm infection were also higher than in surrounding healthy tissues, mainly due to ROS generated by the host’s immune response (Nathan and Cunningham-Bussel 2013). The study shows that the bacteria's production of H2O2 comes to interfere with the host's inflammatory response (Erttmann and Gekara 2019). Due to the limitations of H2O2’s low efficiency, slow onset of action, and high concentration required, it is often catalyzed into hROS to improve bactericidal efficiency. Some nano enzymes with POD activity can catalyze H2O2 and produce hROS for better antibacterial and anti-biofilm effects (Fig. 2) (Wang et al. 2017; Chen et al. 2018b). Ting Pan et al. designed chitosan-grafted Fe-doped carbon dots nano enzymes CS@Fe/CDs. CD exerts the ability to sterilize and disperse EPS by catalyzing the production of -OH from H2O2 (Pan et al. 2022). Although the body can release H2O2 in pathological states, its concentration remains low and does not better stimulate the NDDS to release active substances quickly and efficiently. To increase the level of H2O2 in the microenvironment, a cascade catalytic reaction generator (CaO2/graphene@aluminate) was designed (Yan et al. 2018). First, CaO2 can react with water to produce a large amount of H2O2, which is then catalyzed by graphene to produce hROS, thus destroying biofilm and sterilizing it. According to information, large amounts of H2O are potentially toxic to healthy tissue and can delay wound healing (Loo et al. 2012). A specific increase in H2O2 levels at the site of infection is an effective strategy to reduce toxicity. Currently, a selective increase of H2O2 levels at the site of infection is often widely used as a reaction condition for the effective release of active substances. Yuting Shi et al. designed enzyme-linked reaction-specific H2O2-releasing nanoparticles (Shi et al. 2022). In the weakly acidic environment of biofilm infection, the aldehyde condensation bonds of nanoparticles break and release maltose heptose, which is catalyzed by glycosylase (GA) and glucose oxidase (Gox) to produce large amounts of H2O2, approximately three times more than under physiological conditions. The guanidine group of arginine releases NO under the condition of H2O2. The microenvironmental response improves the accuracy and release of the dispersant, removing a physical barrier to the further effective performance of the antimicrobial agent.
Exogenous response
In recent years, nanoparticles with exogenous conditionally stimulated release dispersants have shown promising results in treating bacterial biofilm infections. Due to their photocontrol ability, high selectivity, and low systemic toxicity have become the ideal exogenous stimulus condition for modulating dispersant release (Fig. 2) (Imberti et al. 2020; Yuan et al. 2020). The dispersant is usually attached to the polymer through a light-crackable joint. Shen, Z. et al. By RAFT polymerization reaction, coumarin chromophore is linked with N-nitrosamines to form (CouNO), which is then coupled with polyethylene oxide (PEO) to form amphiphilic polymer micelles PEO-b-PCouNO spontaneously (Shen et al. 2019). Under visible light, CouNO releases NO spontaneously and releases the loaded Cip when the polymer micelle structure decomposes. The photoresponsive release of NO facilitates removing the physical barrier of P. aeruginosa, which improves the prerequisites for the further antibacterial effect of Cip. Duan, Y. et al. also designed similarly functioning light-responsive vesicles (Duan et al. 2021). Visible light-mediated release of NO and gentamicin (GS) has a better anti-biofilm effect than NO or GS alone. In addition, multifunctional nanoparticles with photothermal responsive release dispersants have been developed. Polydopamine (PDA) has received much attention recently as an alternative to photothermal-sensitive materials due to its excellent biodegradability and photo conversion efficiency (Yuan et al. 2020). Yu, S. et al. prepared Fe3O4@PDA@PAMAM@NONOate containing Fe3O4, PDA, poly(amino) dendrimers (PAMAM), and NONOate (Yu et al. 2018). Under laser irradiation, Fe3O4 and PDAgenerate heat, increasing the local temperature. NONOate rapidly releases NO, realizing the on-demand triggered NO release under intermittent laser irradiation.
Strong permeability
EPS, which acts as a physical barrier, severely hinders further penetration of the antimicrobial agent. Positively charged substances can disrupt the intact structure of EPS, are highly permeable, and are often modified on the surface of nanoparticles or used as drug carriers. For example, Ma et al. synthesized CS nanoparticles loaded with curcumin, which enhanced curcumin’s permeability by disrupting biofilms’ structural integrity through electrostatic interactions (Ma et al. 2020). However, the positively charged nanoparticles are easily removed by the body’s immune system and are difficult to reach the infection site through blood circulation. Poly(β-amino) ester (PAE) is a pH-responsive polymer material positively charged by protonation under acidic conditions and is now widely used. PAE tri-block charge reversal micelles for vancomycin delivery prolong the blood circulation time (Chu et al. 2016). Based on in vivo fluorescence imaging results, the 24 h accumulation of charge-reversing micelles at subcutaneous infection sites in mice was 2.7 and 1.8 times greater than vancomycin and positively charged micelles. Charge-reversing nanoparticles that are superior to positively charged nanoparticles are often used to deliver antimicrobial agents. Liu, Y. et al. prepared charge reversal polymer micelles (MSPM) composed of PAE (Liu et al. 2016). Under acidic infection conditions, MSPM positively charged to enhance the permeability of the biofilm, and its permeability was significantly higher than that of triclosan, polymeric micelles composed of triclosan and polyethylene glycol (PEG) (SSPM), resulting in satisfactory therapeutic efficacy. Positively charged nanoparticles with strong permeability effectively deliver antimicrobial agents to the biofilm’s interior, bypassing the biofilm’s resistance to antimicrobial agent penetration.
Dispersant-based nanoparticle delivered antimicrobial agents in the treatment of biofilm-related diseases
Biofilm is a common cause of various intractable diseases (e.g., traumatic wound infections, lung infections, subcutaneous abscesses, urethritis, etc.) (Mihai et al. 2022). Dispersants can remove the protective shield of bacteria but usually need to use with an antimicrobial agent, which is necessary to eradicate the bacterial biofilm and avoid new infections. The application of dispersants and antimicrobial agents in different forms of NDDS for biofilm-associated infections will be described next.
Nanoparticles co-modified with dispersants and antimicrobial agents
NO is an important signaling molecule in dispersing bacterial biofilms. In addition, it also has some ability to promote healing. NO donors co-modified with antimicrobial agents on nanoparticles have achieved the expected results in treating bacterial biofilm-associated infections. For example, azide-modified CS binds to PAMAM via an electroshock reaction, introducing abundant primary amines to load methicillin (Met) and NONOates (Liu et al. 2020). Under physiological conditions, NO is continuously released, along with antimicrobial agents. This delivery system resulted in over 85% dispersion of MRSA biofilm, significantly killing biofilm bacteria. On day 7 of treatment for wound infection, MRSA was barely observable in the wound exudate, demonstrating the fastest rate of wound healing and preventing persistent severe wound infection. Previous researchers have successfully designed specific stimulus-responsive nanoparticles to enhance the release of dispersants and antimicrobial agents. Alpha-cyclodextrin (α-CD) is conjugated with NO donor through GSH-sensitive bonding to form NO prodrug (α-CD-NO), which then binds to polyethylene glycol (PEG) block peptide copolymer through host–guest interaction to create smart nanocarriers α-CD-Ce6-NO-DA (Fig. 3a) (Hu et al. 2020). The nanocarriers are positively charged by charge reversal under acidic conditions, which promotes effective permeation of the nanocarriers within the biofilm. The nanoparticles permeating the biofilm responded to overexpressed GSH, triggering a rapid release of NO, while Ce6 responded to light to produce ROS (Fig. 3a). NO has active nitrogen by reacting with ROS and exerts the desired effect in combination with photodynamic therapy (PDT). In vitro fluorescence imaging showed that the fluorescence of nanoparticles was enhanced 1 h after intravenous injection and stayed at the infected site for 24 h (Fig. 3b). Under the light, it enhances nanoparticles’ antibacterial effect and promotes earlier scarring and faster wound healing (Fig. 3c).
a Schematic diagram of the preparation of α-CD-Ce6-NO-DA and the mechanism of MRSA biofilm removal. b Fluorescence imaging of MRSA biofilm infection in mice treated with α-CD-Ce6-NO-DA. c Histological micrographs of α-CD-Ce6-NO-DA treated infected skin. Reprinted with permission from Ref. (Hu et al. 2020) Copyright 2020, American Chemical Society
Dispersant modified antimicrobial nanoparticles/nanoparticles loaded with antimicrobial agents
Enzyme modified nanoparticles
Enzymatic dispersants break the three-dimensional structure of EPS by degrading extracellular polysaccharides, proteins, and eDNA within the biofilm. However, it has a poor bactericidal effect, often combined with the bactericidal ability of antimicrobial agents, which is a powerful countermeasure to eradicate biofilm and avoid reinfection. Currently, enzyme dispersants are generally modified on the surface of nanoparticles in a non-covalent manner to exert the desired effect. For example, serine proteases are electrostatically adsorbed on the surface of polyacrylic acid nano gels encapsulating ciprofloxacin (Cip) to enhance the impact of Cip on bacterial activity by hydrolyzing EPS (Weldrick et al. 2019). It is well known that novel nanoparticles such as Au, Ag, and Zn kill bacteria by converting heat energy by light or triggering the production of ROS, among which Ag nanoparticles have been used as broad-spectrum antimicrobial agents (Parham et al. 2016; Kim et al. 2018). DNase-functionalized gold nanoparticles (DNase-AuNCs) were designed (Xie et al. 2020). DNase combined with Ag phototherapy to achieve the desired effect of biofilm removal. Similar results have been achieved with invisible aligners, hinting at potential applications in medical devices. In addition, enzymatic dispersants can also modify the surface of nanoparticles by covalent means. It is well documented that carbon monoxide (CO) gas has a bactericidal effect by a mechanism related to targeting the bacterial respiratory chain to enhance ROS production and has good anti-inflammatory properties (Davidge et al. 2009; Motterlini and Otterbein 2010). DNase binds to the surface of polydopamine nanoparticles (MPDA) loaded with CO donor (FeCO) via Schiff base reaction or Michael addition reaction to form DNase-CO@MPDA nanoparticles (Fig. 4a) (Yuan et al. 2021). DNase-MPDA significantly disassembled the eDNA in the biofilm and achieved the disruption of the dense structure of the MRSA biofilm (Fig. 4 b). the NIR-triggered CO rapidly penetrates the biofilm’s interior and affects the bacteria’s viability. Under NIR-treated conditions, DNase-CO@MPDA reduced the inflammatory response and promoted wound healing by removing MRSA biofilm from abscess wounds, hindering the risk of systemic infection (Fig. 4c).
a Schematic diagram of DNase-CO@MPDA synergistic photothermal therapy (PTT) to enhance MRSA biofilm removal. b Residual amount of eDNA in MRSA biofilms after DNase-CO@MPDA treatment. c Histological micrographs of DNaseCO@MPDA-treated infected skin. Reprinted with permission from Ref. (Yuan et al. 2021) Copyright 2021, Wiley
D-amino acid modified nanoparticles
D-AA may promote biofilm dispersion by affecting the structure of bacterial cell walls (De Pedro et al. 2003; Pidgeon and Pires 2017). The D-AA-modified nanoparticles report to have the property of dispersing biofilm, which removed the barrier for the adequate performance of antimicrobial agents (Johansson et al. 2011; Wei et al. 2015). D-cysteine (D-cys)-functionalized silver nanoparticles embedded in dopamine-coated stainless steel surfaces exert synergistic “dispersing and bactericidal” effects (Huang et al. 2020). D-cys not only inhibit biofilm formation but also disperse the physical barrier of biofilm so that Ag kills the dispersed bacteria and prevents further infection. Environmentally sensitive nanoparticles are widely designed to improve the selectivity of dispersants and drug release. Fan, Q. et al. attached D-Ty to CA-Tyr by cis-aconitic bonding and then bound electrostatically to the surface of polymeric micelles to form CM/Cy5.5@Tyr (Fan et al. 2021). When an acidic microenvironment reaches, the breakage of the acid-unstable cis-aconitine bond leads to the release of D-Ty with accompanying charge reversal that disperses and penetrates the dense biofilm to enhance the antibacterial effect of AZM. The study shows that the decomposition capacity of biofilms reached 82%, almost destroying the three-dimensional structure of biofilms. Treatment of lung infections has shown a reduction in the number of bacteria in the lungs and a decrease in fibrosis.
Rhamnolipid modified nanoparticles
RHL has the advantages of low toxicity, excellent biocompatibility, and removal of mature biofilms (Irie et al. 2005; Silva et al. 2017). The frequent use of RHL as a drug delivery vehicle has been reported (Niaz et al. 2019; Marangon et al. 2020). Li, P. et al. formed nanoparticles (PEG/CLR/RHL LPNs) loaded with clarithromycin (CLR) by amphiphilic self-loading of RHL and liposomes (Li et al. 2019). The mucus barrier biofilm model shows nanoparticles can effectively penetrate mucus, disperse H. pylori biofilm, and exert CLR antibacterial activity. Multifunctional self-assembled nanospheres (BD/RHL NDs) then prepare using lipophilic alkyl berberine derivatives (BDS) and RHL to overcome the dual barrier of the mucus layer and biofilm for the treatment of H. pylori gastritis (Fig. 5a) (Shen et al. 2020). In in vitro experiments, RHL-modified drug-loaded microspheres removed bacterial biofilms more effectively than drug-loaded microspheres alone, with 100 μg/mLC10-BD/RHL NDs removing 90.4% of bacterial biofilms (Fig. 5b). C10-BD/RHL NDs in treating H. pylori infection in the stomach reduced the number of H. pylori to nearly 0.6 log10 as measured by qPCR, with no abnormalities in the gastric mucosa. At the same time, the other groups had varying degrees of severe infiltration of inflammatory cells (Fig. 5c). RHL modified nanoparticles are powerful in eradicating H. pylori biofilms and reducing the damage caused by the inflammatory response associated with bacterial infection, thus cutting off a critical step in the recurrence of biofilm infections.
a Schematic diagram of the preparation of BD/RHL NDs and the mechanism of H. pylori biofilm remova. b Residual amount of H. pylori biofilm after BD/RHL NDs treatment. c Histological micrographs of H. pylori gastritis treated with BD/RHL NDs. Reprinted with permission from Ref. (Shen et al. 2020) Copyright 2020 Elsevier
Nanoparticles loaded with dispersants and antimicrobial agents
To avoid the destruction of dispersants by the immune system in vivo, loading dispersants into nanoparticles is often a standard means of effective delivery to the site of infection (Islan et al. 2015; Tan et al. 2018). Lipid nanoparticles loaded with levofloxacin (LV) and DNase for pulmonary delivery are a new alternative to improve current CF infections (Islan et al. 2016). DNase can reduce the viscoelasticity of lung mucus in CF patients, which contributes to the diffusion of LV and thus enhances the antimicrobial activity of LV. In addition, nanoparticles loaded with nano enzymes are also a standard form. For example, Yan, Z. et al. encapsulated CaO2 and heme-containing graphene (H-G) into alginate (Yan et al. 2018). It converted H2O2 to ROS by a local cascade reaction at the site of bacterial infection. It disrupted the main components of the biofilm (bacteria, polysaccharides, proteins, and nucleic acids), dispersing 81.5% of the biofilm and almost killing S. aureus in the biofilm. After 7 days of treatment, it killed more than 90% of the bacteria on the implanted catheter and promoted wound crusting and healing in the rats. Combining dispersants and antimicrobial agents through NDDS is an effective strategy to encourage physical barrier dispersion and hinder new infections caused by bacteria.
Summary and challenges
The biofilm forms a complex physicochemical barrier that protects the encapsulated bacteria from antimicrobial drug treatment to some extent, further promoting the development of bacterial drug resistance. Dispersants are an effective means of breaking down physical barriers. Nanoparticles with stimulated response release dispersant to increase the dispersant accumulation at the infection site. It facilitates the ability to exert biofilm decomposition, which is a prerequisite for further antimicrobial action. However, its intelligence, stability, and sensitivity need further improvement. The bacterial biofilm consists of the inner bacteria and the outer layer of EPS. Bacterial biofilms treated with dispersants can expose the bacteria inside and, if left untreated, can cause new infections. So far, antimicrobial agent delivery by dispersant-based nanoparticles achieved the desired effect. It is an effective means of eradicating bacterial biofilms and alleviating recurrent infections caused by dispersed bacteria. However, the intrinsic cytotoxicity of nanomaterials remains a challenging issue, limiting further clinical applications of nanomaterials in biofilm infection-related diseases. Therefore, a more in-depth and careful examination of nanomaterials' long-term safety and biocompatibility is an important task.
Data Availability
Data sharing is not suitable for this paper because no new data was created.
References
Alhede M, Bjarnsholt T, Givskov M, Alhede M (2014) Pseudomonas aeruginosa biofilms. Mechanisms of immune evasion. Adv Appl Microbiol 86:1–40. https://doi.org/10.1016/B978-0-12-800262-9.00001-9
Alves FRF, Silva MG, Rôç IN, Siqueira JF (2013) Biofilm biomass disruption by natural substances with potential for endodontic use. Braz Oral Res 27:20–25. https://doi.org/10.1590/S1806-83242013000100004
Ammons MC, Copié V (2013) Mini-review: Lactoferrin: a bioinspired, anti-biofilm therapeutic. Biofouling 29:443–455. https://doi.org/10.1080/08927014.2013.773317
Andersen JB, Hultqvist LD, Jansen CU, Jakobsen TH, Nilsson M, Rybtke M, Uhd J, Fritz BG, Seifert R, Berthelsen J, Nielsen TE, Qvortrup K, Givskov M, Tolker-Nielsen T (2021) Identification of small molecules that interfere with c-di-GMP signaling and induce dispersal of pseudomonas aeruginosa biofilms. Biofilms Microbiomes 7:59. https://doi.org/10.1038/s41522-021-00225-4
Bangert MK, Hasbun R (2019) Neurological and psychiatric adverse effects of antimicrobials. CNS Drugs 33:727–753. https://doi.org/10.1007/s40263-019-00649-9
Banin E, Brady KM, Greenberg EP (2006) Chelator-induced dispersal and killing of pseudomonas aeruginosa cells in a biofilm. Appl Environ Microbiol 72:2064–2069. https://doi.org/10.1128/AEM.72.3.2064-2069.2006
Barraud N, Hassett DJ, Hwang SH, Rice SA, Kjelleberg S, Webb JS (2006) Involvement of nitric oxide in biofilm dispersal of pseudomonas aeruginosa. J Bacteriol 188:7344–7353. https://doi.org/10.1128/JB.00779-06
Barraud N, Schleheck D, Klebensberger J, Webb JS, Hassett DJ, Rice SA, Kjelleberg S (2009) Nitric oxide signaling in pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic di-GMP levels, and enhanced dispersal. J Bacteriol 191:7333–7342. https://doi.org/10.1128/JB.00975-09
Bayer AS, Park S, Ramos MC, Nast CC, Eftekhar F, Schiller NL (1992) Effects of alginase on the natural history and antibiotic therapy of experimental endocarditis caused by mucoid pseudomonas aeruginosa. Infect Immun 60:3979–3985. https://doi.org/10.1128/iai.60.10.3979-3985.1992
Bhattacharjee A, Nusca TD, Hochbaum AI (2016) Rhamnolipids mediate an interspecies biofilm dispersal signaling pathway. ACS Chem Biol 11:3068–3076. https://doi.org/10.1021/acschembio.6b00750
Blanco-Cabra N, Paetzold B, Ferrar T, Mazzolini R, Torrents E, Serrano L, LLuch-Senar M (2020) Characterization of different alginate lyases for dissolving pseudomonas aeruginosa biofilms. Sci Rep 10:1–10. https://doi.org/10.1038/s41598-020-66293-2
Boles BR, Thoendel M, Singh PK (2005) Rhamnolipids mediate detachment of pseudomonas aeruginosa from biofilms. Mol Microbiol 57:1210–1223. https://doi.org/10.1111/j.1365-2958.2005.04743.x
Bridges AA, Bassler BL (2019) The intragenus and interspecies quorum-sensing autoinducers exert distinct control over Vibrio cholerae biofilm formation and dispersal. PLoS Biol 17:1–28. https://doi.org/10.1371/journal.pbio.3000429
Cai YM, Zhang YD, Yang L (2021) NO donors and NO delivery methods for controlling biofilms in chronic lung infections. Appl Microbiol Biotechnol 105:3931–3954. https://doi.org/10.1007/s00253-021-11274-2
Cai YM, Webb JS (2020) Optimization of nitric oxide donors for investigating biofilm dispersal response in Pseudomonas aeruginosa clinical isolates. Appl Microbiol Biotechnol 104:8859–8869. https://doi.org/10.1007/s00253-020-10859-7
Cai YM, Hutchin A, Craddock J, Walsh MA, Webb JS, Tews I (2020) Differential impact on motility and biofilm dispersal of closely related phosphodiesterases in Pseudomonas aeruginosa. Sci Rep 10:1–12. https://doi.org/10.1038/s41598-020-63008-5
Chacón KN, Mealman TD, McEvoy MM, Blackburn NJ (2014) Tracking metal ions through a Cu/Ag efflux pump assigns the functional roles of the periplasmic proteins. Proc Natl Acad Sci USA 111:15373–15378. https://doi.org/10.1073/pnas.1411475111
Chang RYK, Li M, Chow MYT, Ke WR, Tai W, Chan HK (2022) A dual action of D-amino acids on anti-biofilm activity and moisture-protection of inhalable ciprofloxacin powders. Eur J Pharm Biopharm 173:132–140. https://doi.org/10.1016/j.ejpb.2022.03.003
Chen NH, Couñago RM, Djoko KY, Jennings MP, Apicella MA, Kobe B, McEwan AG (2013) A glutathione-dependent detoxification system is required for formaldehyde resistance and optimal survival of neisseria meningitidis in biofilms. Antioxid Redox Signal 18:743–755. https://doi.org/10.1089/ars.2012.4749
Chen Z, Ji H, Liu C, Bing W, Wang Z, Qu X (2016) A multinuclear metal complex based DNase-mimetic artificial enzyme: matrix cleavage for combating bacterial biofilms. Angewandte Chemie - International Edition 55:10732–10736. https://doi.org/10.1002/anie.201605296
Chen Q, Zhu C, Huo D, Xue J, Cheng H, Guan B, Xia Y (2018a) Continuous processing of phase-change materials into uniform nanoparticles for near-infrared-triggered drug release. Nanoscale 10:22312–22318. https://doi.org/10.1039/c8nr07027j
Chen Z, Wang Z, Ren J, Qu X (2018b) Enzyme mimicry for combating bacteria and biofilms. Acc Chem Res 51:789–799. https://doi.org/10.1021/acs.accounts.8b00011
Chen M, Wei J, Xie S, Tao X, Zhang Z, Ran P, Li X (2019) Bacterial biofilm destruction by size/surface charge-adaptive micelles. Nanoscale 11:1410–1422. https://doi.org/10.1039/c8nr05575k
Chu L, Gao H, Cheng T, Zhang Y, Liu J, Huang F, Yang C, Shi L, Liu J (2016) A charge-adaptive nanosystem for prolonged and enhanced: in vivo antibiotic delivery. Chem Commun 52:6265–6268. https://doi.org/10.1039/c6cc01269h
Chua SL, Liu Y, Yam JKH, Chen Y, Vejborg RM, Tan BGC, Kjelleberg S, Tolker-Nielsen T, Givskov M, Yang L (2014) Dispersed cells represent a distinct stage in the transition from bacterial biofilm to planktonic lifestyles. Nat Commun 5:1–12. https://doi.org/10.1038/ncomms5462
Davidge KS, Sanguinetti G, Yee CH, Cox AG, McLeod CW, Monk CE, Mann BE, Motterlini R, Poole RK (2009) Carbon monoxide-releasing antibacterial molecules target respiration and global transcriptional regulators. J Biol Chem 284:4516–4524. https://doi.org/10.1074/jbc.M808210200
Davies D (2003) Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discovery 2:114–122. https://doi.org/10.1038/nrd1008
Davies DG, Marques CNH (2009) A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J Bacteriol 191:1393–1403. https://doi.org/10.1128/JB.01214-08
De Pedro MA, Young KD, Höltje JV, Schwarz H (2003) Branching of escherichia coli cells arises from multiple sites of inert peptidoglycan. J Bacteriol 185:1147–1152. https://doi.org/10.1128/JB.185.4.1147-1152.2003
Delfino D, Mori G, Rivetti C, Grigoletto A, Bizzotto G, Cavozzi C, Malatesta M, Cavazzini D, Pasut G, Percudani R (2021) Actin-resistant dnase1L2 as a potential therapeutics for cf lung disease. Biomolecules 11:1–20. https://doi.org/10.3390/biom11030410
Ding M, Zhao W, Song LJ, Luan SF (2022) Stimuli-responsive nanocarriers for bacterial biofilm treatment. Rare Met 41:482–498. https://doi.org/10.1007/s12598-021-01802-4
Disease KP (2009) Aerosolized bovine lactoferrin reduces neutrophils and pro-inflammatory cytokines in mouse models of pseudomonas aeruginosa lung infections. Biochem Cell Biol 3752:1–27. https://doi.org/10.1139/bcb-2016-0050
Dong X, Liu HJ, Feng HY, Yang SC, Liu XL, Lai X, Lu Q, Lovell JF, Chen HZ, Fang C (2019) Enhanced drug delivery by nanoscale integration of a nitric oxide donor to induce tumor collagen depletion. Nano Lett 19:997–1008. https://doi.org/10.1021/acs.nanolett.8b04236
Dow JM, Crossman L, Findlay K, He YQ, Feng JX, Tang JL (2003) Biofilm dispersal in xanthomonas campestris is controlled by cell-cell signaling and is required for full virulence to plants. Proc Natl Acad Sci USA 100:10995–11000. https://doi.org/10.1073/pnas.1833360100
Duan Y, Zhang M, Shen Z, Zhang M, Zheng B, Cheng S, Hu J (2021) Photoresponsive vesicles enabling sequential release of nitric oxide (NO) and gentamicin for efficient biofilm eradication. Macromol Rapid Commun 42:1–8. https://doi.org/10.1002/marc.202000759
Erttmann SF, Gekara NO (2019) Hydrogen peroxide release by bacteria suppresses inflammasome-dependent innate immunity. Nat Commun 10:1–13. https://doi.org/10.1038/s41467-019-11169-x
Fan Q, Wang C, Guo R, Jiang X, Li W, Chen X, Li K, Hong W (2021) Step-by-step dual stimuli-responsive nanoparticles for efficient bacterial biofilm eradication. Biomaterials Science 9:6889–6902. https://doi.org/10.1039/d1bm01038g
Fang K, Park OJ, Hong SH (2020) Controlling biofilms using synthetic biology approaches. Biotechnol Adv 40:107518. https://doi.org/10.1016/j.biotechadv.2020.107518
Fei Y, Wu J, An HW, Zhu K, Peng B, Cai J, Zhang Y, Li LL, Wang H, Huang Z (2020) Identification of new nitric oxide-donating peptides with dual biofilm eradication and antibacterial activities for intervention of device-related infections. J Med Chem 63:9127–9135. https://doi.org/10.1021/acs.jmedchem.9b01832
Fleming D, Rumbaugh KP (2017) Approaches to dispersing medical biofilms. Microorganisms 5. https://doi.org/10.3390/microorganisms5020015
Fleming D, Chahin L, Rumbaugh K (2017) Glycoside hydrolases degrade polymicrobial bacterial biofilms in wounds. Antimicrob Agents Chemothe 61. https://doi.org/10.1128/AAC.01998-16
Fleming D, Rumbaugh K (2018) The consequences of biofilm dispersal on the host. Sci Rep 8:1–7. https://doi.org/10.1038/s41598-018-29121-2
Fulaz S, Hiebner D, Barros CHN, Devlin H, Vitale S, Quinn L, Casey E (2019) Ratiometric imaging of the in situ pH distribution of biofilms by use of fluorescent mesoporous silica nanosensors. ACS Appl Mater Interfaces 11:32679–32688. https://doi.org/10.1021/acsami.9b09978
Gales G, Penninckx M, Block JC, Leroy P (2008) Role of glutathione metabolism status in the definition of some cellular parameters and oxidative stress tolerance of saccharomyces cerevisiae cells growing as biofilms. FEMS Yeast Res 8:667–675. https://doi.org/10.1111/j.1567-1364.2008.00401.x
Gao L, Giglio KM, Nelson JL, Sondermann H, Travis AJ (2014) Ferromagnetic nanoparticles with peroxidase-like activity enhance the cleavage of biological macromolecules for biofilm elimination. Nanoscale 6:2588–2593. https://doi.org/10.1039/c3nr05422e
Green HD, Jones AM (2022) Managing pulmonary infection in adults with cystic fibrosis: adult cystic fibrosis series. Chest 162:66–75. https://doi.org/10.1016/j.chest.2022.02.007
Guilhen C, Charbonnel N, Parisot N, Gueguen N, Iltis A, Forestier C, Balestrino D (2016) Transcriptional profiling of klebsiella pneumoniae defines signatures for planktonic, sessile and biofilm-dispersed cells. BMC Genomics 17:237. https://doi.org/10.1186/s12864-016-2557-x
Guilhen C, Miquel S, Charbonnel N, Joseph L, Carrier G, Forestier C, Balestrino D (2019) Colonization and immune modulation properties of klebsiella pneumoniae biofilm-dispersed cells. Biofilms and Microbiomes 5:25. https://doi.org/10.1038/s41522-019-0098-1
Harmsen M, Yang L, Pamp SJ, Tolker-Nielsen T (2010) An update on pseudomonas aeruginosa biofilm formation, tolerance, and dispersal. FEMS Immunol Med Microbiol 59:253–268. https://doi.org/10.1111/j.1574-695X.2010.00690.x
Harrison ZL, Awais R, Harris M, Raji B, Hoffman BC, Baker DL, Jennings JA (2021) 2-heptylcyclopropane-1-carboxylic acid disperses and inhibits bacterial biofilms. Front Microbiol 12:1–11. https://doi.org/10.3389/fmicb.2021.645180
Hu D, Deng Y, Jia F, Jin Q, Ji J (2020) Surface charge switchable supramolecular nanocarriers for nitric oxide synergistic photodynamic eradication of biofilms. ACS Nano 14:347–359. https://doi.org/10.1021/acsnano.9b05493
Hu H, Kang X, Shan Z, Yang X, Bing W, Wu L, Ge H, Ji H (2022) A DNase-mimetic artificial enzyme for the eradication of drug-resistant bacterial biofilm infections. Nanoscale 14:2676–2685. https://doi.org/10.1039/d1nr07629a
Huang L, Lou Y, Zhang D, Ma L, Qian H, Hu Y, Ju P, Xu D, Li X (2020) D-Cysteine functionalised silver nanoparticles surface with a “disperse-then-kill” antibacterial synergy. Chem Eng J 381:122662. https://doi.org/10.1016/j.cej.2019.122662
Hube B, Naglik J (2001) Candida albicans proteinases: resolving the mystery of a gene family. Microbiology 147:1997–2005. https://doi.org/10.1099/00221287-147-8-1997
Hughes HQ, Floyd KA, Hossain S, Anantharaman S, Kysela DT, Zöldi M, Barna L, Yu Y, Kappler MP, Dalia TN, Podicheti RC, Rusch DB, Zhuang M, Fraser CL, Brun Y V, Jacobson SC, McKinlay JB, Yildiz FH, Boon EM, Dalia AB (2022) Nitric oxide stimulates type IV MSHA pilus retraction in Vibrio cholerae via activation of the phosphodiesterase CdpA. Proc Natl Acad Sci U S A 119. https://doi.org/10.1073/pnas.2108349119
Hynes WL, Walton SL (2000) Hyaluronidases of Gram-positive bacteria. FEMS Microbiol Lett 183:201–207. https://doi.org/10.1016/S0378-1097(99)00669-2
Imberti C, Zhang P, Huang H, Sadler PJ (2020) New designs for phototherapeutic transition metal complexes. Angew Chem Int Ed 59:61–73. https://doi.org/10.1002/anie.201905171
Irie Y, O’Toole GA, Yuk MH (2005) Pseudomonas aeruginosa rhamnolipids disperse bordetella bronchiseptica biofilms. FEMS Microbiol Lett 250:237–243. https://doi.org/10.1016/j.femsle.2005.07.012
Islan GA, Dini C, Bartel LC, Bolzán AD, Castro GR (2015) Characterization of smart auto-degradative hydrogel matrix containing alginate lyase to enhance levofloxacin delivery against bacterial biofilms. Int J Pharm 496:953–964. https://doi.org/10.1016/j.ijpharm.2015.10.050
Islan GA, Tornello PC, Abraham GA, Duran N, Castro GR (2016) Smart lipid nanoparticles containing levofloxacin and DNase for lung delivery. Design and characterization. Colloids Surf, B 143:168–176. https://doi.org/10.1016/j.colsurfb.2016.03.040
Jakubovics NS, Shields RC, Rajarajan N, Burgess JG (2013) Life after death: the critical role of extracellular DNA in microbial biofilms. Lett Appl Microbiol 57:467–475. https://doi.org/10.1111/lam.12134
Ji H, Dong K, Yan Z, Ding C, Chen Z, Ren J, Qu X (2016) Bacterial hyaluronidase self-triggered prodrug release for chemo-photothermal synergistic treatment of bacterial infection. Small 12:6200–6206. https://doi.org/10.1002/smll.201601729
Ji H, Hu H, Tang Q, Kang X, Liu X, Zhao L, Jing R, Wu M, Li G, Zhou X, Liu J, Wang Q, Cong H, Wu L, Qin Y (2022) Precisely controlled and deeply penetrated micro-nano hybrid multifunctional motors with enhanced antibacterial activity against refractory biofilm infections. J Hazard Mater 436:129210. https://doi.org/10.1016/j.jhazmat.2022.129210
Jiang Y, Geng M, Bai L (2020) Targeting biofilms therapy: current research strategies and development hurdles. Microorganisms 8:1–34. https://doi.org/10.3390/microorganisms8081222
Johansson EMV, Kadam RU, Rispoli G, Crusz SA, Bartels KM, Diggle SP, Cámara M, Williams P, Jaeger KE, Darbre T, Reymond JL (2011) Inhibition of pseudomonas aeruginosa biofilms with a glycopeptide dendrimer containing D-amino acids. MedChemComm 2:418–420. https://doi.org/10.1039/c0md00270d
Karygianni L, Ren Z, Koo H, Thurnheer T (2020) Biofilm matrixome: extracellular components in structured microbial communities. Trends Microbiol 28:668–681. https://doi.org/10.1016/j.tim.2020.03.016
Khan F, Pham DTN, Oloketuyi SF, Manivasagan P, Oh J, Kim YM (2020) Chitosan and their derivatives: antibiofilm drugs against pathogenic bacteria. Colloids Surf, B 185:110627. https://doi.org/10.1016/j.colsurfb.2019.110627
Kim T, Zhang Q, Li J, Zhang L, Jokerst JV (2018) A gold/silver hybrid nanoparticle for treatment and photoacoustic imaging of bacterial infection. ACS Nano 12:5615–5625. https://doi.org/10.1021/acsnano.8b01362
Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R (2010) D-amino acids trigger biofilm disassembly. Science 328:627–629. https://doi.org/10.1126/science.1188628.D-Amino
Kulbir DS, Devi T, Goswami M, Yenuganti M, Bhardwaj P, Ghosh S, Chandra Sahoo S, Kumar P (2021) Oxygen atom transfer promoted nitrate to nitric oxide transformation: a step-wise reduction of nitrate → nitrite → nitric oxide. Chem Sci 12:10605–10612. https://doi.org/10.1039/d1sc00803j
Kumari S, Sarkar PK (2018) Optimisation of Bacillus cereus biofilm removal in the dairy industry using an in vitro model of cleaning-in-place incorporating serine protease. Int J Dairy Technol 71:512–518. https://doi.org/10.1111/1471-0307.12454
Leiman SA, May JM, Lebar MD, Kahne D, Kolter R, Losick R (2013) D-Amino acids indirectly inhibit biofilm formation in Bacillus subtilis by interfering with protein synthesis. J Bacteriol 195:5391–5395. https://doi.org/10.1128/JB.00975-13
Li P, Chen X, Shen Y, Li H, Zou Y, Yuan G, Hu P, Hu H (2019) Mucus penetration enhanced lipid polymer nanoparticles improve the eradication rate of helicobacter pylori biofilm. J Control Release 300:52–63. https://doi.org/10.1016/j.jconrel.2019.02.039
Li X, Chen D, Xie S (2021) Current progress and prospects of organic nanoparticles against bacterial biofilm. Adv Coll Interface Sci 294:102475. https://doi.org/10.1016/j.cis.2021.102475
Lin B, Li R, Handley TNG, Wade JD, Li W, O’Brien-Simpson NM (2021) Cationic antimicrobial peptides are leading the way to combat oropathogenic infections. ACS Infectious Diseases 7:2959–2970. https://doi.org/10.1021/acsinfecdis.1c00424
Liu J, Ling JQ, Zhang K, Wu CD (2013) Physiological properties of Streptococcus mutans UA159 biofilm-detached cells. FEMS Microbiol Lett 340:11–18. https://doi.org/10.1111/1574-6968.12066
Liu Y, Busscher HJ, Zhao B, Li Y, Zhang Z, Van Der Mei HC, Ren Y, Shi L (2016) Surface-adaptive, antimicrobially loaded, micellar nanocarriers with enhanced penetration and killing efficiency in staphylococcal biofilms. ACS Nano 10:4779–4789. https://doi.org/10.1021/acsnano.6b01370
Liu Y, Lin A, Liu J, Chen X, Zhu X, Gong Y, Yuan G, Chen L, Liu J (2019) Enzyme-responsive mesoporous ruthenium for combined chemo-photothermal therapy of drug-resistant bacteria. ACS Appl Mater Interfaces 11:26590–26606. https://doi.org/10.1021/acsami.9b07866
Liu S, Cai X, Xue W, Ma D, Zhang W (2020) Chitosan derivatives co-delivering nitric oxide and methicillin for the effective therapy to the methicillin-resistant S aureus infection. Carbohydr Polym 234:115928. https://doi.org/10.1016/j.carbpol.2020.115928
Loo AEK, Wong YT, Ho R, Wasser M, Du T, Ng WT, Halliwell B (2012) Effects of hydrogen peroxide on wound healing in mice in relation to oxidative damage. PLoS ONE 7:e49215. https://doi.org/10.1371/journal.pone.0049215
Ma Q, Yang Z, Pu M, Peti W, Wood TK (2011a) Engineering a novel c-di-GMP-binding protein for biofilm dispersal. Environ Microbiol 13:631–642. https://doi.org/10.1111/j.1462-2920.2010.02368.x
Ma Q, Zhang G, Wood TK (2011b) Escherichia coli BdcA controls biofilm dispersal in pseudomonas aeruginosa and rhizobium meliloti. BMC Res Notes 4:447. https://doi.org/10.1186/1756-0500-4-447
Ma S, Moser D, Han F, Leonhard M, Schneider-Stickler B, Tan Y (2020) Preparation and antibiofilm studies of curcumin loaded chitosan nanoparticles against polymicrobial biofilms of candida albicans and staphylococcus aureus. Carbohyd Polym 241:116254. https://doi.org/10.1016/j.carbpol.2020.116254
Ma Y, Zohaib Aslam M, Wu M, Nitin N, Sun G (2022) Strategies and perspectives of developing anti-biofilm materials for improved food safety. Food Res Int 159:111543. https://doi.org/10.1016/j.foodres.2022.111543
Marangon CA, Martins VCA, Ling MH, Melo CC, Plepis AMG, Meyer RL, Nitschke M (2020) Combination of rhamnolipid and chitosan in nanoparticles boosts their antimicrobial efficacy. ACS Appl Mater Interfaces 12:5488–5499. https://doi.org/10.1021/acsami.9b19253
Martínez-García S, Rodríguez-Martínez S, Cancino-Diaz ME (2018) Cancino-Diaz JC, Extracellular proteases of staphylococcus epidermidis: roles as virulence factors and their participation in biofilm. APMIS 126:177–185. https://doi.org/10.1111/apm.12805
Mihai MM, Holban A-M, Ion A, Bălăceanu B, Gurău C-D, Lazăr V (2022) Nano-targeted drug delivery approaches for biofilm-associated infections. Micro Nano Technol 97–138. https://doi.org/10.1016/B978-0-323-90792-7.00008-7
Motterlini R, Otterbein LE (2010) The therapeutic potential of carbon monoxide. Nat Rev Drug Discovery 9:728–743. https://doi.org/10.1038/nrd3228
Mu H, Guo F, Niu H, Liu Q, Wang S, Duan J (2014) Chitosan improves anti-biofilm efficacy of gentamicin through facilitating antibiotic penetration. Int J Mol Sci 15:22296–22308. https://doi.org/10.3390/ijms151222296
Naclerio GA, Sintim HO (2021) Starving bacteria of iron: a potential strategy to disperse bacterial biofilms. J Med Chem 64:7272–7274. https://doi.org/10.1021/acs.jmedchem.1c00749
Nagant C, Pitts B, Stewart PS, Feng Y, Savage PB, Dehaye JP (2013) Study of the effect of antimicrobial peptide mimic, CSA-13, on an established biofilm formed by pseudomonas aeruginosa. MicrobiologyOpen 2:318–325. https://doi.org/10.1002/mbo3.77
Nathan C, Cunningham-Bussel A (2013) Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat Rev Immunol 13:349–361. https://doi.org/10.1038/nri3423
Ng VWL, Ke X, Lee ALZ, Hedrick JL, Yang YY (2013) Synergistic co-delivery of membrane-disrupting polymers with commercial antibiotics against highly opportunistic bacteria. Adv Mater 25:6730–6736. https://doi.org/10.1002/adma.201302952
Niaz T, Shabbir S, Noor T, Imran M (2019) Antimicrobial and antibiofilm potential of bacteriocin loaded nano-vesicles functionalized with rhamnolipids against foodborne pathogens. Lwt 116:108583. https://doi.org/10.1016/j.lwt.2019.108583
Okshevsky M, Meyer RL (2015) The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit Rev Microbiol 41:341–352. https://doi.org/10.3109/1040841X.2013.841639
Okuda KI, Zendo T, Sugimoto S, Iwase T, Tajima A, Yamada S, Sonomoto K, Mizunoe Y (2013) Effects of bacteriocins on methicillin-resistant staphylococcus aureus biofilm. Antimicrob Agents Chemother 57:5572–5579. https://doi.org/10.1128/AAC.00888-13
Orgaz B, Lobete MM, Puga CH, Jose CS (2011) Effectiveness of chitosan against mature biofilms formed by food related bacteria. Int J Mol Sci 12:817–828. https://doi.org/10.3390/ijms12010817
Pan T, Chen H, Gao X, Wu Z, Ye Y, Shen Y (2022) Engineering efficient artificial nanozyme based on chitosan grafted Fe-doped-carbon dots for bacteria biofilm eradication. J Hazard Mater 435:128996. https://doi.org/10.1016/j.jhazmat.2022.128996
Parham S, Wicaksono DHB, Bagherbaigi S, Lee SL, Nur H (2016) Antimicrobial treatment of different metal oxide nanoparticles: a critical review. J Chin Chem Soc 63:385–393. https://doi.org/10.1002/jccs.201500446
Pettigrew MM, Marks LR, Kong Y, Gent JF, Roche-Hakansson H, Hakansson AP (2014) Dynamic changes in the streptococcus pneumoniae transcriptome during transition from biofilm formation to invasive disease upon influenza a virus infection. Infect Immun 82:4607–4619. https://doi.org/10.1128/IAI.02225-14
Pidgeon SE, Pires MM (2017) Cell wall remodeling of staphylococcus aureus in live caenorhabditis elegans. Bioconjug Chem 28:2310–2315. https://doi.org/10.1021/acs.bioconjchem.7b00363
Ramírez-Larrota JS, Eckhard U (2022) An introduction to bacterial biofilms and their proteases, and their roles in host infection and immune evasion. Biomolecules 12:306. https://doi.org/10.3390/biom12020306
Redman WK, Welch GS, Rumbaugh KP (2020) Differential efficacy of glycoside hydrolases to disperse biofilms. Front Cell Infect Microbiol 10:1–7. https://doi.org/10.3389/fcimb.2020.00379
Rofeal MG, Elzoghby AO, Helmy MW, Khalil R, Khairy H, Omar S (2020) Dual therapeutic targeting of lung infection and carcinoma using lactoferrin-based green nanomedicine. ACS Biomater Sci Eng 6:5685–5699. https://doi.org/10.1021/acsbiomaterials.0c01095
Rumbaugh KP, Sauer K (2020) Biofilm dispersion. Nat Rev Microbiol 18:571–586. https://doi.org/10.1038/s41579-020-0385-0
Ryan RP, Dow JM (2011) Communication with a growing family: diffusible signal factor (DSF) signaling in bacteria. Trends Microbiol 19:145–152. https://doi.org/10.1016/j.tim.2010.12.003
Sanchez CJ, Prieto EM, Krueger CA, Zienkiewicz KJ, Romano DR, Ward CL, Akers KS, Guelcher SA, Wenke JC (2013) Effects of local delivery of d-amino acids from biofilm-dispersive scaffolds on infection in contaminated rat segmental defects. Biomaterials 34:7533–7543. https://doi.org/10.1016/j.biomaterials.2013.06.026
Schallenberger MA, Niessen S, Shao C, Fowler BJ, Romesberg FE (2012) Type I signal peptidase and protein secretion in staphylococcus aureus. J Bacteriol 194:2677–2686. https://doi.org/10.1128/JB.00064-12
Scherr TD, Heim CE, Morrison JM, Kielian T (2014) Hiding in plain sight: interplay between staphylococcal biofilms and host immunity. Front Immunol 5:1–7. https://doi.org/10.3389/fimmu.2014.00037
Shen Z, He K, Ding Z, Zhang M, Yu Y, Hu J (2019) Visible-light-triggered self-reporting release of nitric oxide (NO) for bacterial biofilm dispersal. Macromolecules 52:7668–7677. https://doi.org/10.1021/acs.macromol.9b01252
Shen Y, Zou Y, Chen X, Li P, Rao Y, Yang X, Sun Y, Hu H (2020) Antibacterial self-assembled nanodrugs composed of berberine derivatives and rhamnolipids against helicobacter pylori. J Control Release 328:575–586. https://doi.org/10.1016/j.jconrel.2020.09.025
Shi Y, Cao Y, Cheng J, Yu W, Liu M, Yin J, Huang C, Liang X, Zhou H, Liu H, Yang Z, Fang Y, Wei H, Zhao G (2022) Construction of self-activated nanoreactors for cascade catalytic anti-biofilm therapy based on H2O2 self-generation and switch-On NO release. Adv Func Mater 32:1–13. https://doi.org/10.1002/adfm.202111148
Silva SSE, Carvalho JWP, Aires CP, Nitschke M (2017) Disruption of staphylococcus aureus biofilms using rhamnolipid biosurfactants. J Dairy Sci 100:7864–7873. https://doi.org/10.3168/jds.2017-13012
Sobke A, Klinger M, Hermann B, Sachse S, Nietzsche S, Makarewicz O, Keller PM, Pfister W, Straubea E (2012) The urinary antibiotic 5-nitro-8-hydroxyquinoline (nitroxoline) reduces the formation and induces the dispersal of pseudomonas aeruginosa biofilms by chelation of iron and zinc. Antimicrob Agents Chemother 56:6021–6025. https://doi.org/10.1128/AAC.01484-12
Strempel N, Strehmel J, Overhage J (2014) Potential application of antimicrobial peptides in the treatment of bacterial biofilm infections. Curr Pharm Des 21:67–84. https://doi.org/10.2174/1381612820666140905124312
Tamara FR, Lin C, Mi FL, Ho YC (2018) Antibacterial effects of chitosan/cationic peptide nanoparticles. Nanomaterials 8:1–15. https://doi.org/10.3390/nano8020088
Tan Y, Ma S, Leonhard M, Moser D, Haselmann GM, Wang J, Eder D, Schneider-Stickler B (2018) Enhancing antibiofilm activity with functional chitosan nanoparticles targeting biofilm cells and biofilm matrix. Carbohyd Polym 200:35–42. https://doi.org/10.1016/j.carbpol.2018.07.072
Tang Y, Huang QX, Zheng DW, Chen Y, Ma L, Huang C, Zhang XZ (2022) Engineered bdellovibrio bacteriovorus: a countermeasure for biofilm-induced periodontitis. Mater Today 53:71–83. https://doi.org/10.1016/j.mattod.2022.01.013
Tao F, Swarup S, Zhang LH (2010) Quorum sensing modulation of a putative glycosyltransferase gene cluster essential for Xanthomonas campestris biofilm formation. Environ Microbiol 12:3159–3170. https://doi.org/10.1111/j.1462-2920.2010.02288.x
Thallinger B, Prasetyo EN, Nyanhongo GS, Guebitz GM (2013) Antimicrobial enzymes: an emerging strategy to fight microbes and microbial biofilms. Biotechnol J 8:97–109. https://doi.org/10.1002/biot.201200313
Thorn CR, Howell PL, Wozniak DJ, Prestidge CA, Thomas N (2021) Enhancing the therapeutic use of biofilm-dispersing enzymes with smart drug delivery systems. Adv Drug Deliv Rev 179:113916. https://doi.org/10.1016/j.addr.2021.113916
Tian S, van der Mei HC, Ren Y, Busscher HJ, Shi L (2021) Recent advances and future challenges in the use of nanoparticles for the dispersal of infectious biofilms. J Mater Sci Technol 84:208–218. https://doi.org/10.1016/j.jmst.2021.02.007
Uppuluri P, Zaldívar A, Anderson MZ, Dunn MJ, Berman J, Ribot JLL, Köhler JR (2018) Candida albicans dispersed cells are developmentally distinct. mBio 9:1–16. https://doi.org/10.1128/mbio.01338-18
Vatansever F, de Melo WCMA, Avci P, Vecchio D, Sadasivam M, Gupta A, Chandran R, Karimi M, Parizotto NA, Yin R, Tegos GP, Hamblin MR (2013) Antimicrobial strategies centered around reactive oxygen species - bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol Rev 37:955–989. https://doi.org/10.1111/1574-6976.12026
Velliyagounder K, Bahdila D, Pawar S, Fine DH (2018) Role of lactoferrin and lactoferrin-derived peptides in oral and maxillofacial diseases. Oral Dis 25:625–669. https://doi.org/10.1111/odi.12868
Verderosa AD, Totsika M, Fairfull-Smith KE (2019) Bacterial biofilm eradication agents: a current review. Front Chem 7:1–17. https://doi.org/10.3389/fchem.2019.00824
Wang PG, Xian M, Tang X, Wu X, Wen Z, Cai T, Janczuk AJ (2002) Nitric oxide donors: chemical activities and biological applications. Chem Rev 102:1091–1134. https://doi.org/10.1021/cr000040l
Wang R, Queck SY, Otto M, Wang R, Khan BA, Cheung GYC, Bach TL (2011) Staphylococcus epidermidis surfactant peptides promote biofilm maturation and dissemination of biofilm-associated infection in mice Find the latest version : surfactant peptides promote biofilm maturation and dissemination of biofilm-associated inf. J Clin Investig 121:238–248. https://doi.org/10.1172/JCI42520.238
Wang X, Meier RJ, Wolfbeis OS (2013) Fluorescent pH-sensitive nanoparticles in an agarose matrix for imaging of bacterial growth and metabolism. Angew Chem 125:424–427. https://doi.org/10.1002/ange.201205715
Wang T, Flint S, Palmer J (2019) Magnesium and calcium ions: roles in bacterial cell attachment and biofilm structure maturation. Biofouling 35:959–974. https://doi.org/10.1080/08927014.2019.1674811
Wang Y, Shen X, Ma S, Guo Q, Zhang W, Cheng L, Ding L, Xu Z, Jiang J, Gao L (2020) Oral biofilm elimination by combining iron-based nanozymes and hydrogen peroxide-producing bacteria. Biomater Sci 8:2447–2458. https://doi.org/10.1039/c9bm01889a
Wang T, Rong F, Tang Y, Li M, Feng T, Zhou Q, Li P, Huang W (2021) Targeted polymer-based antibiotic delivery system: a promising option for treating bacterial infections via macromolecular approaches. Prog Polym Sci 116:101389. https://doi.org/10.1016/j.progpolymsci.2021.101389
Wang Z, Dong K, Liu Z, Zhang Y, Chen Z, Sun H, Ren J, Qu X (2017) Activation of biologically relevant levels of reactive oxygen species by Au/g-C3N4 hybrid nanozyme for bacteria killing and wound disinfection. Biomaterials 113:145–157. https://doi.org/10.1016/j.biomaterials.2016.10.041
Wei W, Bing W, Ren J, Qu X (2015) Near infrared-caged D-amino acids multifunctional assembly for simultaneously eradicating biofilms and bacteria. Chem Commun 51:12677–12679. https://doi.org/10.1039/c5cc04729c
Weldrick PJ, Hardman MJ, Paunov VN (2019) Enhanced clearing of wound-related pathogenic bacterial biofilms using protease-functionalized antibiotic nanocarriers. ACS Appl Mater Interfaces 11:43902–43919. https://doi.org/10.1021/acsami.9b16119
Xie Y, Zheng W, Jiang X (2020) Near-infrared light-activated phototherapy by gold nanoclusters for dispersing biofilms. ACS Appl Mater Interfaces 12:9041–9049. https://doi.org/10.1021/acsami.9b21777
Yan Z, Bing W, Ding C, Dong K, Ren J, Qu X (2018) A H2O2-free depot for treating bacterial infection: Localized cascade reactions to eradicate biofilms: In vivo. Nanoscale 10:17656–17662. https://doi.org/10.1039/c8nr03963a
Yang L, Feura ES, Ahonen MJR, Schoenfisch MH (2018) Nitric oxide-releasing macromolecular scaffolds for antibacterial applications. Adv Healthcare Mater 7:1–18. https://doi.org/10.1002/adhm.201800155
Yasir M, Willcox MDP, Dutta D (2018) Action of antimicrobial peptides against bacterial biofilms. Materials 11:2468. https://doi.org/10.3390/ma11122468
Yu S, Li G, Liu R, Ma D, Xue W (2018) Dendritic Fe3O4 @poly(dopamine)@PAMAM nanocomposite as controllable NO-releasing material: a synergistic photothermal and NO antibacterial study. Adv Func Mater 28:1707440. https://doi.org/10.1002/adfm.201707440
Yuan Z, Lin C, He Y, Tao B, Chen M, Zhang J, Liu P, Cai K (2020) Near-infrared light-triggered nitric-oxide-enhanced photodynamic therapy and low-temperature photothermal therapy for biofilm elimination. ACS Nano 14:3546–3562. https://doi.org/10.1021/acsnano.9b09871
Yuan Z, Lin C, Dai L, He Y, Hu J, Xu K, Tao B, Liu P, Cai K (2021) Near-infrared light-activatable dual-action nanoparticle combats the established biofilms of methicillin-resistant staphylococcus aureus and its accompanying inflammation. Small 17:1–16. https://doi.org/10.1002/smll.202007522
Zhou L, Zhang LH, Cámara M, He YW (2017) The DSF family of quorum sensing signals: diversity, biosynthesis, and turnover. Trends Microbiol 25:293–303. https://doi.org/10.1016/j.tim.2016.11.013
Zhu X, Chen X, Jia Z, Huo D, Liu Y, Liu J (2021) Cationic chitosan@Ruthenium dioxide hybrid nanozymes for photothermal therapy enhancing ROS-mediated eradicating multidrug resistant bacterial infection. J Colloid Interface Sci 603:615–632. https://doi.org/10.1016/j.jcis.2021.06.073
Acknowledgements
This work was supported by the College of Veterinary Medicine of Sichuan Agricultural University. We acknowledge the partial support the State Key Laboratory of Crop Gene Exploration and Utilization in Southwest China.
Author information
Authors and Affiliations
Contributions
XL, first author, responsible for collecting relevant information, writing the entire content of the manuscript, and drawing; SL and YW, co-first author, responsible for collecting information related to reinfection caused by dispersed bacteria; YC, responsible for inserting references to the paper; WZ and GS, responsible for checking the format of the first draft; HL and FX, responsible for touching up the full text; JL and GP, rsponsible for testing the format of references; HF, corresponding author, mainly responsible for submission.
Corresponding author
Ethics declarations
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaojuan Li was the first author, and Shiyu Lin and Yueli Wang contributed equally to this study as co-first authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, X., Lin, S., Wang, Y. et al. Application of biofilm dispersion-based nanoparticles in cutting off reinfection. Appl Microbiol Biotechnol 108, 386 (2024). https://doi.org/10.1007/s00253-024-13120-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-024-13120-7