Abstract
ADP-activated β-d-manno-heptoses (ADP-β-d-manno-heptoses) are precursors for the biosynthesis of the inner core of lipopolysaccharide in Gram-negative bacteria. Recently, ADP-d-glycero-β-d-manno-heptose (ADP-d,d-manno-heptose) and its C-6′′ epimer, ADP-l-glycero-β-d-manno-heptose (ADP-l,d-manno-heptose), were identified as potent pathogen-associated molecular patterns (PAMPs) that can trigger robust innate immune responses. Although the production of ADP-d,d-manno-heptose has been studied in several different pathogenic Gram-negative bacteria, current knowledge of ADP-β-d-manno-heptose biosynthesis in Vibrio strains remains limited. Here, we characterized the biosynthetic enzymes of ADP-d,d-manno-heptose and the epimerase that converts it to ADP-l,d-manno-heptose from Vibrio cholerae (the causative agent of pandemic cholera) and Vibrio parahaemolyticus (non-cholera pathogen causing vibriosis with clinical manifestations of gastroenteritis and wound infections) in comparison with their isozymes from Escherichia coli. Moreover, we discovered that β-d-mannose 1-phosphate, but not α-d-mannose 1-phosphate, could be activated to its ADP form by the nucleotidyltransferase domains of bifunctional kinase/nucleotidyltransferases HldEVC (from V. cholerae) and HldEVP (from V. parahaemolyticus). Kinetic analyses of the nucleotidyltransferase domains of HldEVC and HldEVP together with the E. coli–derived HldEEC were thus carried out using β-d-mannose 1-phosphate as a mimic sugar substrate. Overall, our works suggest that V. cholerae and V. parahaemolyticus are capable of synthesizing ADP-β-d-manno-heptoses and lay a foundation for further physiological function explorations on manno-heptose metabolism in Vibrio strains.
Key points
• Vibrio strains adopt the same biosynthetic pathway as E. coli in synthesizing ADP-β-d-manno-heptoses.
• HldEs from two Vibrio strains and E. coli could activate β-d-mannose 1-phosphate to ADP-β-d-mannose.
• Comparable nucleotidyltransfer efficiencies were observed in the kinetic studies of HldEs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
ADP-activated β-d-manno-heptoses (ADP-β-d-manno-heptoses), including ADP-d-glycero-β-d-manno-heptose (ADP-d,d-manno-heptose) and ADP-l-glycero-β-d-manno-heptose (ADP-l,d-manno-heptose), are common building blocks of lipopolysaccharides (LPSs) in Gram-negative bacteria. In some cases, blockade of β-d-manno-heptose biosynthesis can remarkably increase bacterial sensitivity to antibiotics and reduce bacterial virulence (Raetz and Whitfield 2002). In addition, ADP-activated β-d-manno-heptoses are sugar donors of heptosylation of bacterial autotransporters that deliver virulence factors to the bacterial surface (Lu et al. 2014). They are also key intermediates of the biosynthesis of nucleoside antibiotics like septacidins and spicamycins with fascinating antifungal and antitumor bioactivities (Guo et al. 2021; Tang et al. 2018). Recently, ADP-β-d-manno-heptoses from several pathogenic bacteria like Yersinia pseudotuberculosis and Campylobacter jejuni were identified as potent pathogen-associated molecular patterns (PAMPs), which can be sensed by alpha-protein kinase 1 (ALPK1) and trigger robust innate immune responses via nuclear factor kappa-B (NF-κB) signaling pathway (Zhou et al. 2018; Cui et al. 2021). Moreover, two intermediates of ADP-β-d-manno-heptose biosynthesis, d-glycero-β-d-manno-heptose 1,7-bisphosphate (HBP) and d-glycero-β-d-manno-heptose 1-phosphate (H1P), were proposed to be PAMPs that can elicit NF-κB-mediated innate immune responses during studies on pathogens like Helicobacter pylori and Neisseria meningitides (Gaudet et al. 2015; Malott et al. 2013; Zimmermann et al. 2017; Garcia-Weber and Arrieumerlou 2021), underlining the immunological roles of the β-d-manno-heptose metabolites.
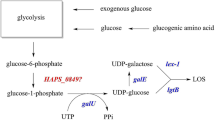
The biosynthesis of ADP-β-d-manno-heptoses has been well elucidated in several bacteria. They are derived from d-sedoheptulose 7-phosphate (S7P), an intermediate of the pentose phosphate pathway. In Escherichia coli, S7P is converted to d-glycero-d-manno-heptose 7-phosphate (H7P) by an isomerase GmhAEC, and the kinase domain of HldEEC, a bifunctional kinase/nucleotidyltransferase, catalyzes the phosphorylation of the anomeric carbon of H7P to generate HBP. After the C-7′′ phosphate group of HBP is removed by a phosphatase GmhBEC, H1P is activated by the nucleotidyltransferase domain of HldEEC to generate ADP-d,d-manno-heptose (Fig. 1a) (Kneidinger et al. 2002). ADP-d,d-manno-heptose can be converted to its C-6″ epimer, ADP-l,d-manno-heptose, by an NAD+ dependent epimerase HldDEC (Fig. 2a) (Morrison and Tanner 2007). It is noteworthy that, in some bacteria (e.g., Burkholderia pseudomallei), the manno-heptose kinase and nucleotidyltransferase are not bifunctional proteins like HldEEC, but two mono-functional enzymes (Park et al. 2018).
Characterization of the biosynthetic enzymes of ADP-d,d-manno-heptose from two pathogenic Vibrio strains. a The biosynthetic pathway of ADP-d,d-manno-heptose. S7P, d-sedoheptulose 7-phosphate; H7P, d-glycero-β-d-manno-heptose 7-phosphate; HBP, d-glycero-β-d-manno-heptose 1,7-biphosphate; H1P, d-glycero-β-d-manno-heptose 1-phosphate. b Structural comparison of bifunctional HldEs from Vibrio strains with E. coli–derived HldEEC. HldEVC and HldEVP are bifunctional kinase/nucleotidyltransferses from V. cholerae O1 2010EL-1786 and V. parahaemolyticus CGMCC 1.1997, respectively. c SDS-PAGE analysis of N-His6 tagged ADP-d,d-manno-heptose biosynthetic enzymes. Lane M, protein marker. GmhAVC, GmhBVC, and HldEVC are from V. cholerae O1 2010EL-1786; GmhAVP, GmhBVP, and HldEVP are from V. parahaemolyticus CGMCC 1.1997; GmhAEC, GmhBEC, and HldEEC are from E. coli BL21. d HPLC profiles of the enzymatic assays of the ADP-d,d-manno-heptose synthetic enzymes using S7P and ATP as substrates. The detection wavelength was set at 254 nm. Enzymes from V. cholerae O1 2010EL-1786, V. parahaemolyticus CGMCC 1.1997, and E. coli are indicated in blue, rose, and black, respectively
Characterization of the ADP-d,d-manno-heptose C-6′′ epimerases from two pathogenic Vibrio strains. a A proposed reaction to form ADP-l,d-manno-heptose catalyzed by ADP-d,d-manno-heptose C-6′′ epimerase. b SDS-PAGE analysis of N-His6 tagged ADP-d,d-manno-heptose C-6′′ epimerases. Lane M, protein marker. HldDVC, HldDVP, and HldDEC are from V. cholerae O1 2010EL-1786, V. parahaemolyticus CGMCC 1.1997, E. coli BL21 (DE3), respectively. c Representative HPLC profiles of the enzymatic assays of ADP-d,d-manno-heptose C-6′′ epimerases. The detection wavelength was set at 254 nm
The Vibrio genus is ubiquitously found in diverse aquatic and marine habitats. It comprises more than 12 species that can cause human infections. Human diseases caused by pathogenic Vibrio species are divided into two types: cholera and non-cholera infections (Baker-Austin et al. 2018). V. cholerae is the causative agent of pandemic cholera, an acute watery diarrheal disease spreading via the fecal-oral and person-to-person transmission (Kanungo et al. 2022). Of the non-cholera pathogens, V. parahaemolyticus and Vibrio vulnificus are the representative species causing vibriosis including gastroenteritis, wound infection, and sepsis (Baker-Austin et al. 2018). Several virulence factors, e.g., enterotoxin, hemolysin, and LPS contribute considerably to the pathogenicity of Vibrio, thus being studied intensively (Zhang and Austin 2005). In V. cholerae, the LPS inner core is usually composed of at least three l-glycero-α-d-manno-heptoses, which are loaded by inverting glycosyltransferases employing ADP-l,d-manno-heptose as a sugar donor (Chatterjee and Chaudhuri 2003). However, little is known about the biosynthesis of ADP-activated β-d-manno-heptoses in Vibrio, let alone the influences of those ADP-β-d-manno-heptoses on Vibrio pathogenesis.
In this work, we analyzed the genomes of two pathogenic Vibrio strains, V. cholerae O1 2010EL-1786 and V. parahaemolyticus CGMCC 1.1997 (ATCC 17802), and found that they possess the same set of ADP-β-d-manno-heptose biosynthetic enzymes as E. coli. Interestingly, though they display high protein sequence similarities with the E. coli–derived homologues (>60%), the predicted structural differences between the nucleotidyltransferase domains of the bifunctional HldEs aroused our interests to study their functions elaborately. The catalytic activities of those Vibrio enzymes were identified by in comparative investigations along with their isozymes from E. coli. Restricted by the availability of H1P, quantitative studies on the β-d-manno-heptose nucleotidyltransferases are difficult. Here, we show that the bifunctional HldEs from both Vibrio and E. coli could activate β-d-mannose 1-phosphate, but not α-d-mannose 1-phosphate, to form ADP-β-d-mannose. And the kinetic analyses of their nucleotidyltransferase domains were performed using β-d-mannose 1-phosphate, which revealed that they have comparable nucleotidyltransfer efficiencies. Taken together, we characterized the enzymes involved in ADP-β-d-manno-heptose synthesis in Vibrio strains and laid a foundation for further investigations on the influence of heptose metabolism on the virulence of Vibrio.
Materials and methods
Bacterial strains and plasmids
Bacterial strains and plasmids used in this study are listed in Table S1. E. coli JM109 was used for general DNA cloning. E. coli BL21 (DE3) ΔgmhA ΔgmhB ΔhldE mutant was used for protein expression (Tang et al. 2022). LB broth and agar were used for the growth of E. coli strains at 37 °C. V. parahaemolyticus CGMCC 1.1997 was cultured at 37 °C in LB broth and used as the template for cloning the genes involving in ADP-β-d-manno-heptose biosynthesis from its genome (NCBI RefSeq assembly, GCF_001011015.1). ADP-β-d-manno-heptose biosynthetic genes from V. cholerae O1 2010EL-1786 (NCBI RefSeq assembly, GCF_000166455.1) were obtained by DNA synthesis.
DNA manipulation and sequence analysis
All PCR primers used in this study were synthesized by GENEWZ Co. (Suzhou, China) and listed in Table S2. DNA synthesis and sequencing were carried out in GENEWIZ Co. (Suzhou, China). PCRs were performed with PrimeSTAR HS DNA polymerase (Takara, Shiga, Japan) or Taq DNA polymerase (TransGene, Beijing, China) according to the manufacturers’ instructions. A BLASTP search was used to predict protein functions (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Construction of the protein expression plasmids
To construct the expression plasmids of ADP-β-d-manno-heptose biosynthetic enzymes from V. cholerae O1 2010EL-1786, the 0.6-kb isomerase gene gmhAVC, the 0.6-kb phosphatase gene gmhBVC, the 1.4-kb bifunctional kinase/nucleotidyltransferase gene hldEVC, and the 0.9-kb epimerase gene hldDVC were synthesized and inserted into the NdeI/BamHI sites of pET28a to afford plasmids pET28a-gmhAVC, pET28a-gmhBVC, pET28a-hldEVC, and pET28a-hldDVC. For the construction of plasmids pET28a-gmhAVP, pET28a-gmhBVP, pET28a-hldEVP, and pET28a-hldDVP, the linear pET28a fragment was amplified from the pET28a plasmid and digested with DpnI to eliminate the template plasmid. The 0.6-kb gmhAVP gene, the 0.6-kb gmhBVP gene, the 1.4-kb hldEVP gene, and the 0.9-kb hldDVP gene were amplified from the genome of V. parahaemolyticus CGMCC 1.1997 using primer pairs GmhA-VP-1/GmhA-VP-2, GmhB-VP-1/GmhB-VP-2, HldE-VP-1/HldE-VP-2, and HldD-VP-1/HldD-VP-2, respectively. Each of the four fragments contained homologous sequences for ligation independent cloning at its both ends and was ligated with the linear pET28a fragment by one-step clone strategy. The desired plasmids were verified by DNA sequencing.
Protein expression and purification
All the proteins were expressed in E. coli BL21 (DE3) ΔgmhAEC ΔgmhBEC ΔhldEEC mutant. A single transformant of the E. coli BL21 ΔgmhAEC ΔgmhBEC ΔhldEEC strain harboring a specific protein expression plasmid was inoculated into LB medium with 50 μg/mL kanamycin and cultured overnight at 37 °C, 220 rpm. Subsequently, the overnight culture was inoculated into LB with 50 μg/mL kanamycin at 1:100 dilution and incubated at 37 °C, 220 rpm until OD600 reached 0.6. The expression of the candidate protein was then induced by adding isopropyl β-d-thiopyranogalactoside (IPTG) to a final concentration of 0.1 mM and cultured at 16 °C, 180 rpm for 18 h.
Protein purifications were carried out with Ni-NTA affinity column at 4 °C following the manufacturer’s instructions. After harvesting the cell pellets by centrifugation, we resuspended them in binding buffer (20 mM Tris-HCl, 500 mM NaCl, 5 mM imidazole, 5% glycerol, pH 7.9) for sonication. Then, the cell debris was removed by centrifugation and the supernatant was loaded onto Ni-NTA affinity column pre-equilibrated with binding buffer. After being washed with washing buffer (20 mM Tris-HCl, 500 mM NaCl, 60 mM imidazole, 5% glycerol, pH 7.9) and elution buffer (20 mM Tris-HCl, 500 mM NaCl, 500 mM imidazole, 5% glycerol, pH 7.9) sequentially, the desired fractions were combined, desalted with PD-10 columns (GE Healthcare, USA), and concentrated by ultracentrifugation using an Amicon Ultra Centrifugal Filter device (Merck Millipore, USA; molecular mass cutoff of 10 kDa for the bifunctional HldEs and 3 kDa for the other proteins). The purified proteins were stored in 20 mM HEPES buffer (pH 8.0) with 200 mM NaCl and 10% glycerol at −80 °C (Li et al. 2021). Protein concentrations were measured by the Bradford assay (Bradford 1976).
Assays of the ADP-d,d-manno-heptose biosynthetic enzymes
The catalytic activities of ADP-d,d-manno-heptose biosynthetic enzymes were studied with the four-step assays using S7P and ATP as substrates. For the characterization of GmhAVC, GmhBVC, and HldEVC from V. cholerae O1 2010EL-1786, the reactions were performed in a 50-μL volume mixture containing 20 mM HEPES buffer (10% glycerol, 200 mM NaCl, pH 8.0), 2 mM MgCl2, 2 mM KCl, 2 mM ATP, 0.2 mM S7P, 5 μM GmhAVC, 5 μM GmhBVC, and 5 μM HldEVC at 30 °C for 2 h. And the well-studied combination of GmhAEC, GmhBEC, and HldEEC from E. coli was performed at the same conditions as a positive control. To check the catalytic activity of GmhAVC or GmhBVC or HldEVC, its corresponding isoenzyme in the combination of GmhAEC + GmhBEC + HldEEC was replaced by the one from V. cholerae O1 2010EL-1786. The assays of ADP-d,d-manno-heptose biosynthetic enzymes from V. parahaemolyticus CGMCC 1.1997 and the compensation experiments of their isoenzymes from E. coli were performed similarly as the enzymes from V. cholerae O1 2010EL-1786. All of the reactions were quenched by mixing vigorously with an equal volume of chloroform. After centrifugation, the chloroform layer was removed and 10 μL of aqueous sample was subjected to HPLC analysis (Tang et al. 2022).
Enzymatic assays of HldDVC, HldDVP, and HldDEC
The enzymatic reactions of C-6″ epimerases, HldDVC, HldDVP, and HldDEC, were performed at the same conditions and here takes HldDVC as an example. The enzymatic assay of HldDVC was set in a 50-μL volume mixture containing 20 mM HEPES buffer (10% glycerol, 200 mM NaCl, pH 8.0), 2 mM NAD+, 0.2 mM ADP-d,d-manno-heptose, and 5 μM HldDVC at 30 °C for 1 h. The reaction was quenched by mixing vigorously with an equal volume of chloroform. After centrifugation, the chloroform layer was removed and 10 μL of aqueous sample was subjected to HPLC analysis.
Enzymatic assays of HldE using β-d-mannose 1-phosphate as a substrate
To test whether HldE could use β-d-mannose 1-phosphate as a substrate, the enzymatic reaction was carried out in a 50 μL volume mixture containing 20 mM HEPES buffer (10% glycerol, 200 mM NaCl, pH 8.0), 2 mM MgCl2, 2 mM ATP, 0.2 mM β-d-mannose 1-phosphate (Supplementary Scheme 1 and Fig. S1), and 5 μM HldEVC or HldEVP or HldEEC at 30 °C for 2 h. To test whether α-d-mannose 1-phosphate can be taken by HldE, the reactions were performed at the same conditions except that β-d-mannose 1-phosphate was replaced by α-d-mannose 1-phosphate. All the reactions were quenched by mixing vigorously with an equal volume of chloroform. After centrifugation, the chloroform layer was removed and 10 μL of aqueous sample was subjected to HPLC analysis.
Preparation of ADP-β-d-mannose
ADP-β-d-mannose was enzymatically prepared by assays in a 100-μL volume mixture containing 20 mM HEPES buffer (10% glycerol, 200 mM NaCl, pH 8.0), 2 mM MgCl2, 2 mM KCl, 5 mM ATP, 1 mM β-d-mannose 1-phosphate, and 20 μM HldEEC at 30 °C for 6 h. The reaction mixture was filtrated with an Amicon Ultra 10 kDa centrifugal filter (Merck Millipore, MA, USA) to remove the proteins. ADP-β-d-mannose was purified with a Dionex CarboPac™ PA1 BioLC™ Semi-Prep column (9 × 250 mm, Thermo Fisher Scientific, Sunnyvale, CA, USA) on a Shimadzu HPLC system (Shimadzu, Kyoto, Japan) at a detection wavelength of 254 nm. The column was developed at a flow rate of 2.0 mL/min with solvent A (ddH2O) and solvent B (1.0 M CH3COONH4). The percentage of solvent B was changed using the following gradient: 0–2 min, 40%; 2–7 min, 40%–70%; 7–15 min, 70%; 15–16 min, 70%–90%; 16–23 min, 90%; 23–24 min, 90%–40%; and 25–28 min, 40%. The fractions of the desired product were collected, concentrated via lyophilization, and refined on a Sephadex LH20 column (eluted with ddH2O) to obtain ADP-β-d-mannose.
Enzymatic assays of ADP-d-glycero-β-d-altro-heptose synthesis
To test whether HldEVC from V. cholerae O1 2010EL-1786 can synthesize ADP-d-glycero-β-d-altro-heptose, the enzymatic reactions were performed in a 50-μL volume mixture containing 20 mM HEPES buffer (10% glycerol, 200 mM NaCl, pH 8.0), 2 mM MgCl2, 2 mM KCl, 2 mM ATP, 0.2 mM S7P, 5 μM HygP, 5 μM GmhBVC, and 5 μM HldEVC at 30 °C for 2 h. The enzymatic assays of HldEVP from V. parahaemolyticus CGMCC 1.1997 were performed similarly except that HldEVC and GmhBVC were replaced by HldEVP and GmhBVP. The well-studied combination of HygP, GmhBEC, and HldEEC was performed as a positive control at the same conditions. All of the reactions were quenched by mixing vigorously with an equal volume of chloroform. After centrifugation, the chloroform layer was removed and 10 μL of aqueous sample was subjected to HPLC analysis.
Spectroscopic analysis
Analytical HPLC was performed with a Dionex CarboPac™ PA1 BioLC™ column (4 × 250 mm, Thermo Fisher Scientific, USA) on a Shimadzu HPLC system (Shimadzu, Japan). For the detections of ADP-d,d-manno-heptose and ADP-β-d-mannose, the column was developed with solvent A (ddH2O) and solvent B (1.0 M CH3COONH4) at a flow rate of 1.0 mL/min. The percentage of solvent B was changed using the following gradient: 0–5 min, 15%; 5–30 min, 15%–45%; 30–32 min, 45%–90%; 32–38 min, 90%; 38–40 min, 90%–15%; and 40–45 min, 15%. To detect ADP-l,d-manno-heptose, the percentage of solvent B was changed using the following gradient:0–5 min, 24%; 5–30 min, 24%–27%; 30–32 min, 27%–90%; 32–38 min, 90%; 38–40 min, 90%–24%; and 40–45 min, 24%. To detect ADP-d-glycero-β-d-altro-heptose, the percentage of solvent B was changed using the following gradient: 0–5 min, 15%; 5–30 min, 15%–30%; 30–40 min, 30%–45%; 40–42 min, 45%–90%; 42–52 min, 90%; and 52–53 min, 90%–15%; and 53–63 min, 15%. The detection wavelength was set as 254 nm.
HRMS was performed on an Agilent 1260 HPLC/6520 QTOF-MS instrument with an electrospray ionization source. NMR spectra were recorded at room temperature on a Bruker-500 NMR.
Colorimetric assay
A pyrophosphatase (PPase)-coupled colorimetric assay was developed for monitoring the nucleotidyltransfer activities of HldEVC, HldEVP, and HldEEC. Briefly, the enzymatic assay was carried out in a 50-μL volume mixture in 96-well microtiter plate using ATP (0.2 mM) and β-d-mannose 1-phosphate (0.1 mM) as substrates. The PPase assays revealed that 0.1 U PPase could effectively catalyze the hydrolysis of 0.2 mM PPi at a wide temperature range (from 15 to 45 °C). Therefore, HldE was added together with 0.1 U PPase and the reaction mixture was incubated at the assay temperature for HldE. After 30 min, the reactions were terminated by adding the premixed malachite green and ammonium molybdate agent from the Malachite Green Phosphate Detection Kit (CST, MA, USA) at room temperature for 15 min and monitored by a microplate reader (Synergy H4, BioTek, USA) at 630 nm (Sha et al. 2012). The reactions with boiled HldEs were carried out as negative controls to adjust the interference by substrates and buffer components.
Kinetic studies
Using the developed colorimetric assay, the reaction conditions of HldEVC, HldEVP, and HldEEC were optimized. The optimal pH was determined by performing the enzyme reaction in 20 mM different buffers (citrate-sodium citrate buffer (pH 6.5), HEPES-NaOH (pH 7.0, 7.5, 8.0), Tris-HCl buffer (pH 8.5, 9.0), glycine-NaOH buffer (pH 9.5, 10.0)) at 30 °C. The optimal temperatures of the three enzymes were determined by incubating the reactions at 15 to 45 °C at their optimal pHs. The initial velocities were evaluated by performing the reactions at a different incubation time under the optimal pH and temperature.
The Km and kcat values of HldEVC, HldEVP, and HldEEC against β-d-mannose 1-phosphate and ATP were determined under their optimal pH and temperature. The Km values of the three enzymes against β-d-mannose 1-phosphate were obtained by performing the reactions with different concentrations of β-d-mannose 1-phosphate (5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 75, 100, 125, and 150 μM) and a saturation concentration of ATP (2 mM). The Km values of the three enzymes against ATP were obtained by performing the reactions with different concentrations of ATP (20, 35, 50, 75, 100, 150, 200, 250, 300, 500, 750, and 1000 μM) and a saturation concentration of β-d-mannose 1-phosphate (400 μM). Each parameter was measured in triplicate and the data were analyzed using Origin 2021.
Results
Characterization of the Vibrio enzymes responsible for ADP-d,d-manno-heptose biosynthesis
V. cholerae O1 2010EL-1786 is a causative agent of life-threating cholera isolated from a stool sample of one cholera patient (Reimer et al. 2011); V. parahaemolyticus CGMCC 1.1997 is a gastroenteritis causative strain from a patient suffering with Shirasu food poisoning (Daniel et al. 1999). In silico analysis of their genome sequences (NCBI RefSeq assemblies: GCF_000166455.1 (V. cholerae O1 2010EL-1786) and GCF_001011015.1 (V. parahaemolyticus CGMCC 1.1997)) revealed that both of the two strains contain the necessary genes responsible for ADP-β-d-manno-heptose biosynthesis. Sequence alignments revealed that the ADP-β-d-manno-heptose biosynthetic enzymes from the two Vibrio strains display more than 60% amino acid sequence identities with their homologues from E. coli (Table S3). AlphaFold prediction and subsequent structure analysis revealed that the overall structures of the isomerases, phosphatases, and ADP-l,d-manno-heptose C-6″ epimerases from both Vibrio strains are quite similar to their homologues from E. coli (Figs. S2 and S3), while prominent conformation differences that deviate severely from HldEEC were observed in both of the two HldEs from Vibrio, especially the nucleotidyltransferase domain of HldEVP (Fig. 1c), which aroused our interests to study their functions elaborately. We cloned the genes encoding β-d-manno-heptose isomerase (gmhAVP), phosphatase (gmhBVP), and bifunctional kinase/nucleotidyltransferase (hldEVP) from V. parahaemolyticus CGMCC 1.1997. The genes encoding the same set of isozymes from V. cholerae O1 2010EL-1786, gmhAVC, gmhBVC, and hldEVC, were codon optimized and synthesized. The six genes were expressed as N-His6 tagged proteins using a “clean” chassis strain E. coli BL21 (DE3) ΔgmhA ΔgmhB ΔhldE (Tang et al. 2022), in which the native β-d-manno-heptose biosynthetic genes were knocked out (Fig. 1c).
The catalytic activities of the enzymes responsible for ADP-d,d-manno-heptose synthesis in V. cholerae O1 2010EL-1786 were investigated by incubating GmhAVC, GmhBVC, and HldEVC with S7P and ATP. A product shared the same retention time with ADP-d,d-manno-heptose was generated and was further identified by HPLC co-injection with the authentic standard of ADP-d,d-manno-heptose. We also tested the hybrid assays by replacing the well-characterized isomerase, phosphatase, or kinase/nucleotidyltransferase in the combination of GmhAEC + GmhBEC + HldEEC, which can synthesize ADP-d,d-manno-heptose efficiently, with the corresponding isozyme from V. cholerae O1 2010EL-1786, and all the combinations converted S7P to ADP-d,d-manno-heptose with comparable efficiencies (Fig. 1d).
When the heptose synthetic enzymes from V. parahaemolyticus CGMCC 1.1997 were incubated with S7P and ATP, ADP-d,d-manno-heptose was also generated in the GmhAVP + GmhBVP + HldEVP combination, but not in the control reaction with boiled HldEVP. And the enzymes from V. parahaemolyticus CGMCC 1.1997 could also functionally replace their isozymes in the GmhAEC + GmhBEC + HldEEC combination (Fig. 1d). HPLC analyses showed that about 72 μM, 64 μM, and 80 μM of S7P (200 μM) were converted to ADP-d,d-manno-heptose by HldEVC, HldEVP, and HldEEC together with its cognate GmhA and GmhB, respectively, at 30 °C, pH 8.0 (20 mM HEPES) for 2 h. The comparable production of ADP-d,d-manno-heptose could be explained by docking analysis of ATP in the binding pockets of the nucleotidyltransferase domains of HldEs, which showed that despite the differences in tertiary structures of the HldEs, they may adopt similar “horseshoe” binding model of ATP to shape ADP-d,d-manno-heptose effectively (Fig. S4). Collectively, these results confirmed that GmhAVC and GmhAVP are S7P isomerases, GmhBVC and GmhBVP are HBP phosphatases, and HldEVC and HldEVP are bifunctional proteins with H7P kinase and H1P nucleotidyltransferase activities.
Conversion of ADP-d,d-manno-heptose to ADP-l,d-manno-heptose by a C-6″ epimerase
To our knowledge, three l,d-manno-heptoses are contained in the LPS inner core of V. cholerae O1 strains as in E. coli, while the LPS structure of V. parahaemolyticus CGMCC 1.1997 remains unknown (Chatterjee and Chaudhuri 2003). In E. coli, ADP-d,d-manno-heptose is converted to ADP-l,d-manno-heptose by an NAD+-dependent C-6″ epimerase HldDEC (Fig. 2a). Bioinformatic analysis revealed that both of the two Vibrio genomes contain an isoenzyme HldDEC, HldDVC (WP_000587795.1), and HldDVP (WP_015296139.1). Therefore, we cloned the epimerase encoding gene hldDVP from V. parahaemolyticus CGMCC 1.1997, and the hldDVC gene from V. cholerae O1 2010EL-1786 was obtained by DNA synthesis after codon optimization. The two epimerases were then expressed as N-His6 tagged proteins in E. coli BL21 (DE3) ΔgmhA ΔgmhB ΔhldE (Fig. 2b).
To verify the function of HldDVC, it was incubated with ADP-d,d-manno-heptose and NAD+. HPLC analysis showed that ADP-d,d-manno-heptose was converted to a compound having the same retention time as the authentic standard of ADP-l,d-manno-heptose, and it was further verified by HRMS analysis (m/z 618.0847 for [M-H]−,C17H27N5O16P2, cacld 618.0855) (Fig. S5). HldDVP could also convert ADP-d,d-manno-heptose to ADP-l,d-manno-heptose under the same assay conditions. Both of the two ADP-d,d-manno-heptose C-6″ epimerase from Vibrio species displayed comparable catalytic efficiencies with the E. coli epimerase HldDEC, which was used as a positive control (Fig. 2c). Taken together, we showed that both V. cholerae O1 2010EL-1786 and V. parahaemolyticus CGMCC 1.1997 possess the complete set of β-d-manno-heptose biosynthetic enzymes for synthesizing ADP-l,d-manno-heptose from S7P, implying that l,d-manno-heptose is widely distributed in the LPSs of different Vibrio strains.
Synthesis of ADP-β-d-mannose by the nucleotidyltransferase domain of HldE
A study on HldEEC suggested that it can convert mannose to ADP-β-d-mannose in the presence of ATP, indicating that the kinase domain of HldEEC is able to add a phosphate group onto the anomeric carbon of mannose to form β-d-mannose 1-phosphate, which is then converted to ADP-β-d-mannose by the nucleotidyltransferase domain of HldEEC (Morrison and Tanner 2007). To verify the substrate promiscuity of the nucleotidyltransferase domain of HldEEC, we chemically synthesized β-d-mannose 1-phosphate as described (Supplementary Scheme 1 and Fig. S1). When HldEEC was incubated with ATP and the mimic sugar substrate, β-d-mannose 1-phosphate, or α-d-mannose 1-phosphate (commercially available), a new peak was observed only in the assay of β-d-mannose 1-phosphate, while not in the assay using α-d-mannose 1-phosphate or the negative control using boiled HldEEC (Fig. 3b; Fig. S6). Subsequently, the product was prepared by a large-scale enzymatic synthesis and confirmed to be ADP-β-d-mannose by careful analyses of its high-resolution mass spectrometry and nuclear magnetic resonance data (Fig. 3c). The β-configuration of the anomeric carbon of mannose was assigned by the NOE correlations of H-1″ with H-3″ and H-5″ (Fig. S7). We also tested the catalytic activities of HldEVC and HldEVP toward β-d-mannose 1-phosphate (200 μM) and α-d-mannose 1-phosphate. Both of the two enzymes could only take β-d-mannose 1-phosphate, activate it into ADP-β-d-mannose as HldEEC, and comparable yields of ADP-β-d-mannose (76 μM for HldEVC, 68 μM for HldEVP, and 76 μM for HldEEC) were detected at 30 °C, pH 8.0 (20 mM HEPES), 2 h (Fig. 3a, b; Fig. S6). The results suggested that the nucleotidyltransferase domain of HldE possesses a certain level of promiscuity toward the size of the sugar substrates, but it has a stringent specificity on the anomeric configuration of sugar 1-phosphate.
Conversion of β-d-mannose 1-phosphate to ADP-β-d-mannose. a A proposed reaction to form ADP-β-d-mannose. b HPLC profiles of the enzymatic assays catalyzed by the nucleotidyltransferase domains of HldEs using β-d-mannose 1-phosphate and ATP as substrates. The detection wavelength was set as 254 nm. c 1H NMR (500 MHz) and 13C NMR (125 MHz) data for ADP-β-d-mannose in D2O
Kinetics of the nucleotidyltransferase domains of HldEVC, HldEVP, and HldEEC
As aforementioned, ADP-β-d-manno-heptoses are important components of LPS. However, due to the difficulties in preparing H1P, the catalytic efficiency of the nucleotidyltransferase domain of HldE, which is responsible for activating H1P into ADP-d,d-manno-heptose, has not been investigated kinetically yet. If the real substrate of a nucleotidyltransferase is unavailable, its mimic substrate can be used instead for kinetic studies (Kim et al. 2020; Kim et al. 2021). With β-d-mannose 1-phosphate in hand, we collected the kinetic parameters of HldE using it and ATP as substrates.
The nucleotidyltransferase domain of HldE cleaves an ATP into an AMP and one molecule of pyrophosphoric acid (PPi). The AMP is employed to activate β-d-pyranose 1-phosphate into ADP-β-d-pyranose, and the PPi can be hydrolyzed into inorganic phosphate (Pi) by a PPase and measured conveniently by a colorimetric assay as shown in Fig. 4a. Pi reacts with the premixed malachite green and ammonium molybdate agent under acidic condition to generate a malachite green complex, and the signal can be easily read at 630 nm. We developed a stable detection process by controlling the reaction and the detection conditions to minimize the background influence. Then, the reaction conditions of HldEVC and HldEVP as well as HldEEC were optimized by measuring the Pi generation during the process. It was showed that the three enzymes reached their maximal activities at 30 °C, and the optimum pHs of HldEVC, HldEVP, and HldEEC were 8.5, 8.0, and 7.5, respectively (Figs. S8 and S9). Subsequently, steady-state kinetic studies were carried out under the optimized conditions. The Km and kcat values against both substrates, β-d-mannose 1-phosphate and ATP, were collected and summarized in Fig. 4b. HldEVC and HldEVP displayed comparable Km and kcat values. HldEEC from E. coli exhibited slightly higher affinities to β-d-mannose 1-phosphate and ATP substrates than HldEs from Vibrio species, while it had comparable turnover numbers (Fig. 4b; Fig. S10).
Determination of the kinetics of the nucleotidyltransferase domains of HldEs. a A scheme of the colorimetric assay for detecting the formed PPi group that is generated by the nucleotidyltransfer reaction. b The kinetic parameters of the nucleotidyltransferase domains of HldEs against different substrates
Discussion
Vibrio genus contains several important pathogenic species (e.g., V. cholerae and V. parahaemolyticus) that can cause human diseases. Many people around the world are suffering waterborne and foodborne vibriosis with symptomatic entities such as watery diarrhea, stomach cramping, nausea, vomiting, fever, and chills (Dutta et al. 2021). ADP-β-d-manno-heptoses participate in the assembly of LPS, a virulence factor of Vibrio infections (Qadri et al. 2003; Chatterjee and Chaudhuri 2003), and are also powerful agonists that could trigger NF-κB-mediated innate immune responses (Janeway Jr. and Medzhitov 2002; Zhou et al. 2018). However, little is known about the formation mechanism of ADP-β-d-manno-heptoses in Vibrio species or the influences of those ADP-sugars on their pathogenesis. In this work, we characterized the manno-heptose biosynthetic enzymes from two Vibrio strains and proposed that Vibrio adopts the same biosynthetic pathway as E. coli to synthesize ADP-d,d-manno-heptose and then ADP-l,d-manno-heptose using S7P as a precursor. In this process, S7P goes through a four-step reaction relay including isomerization, phosphorylation at C-1″, dephosphorylation at C-7″, and nucleotide activation to form ADP-d,d-manno-heptose. ADP-d,d-manno-heptose is further epimerized at C-6″ to generate ADP-l,d-manno-heptose, which is then loaded to lipid A to assemble the inner core of LPS. The results showed that Vibrio strains synthesize ADP-β-d-manno-heptoses as most of the other Gram-negative bacteria. Characterization of the enzymes involved in manno-heptose biosynthesis paves the way for further investigations on the influences of ADP-β-d-manno-heptoses on Vibrio pathogenesis.
Conversion of H1P to ADP-d,d-manno-heptose by nucleotidyltransferase is a key step of β-d-manno-heptose metabolism. It activates the β-d-manno-heptose into its ADP form, which can be further modified by the following C-6″ epimerase and facilitate the addition of β-d-manno-heptose to lipid A (Whitfield and Trent 2014). To date, a number of different H1P nucleotidyltransferases have been studied enzymatically (Kneidinger et al. 2002; Park et al. 2018; Tang et al. 2022). However, none of them, even the well-characterized HldEEC from E. coli, has been investigated kinetically, mainly due to the difficulties in obtaining the sugar substrate, H1P. We chemically synthesized a mimic substrate, β-d-mannose 1-phosphate, and verified that all of the HldEs from E. coli and Vibrio strains could take it and catalyze the conversion of β-d-mannose 1-phosphate to ADP-β-d-mannose with considerable efficiencies. Thus, kinetic analyses of the three HldEs were performed using the synthesized β-d-mannose 1-phosphate as the sugar donor and ATP as the acceptor. The results showed that the two Vibrio enzymes, HldEVC and HldEVP, exhibited comparable nucleotidyltransfer efficiencies, while HldEEC from E. coli outperformed a little bit on affinities of both substrates. Actually, if the real substrate of a nucleotidyltransferase is unavailable, its mimic substrate can be used instead for kinetic studies (Kim et al. 2020; Kim et al. 2021). While this approach may not capture the natural properties of the enzyme, it can still provide us valuable information and enhance our understanding to the targeted enzyme. In addition, our previous works revealed that HldEEC is able to tolerate the 3-epimer of H1P and activate d-glycero-β-d-altro-heptose 1-phosphate into its ADP form (Tang et al. 2018) and the Vibrio strain–derived HldEVC and HldEVP also possess similar abilities to synthesize ADP-d-glycero-β-d-altro-heptose (Fig. S11). Taken collectively, HldEs exhibit a certain level of sugar substrate flexibilities and can take not only different β-d-heptose 1-phosphate but also β-d-mannose 1-phosphate (Adekoya et al. 2018). They may be developed as potent catalysts for the synthesis of various ADP-β-d-sugars that are not easily synthesized by chemical methods.
In summary, we characterized the biosynthetic enzymes of ADP-β-d-manno-heptoses from two pathogenic Vibrio strains, which suggested that Vibrio strains adopt the same biosynthetic pathway as E. coli in synthesizing ADP-β-d-manno-heptoses. Moreover, we showed that the two HldEs from Vibrio could activate β-d-mannose 1-phosphate to its ADP form as well as HldEEC from E. coli and studied the kinetics of the nucleotidyltransferase domains of these three HldEs using this mimic substrate. All of our works enhance our understanding of ADP-β-d-manno-heptose biosynthesis in Vibrio strains and laid a foundation for the following studies on heptose metabolism in Vibrio and its influences on Vibrio pathogenesis.
Data availability
The original GenBank accession numbers of gmhAVC, hldEVC, gmhBVC, and hldDVC from V. cholerae O1 2010EL-1786 are WP_000284054.1, WP_000805769.1, WP_001108094.1, and WP_000587795.1, respectively. The nucleotide sequence of codon-optimized gmhAVC (accession number: OR656557), hldEVC (accession number: OR656559), gmhBVC (accession number: OR656558), and hldDVC (accession number: OR656560) genes for E. coli is uploaded to the NCBI database, and the data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Adekoya IA, Guo CX, Gray-Owen SD, Cox AD, Sauvageau J (2018) d-glycero-β-d-manno-heptose 1-phosphate and d-glycero-β-d-manno-heptose 1,7-biphosphate are both innate immune agonists. J Immunol 201(8):2385–2391. https://doi.org/10.4049/jimmunol.1801012
Baker-Austin C, Oliver JD, Alam M, Ali A, Waldor MK, Qadri F, Martinez-Urtaza J (2018) Vibrio spp. infections. Nat Rev Dis Primers 4(1):1–19. https://doi.org/10.1038/s41572-018-0005-8
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1-2):248–254. https://doi.org/10.1006/abio.1976.9999
Chatterjee SN, Chaudhuri K (2003) Lipopolysaccharides of Vibrio cholerae. BBA Mol Basis Dis 1639(2):65–79. https://doi.org/10.1016/j.bbadis.2003.08.004
Cui J, Duizer C, Bouwman LI, van Rooijen KS, Voogdt CGP, van Putten JPM, de Zoete MR (2021) The ALPK1 pathway drives the inflammatory response to Campylobacter jejuni in human intestinal epithelial cells. PLoS Pathog 17(8):e1009787. https://doi.org/10.1371/journal.ppat.1009787
Daniel LB, Diane SH, Doris TH, Dallas GH (1999) Response of pathogenic Vibrio species to high hydrostatic pressure. Appl Environ Microb 65(6):2776–2780. https://doi.org/10.1128/aem.65.6.2776-2780.1999
Dutta D, Kaushik A, Kumar D, Bag S (2021) Foodborne pathogenic Vibrios: antimicrobial resistance. Front Microbiol 12:638331. https://doi.org/10.3389/fmicb.2021.638331
Garcia-Weber D, Arrieumerlou C (2021) ADP-heptose: a bacterial PAMP detected by the host sensor ALPK1. Cell Mol Life Sci 78(1):17–29. https://doi.org/10.1007/s00018-020-03577-w
Gaudet RG, Sintsova A, Buckwalter CM, Leung N, Cochrane A, Li J, Cox AD, Moffat J, Gray-Owen SD (2015) Cytosolic detection of the bacterial metabolite HBP activates TIFA-dependent innate immunity. Science 348(6240):1251–1255. https://doi.org/10.1126/science.aaa4921
Guo Z, Tang Y, Tang W, Chen Y (2021) Heptose-containing bacterial natural products: structures, bioactivities, and biosyntheses. Nat Prod Rep 38(10):1887–1909. https://doi.org/10.1039/d0np00075b
Janeway CA Jr, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20(1):197–216. https://doi.org/10.1146/annurev.immunol.20.083001.084359
Kanungo S, Azman AS, Ramamurthy T, Deen J, Dutta S (2022) Cholera. Lancet 399(10333):1429–1440. https://doi.org/10.1016/s0140-6736(22)00330-0
Kim S, Jo S, Kim M-S, Shin DH (2020) A study of a potent inhibitor against a GDP-6-deoxy-α-d-manno-heptose biosynthesis pathway as antibiotic candidates. Microb Drug Resist 26(4):385–390. https://doi.org/10.1089/mdr.2019.0
Kim S, Jo S, Kim M-S, Shin DH (2021) A study of inhibitors of d-glycero-β-d-manno-heptose-1-phosphate adenylyltransferase from Burkholderia pseudomallei as a potential antibiotic target. J Enzyme Inhib Med Chem 36(1):776–784. https://doi.org/10.1080/14756366.2021.1900166
Kneidinger B, Marolda C, Graninger M, Zamyatina A, McArthur F, Kosma P, Valvano MA, Messner P (2002) Biosynthesis pathway of ADP-l-glycero-β-d-manno-heptose in Escherichia coli. J Bacteriol 184(2):363–369. https://doi.org/10.1128/jb.184.2.363-369.2002
Li P, Chen M, Tang W, Guo Z, Zhang Y, Wang M, Horsman GP, Zhong J, Lu Z, Chen Y (2021) Initiating polyketide biosynthesis by on-line methyl esterification. Nat Commun 12(1):4499. https://doi.org/10.1038/s41467-021-24846-7
Lu Q, Yao Q, Xu Y, Li L, Li S, Liu Y, Gao W, Niu M, Sharon M, Ben-Nissan G, Zamyatina A, Liu X, Chen S, Shao F (2014) An iron-containing dodecameric heptosyltransferase family modifies bacterial autotransporters in pathogenesis. Cell Host Microbe 16(3):351–363. https://doi.org/10.1016/j.chom.2014.08.008
Malott RJ, Keller BO, Gaudet RG, McCaw SE, Lai CC, Dobson-Belaire WN, Hobbs JL, St Michael F, Cox AD, Moraes TF, Gray-Owen SD (2013) Neisseria gonorrhoeae-derived heptose elicits an innate immune response and drives HIV-1 expression. Proc Natl Acad Sci U S A 110(25):10234–10239. https://doi.org/10.1073/pnas.1303738110
Morrison JP, Tanner ME (2007) A two-base mechanism for Escherichia coli ADP-l-glycero-d-manno-heptose 6-epimerase. Biochemistry 46(12):3916–3924. https://doi.org/10.1021/bi602641m
Park J, Kim H, Kim S, Lee D, Kim MS, Shin DH (2018) Crystal structure of d-glycero-β-d-manno-heptose-1-phosphate adenylyltransferase from Burkholderia pseudomallei. Proteins 86(1):124–131. https://doi.org/10.1002/prot.25398
Qadri F, Alam MS, Nishibuchi M, Rahman T, Alam NH, Chisti J, Kondo S, Sugiyama J, Bhuiyan NA, Mathan MM, Sack DA, Nair GB (2003) Adaptive and inflammatory immune responses in patients infected with strains of Vibrio parahaemolyticus. J Infect Dis 187(7):1085–1096. https://doi.org/10.1086/368257
Raetz CR, Whitfield C (2002) Lipopolysaccharide endotoxins. Annu Rev Biochem 71(1):635–700. https://doi.org/10.1146/annurev.biochem.71.110601.135414
Reimer A, Domselaar G, Stroika S, Walker M, Kent H, Tarr C, Talkington D, Rowe L, Olsen-Rasmussen M, Frace M, Sammons S, Dahourou G, Boncy J, Smith A, Mabon P, Petkau A, Graham M, Gilmour M, Gerner-Smidt P (2011) Comparative genomics of Vibrio cholerae from Haiti, Asia, and Africa. Emerg Infect Dis 17(11):2113–2121. https://doi.org/10.3201/eid1711.110794
Sha S, Zhou Y, Xin Y, Ma Y (2012) Development of a colorimetric assay and kinetic analysis for Mycobacterium tuberculosis d-glucose-1-phosphate thymidylyltransferase. SLAS Discov 17(2):252–257. https://doi.org/10.1177/1087057111421373
Tang W, Guo Z, Cao Z, Wang M, Li P, Meng X, Zhao X, Xie Z, Wang W, Zhou A, Lou C, Chen Y (2018) d-Sedoheptulose-7-phosphate is a common precursor for the heptoses of septacidin and hygromycin B. Proc Natl Acad Sci U S A 115(11):2818–2823. https://doi.org/10.1073/pnas.1711665115
Tang Y, Tang W, Wang M, Zhang Z, Chen Y (2022) A conservative distribution of tridomain NDP-heptose synthetases in actinobacteria. Sci China Life Sci 65(5):1014–1023. https://doi.org/10.1007/s11427-021-2000-2
Whitfield C, Trent MS (2014) Biosynthesis and export of bacterial lipopolysaccharides. Annu Rev Biochem 83(1):99–128. https://doi.org/10.1146/annurev-biochem-060713-035600
Zhang XH, Austin B (2005) Haemolysins in Vibrio species. J Appl Microbiol 98(5):1011–1019. https://doi.org/10.1111/j.1365-2672.2005.02583.x
Zhou P, She Y, Dong N, Li P, He H, Borio A, Wu Q, Lu S, Ding X, Cao Y, Xu Y, Gao W, Dong M, Ding J, Wang DC, Zamyatina A, Shao F (2018) Alpha-kinase 1 is a cytosolic innate immune receptor for bacterial ADP-heptose. Nature 561(7721):122–126. https://doi.org/10.1038/s41586-018-0433-3
Zimmermann S, Pfannkuch L, Al-Zeer MA, Bartfeld S, Koch M, Liu J, Rechner C, Soerensen M, Sokolova O, Zamyatina A, Kosma P, Maurer AP, Glowinski F, Pleissner KP, Schmid M, Brinkmann V, Karlas A, Naumann M, Rother M et al (2017) ALPK1- and TIFA-dependent innate immune response triggered by the Helicobacter pylori type IV secretion system. Cell Rep 20(10):2384–2395. https://doi.org/10.1016/j.celrep.2017.08.039
Funding
This work was supported in part by the National Key Research and Development Program of China (2021YFC2301000), National Natural Science Foundation of China (32025002), and Chinese Academy of Sciences (KFJ-BRP-009, 079GJHZ2022033GC).
Author information
Authors and Affiliations
Contributions
YC conceived the project. ZS, YT, ZW, MW, and ZZ designed and performed experiments. ZS, YT, JJ, and YC analyzed the data. ZS, YT, and YC wrote the manuscript and designed the figures. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 1832 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shi, Z., Tang, Y., Wang, Z. et al. Characterization of the ADP-β-d-manno-heptose biosynthetic enzymes from two pathogenic Vibrio strains. Appl Microbiol Biotechnol 108, 267 (2024). https://doi.org/10.1007/s00253-024-13108-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-024-13108-3