Abstract
Heme is an iron-containing porphyrin compound widely used in the fields of healthcare, food, and medicine. Compared to animal blood extraction, it is more advantageous to develop a microbial cell factory to produce heme. However, heme biosynthesis in microorganisms is tightly regulated, and its accumulation is highly cytotoxic. The current review describes the biosynthetic pathway of free heme, its fermentation production using different engineered bacteria constructed by metabolic engineering, and strategies for further improving heme synthesis. Heme synthetic pathway in Bacillus subtilis was modified utilizing genome-editing technology, resulting in significantly improved heme synthesis and secretion abilities. This technique avoided the use of multiple antibiotics and enhanced the genetic stability of strain. Hence, engineered B. subtilis could be an attractive cell factory for heme production. Further studies should be performed to enhance the expression of heme synthetic module and optimize the expression of heme exporter and fermentation processes, such as iron supply.
Key points
• Strengthening the heme biosynthetic pathway can significantly increase heme production.
• Heme exporter overexpression helps to promote heme secretion, thereby further promoting excessive heme synthesis.
• Engineered B. subtilis is an attractive alternative for heme production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heme is an iron-containing porphyrin compound that is essential for the survival of virtually all living systems (Gallio et al. 2021). It serves as a prosthetic group in proteins involved in multiple biological processes, such as oxygen transport and storage, electron transfer, and oxidative stress detoxification (Beas et al. 2022). Heme has extensive application value. In the field of healthcare, since heme iron is a better bioavailable and tolerable form of iron, it can be suitable for supplementation in pregnancy for the treatment of anemia (Abbas et al. 2020). In the field of food coloring, heme is a safe and natural pigment that is used to replace carcinogenic chromogen nitrite and synthetic pigments. Moreover, with the development of plant-based meat alternatives, heme-containing proteins can be used to generate meat flavors and/or aromas in a variety of food products during the cooking process (Ahmada et al. 2022). In the field of pharmaceuticals, heme is a raw material used for semisynthesis of hematoporphyrin and its derivatives, as well as for protoporphyrin sodium, which is used for treatment of diseases (Cannon 1993; Yarra et al. 2019).
The global heme market size is expected to reach $530 million by 2026. The traditional heme production method extracts hemoglobin from the blood of pigs or other animals and then prepares free heme through enzymatic hydrolysis. However, collection, transportation, and storage of animal blood are relatively troublesome, and obtaining hemoglobin from blood by a solid-phase extraction method is expensive, low-yielding, and time-consuming (Jia et al. 2017). Therefore, developing microbial cell factories to produce heme is more advantageous. For example, Impossible Foods Incorporation (Redwood City, CA) has developed a Pichia pastoris expression system to realize the industrial-scale production of soy leghemoglobin (Fraser et al. 2018). Shao et al. have achieved a leghemoglobin titer of 3.5 g/L by enhancing globin expression and heme biosynthesis (Shao et al. 2022). Zhao et al. summarized the current strategies for microbial synthesis of hemoglobin, such as promoting heme synthesis, discovering superior hemoglobins, balancing heme synthesis and globins expression (Zhao et al. 2021). However, hemoglobin is mainly used for producing artificial meat products and needs to be hydrolyzed to obtain free heme. In recent years, more and more researchers have focused on developing bacterial cell factories through synthetic biology to directly produce free heme (Fig. 1). The free heme production of engineered Escherichia coli was 1,034 mg/L (Choi et al. 2022), the highest yield of heme produced by microbial fermentation reported so far.
The current review provides a concise and comprehensive summary of the progress of microbial synthesis of heme, including the biosynthetic pathway of free heme and its fermentation production with different engineered bacteria, i.e., E. coli, Corynebacterium glutamicum, and Bacillus subtilis. In microorganisms, heme biosynthesis is tightly regulated, showing highly cytotoxic effect at accumulation > 1 µM (Nishinaga et al. 2021; Sassa 2004). Heme is mainly accumulated inside the cell during flask fermentation and outside the cell during fed-batch fermentation in the fermenter (Ko et al. 2021; Yang et al. 2023; Zhao et al. 2018). The secretion ability of heme can be improved by increasing the expression of heme exporter. Based on the comparative investigations of the characteristics of these three engineered bacteria, it is proposed that B. subtilis is expected to be an efficient cell factory for heme production. In addition, strategies for further improving microbial synthesis of heme are proposed, such as enhancing the expression of heme synthetic module, finding the endogenous heme exporter or dynamically regulating the expression of the heterologous heme exporter, and optimizing the fermentation processes such as iron supply.
Biosynthetic pathway of free heme
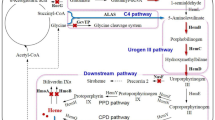
The biosynthetic pathway of heme is divided into three modules in microorganisms, including 5-aminolevulinic acid (ALA), uroporphyrinogen (urogen) III, and heme synthetic modules (Fig. 2).
Biosynthetic pathway of heme in microorganisms. GluRS, glutamyl-tRNA synthase; GtrR, glutamyl-tRNA reductase; GsaM, glutamate-1-semialdehyde-2,1-aminomutase; AlaS, 5-aminolevulinic acid synthase; PbgS, porphobilinogen synthase; HmbS, hydroxymethylbilane synthase; UroS, uroporphyrinogen (urogen) III synthase; UroD, urogen III decarboxylase; CgoX, coproporphyrinogen (coprogen) oxidase; CpfC, coproporphyrin ferrochelatase; ChdC, coproheme decarboxylase; CgdC, oxygen-dependent coprogen III oxidase; CgdH, oxygen-independent coprogen III dehydrogenase; PgoX, protoporphyrinogen oxidase; PgdH1 and PgdH2, protoporphyrinogen dehydrogenase; PpfC, protoporphyrin ferrochelatase; SUMT, S-adenosyl-L-methionine urogen III methyltransferase; SicC, precorrin-2 dehydrogenase; SirB, sirohydrochlorin ferrochelatase; AhbA and AhbB, siroheme decarboxylase; AhbC, Fe-coproporphyrin synthase; AhbD, coproheme decarboxylase
ALA synthetic module
ALA is an important precursor for heme synthesis with two biosynthetic pathways of C4 and C5 (Fig. 2). The C4 pathway exists in humans, animals, fungi, and purple non-sulfur phototrophic bacteria (Stojanovski et al. 2019; Zhao et al. 2021), such as Rhodobacter capsulatus and Rhodobacter sphaeroides. ALA synthase (AlaS) is the only enzyme in this pathway that catalyzes the condensation of glycine and succinyl-CoA to form ALA. The C5 pathway is present in archaea, plants, and other bacteria (Layer 2021; Zhao et al. 2021), such as Salmonella arizona, E. coli, and B. subtilis. It involves glutamyl-tRNA synthase (GluRS), glutamyl-tRNA reductase (GtrR), and glutamate-1-semialdehyde 2,1-aminomutase (GsaM), catalyzing L-glutamate to generate ALA. In S. arizona and E. coli, HemA is sensitive to hydrolase and is regulated by heme. GtrRM is obtained by inserting two lysine residues (KK) between the Thr2 and Leu3 residues of native GtrR, relieving feedback inhibition (Yu et al. 2015; Zhao et al. 2018). In B. subtilis, GtrR is negatively regulated by the negative regulatory protein HemX, decreasing the steady-state cellular concentration of GtrR protein by controlling its synthesis rate (Johansson and Hederstedt 1999; Schroder et al. 1994). This regulation is released by knocking out hemX (Yang et al. 2023). In addition, there are a few microorganisms with both of the C4 and C5 pathways, such as Euglena gracilis (Weinstein and Beale 1983).
Urogen III synthetic module
The synthesis of ALA to urogen III includes three enzymatic reactions and is a common pathway among all microorganisms. First, two ALA molecules are condensed and cyclized to generate one porphobilinogen molecule catalyzed by porphobilinogen synthase (PbgS) (Hansson et al. 1991; Lu et al. 2010). Then, four porphobilinogen molecules react with one water molecule to form one hydroxymethylbilane molecule and release four ammonia molecules catalyzed by hydroxymethylbilane synthase (HmbS) (Hansson et al. 1991). Finally, urogen III synthase (UroS) catalyzes the dehydration of hydroxymethylbilane to generate urogen III (Hansson et al. 1991; Stamford et al. 1995). That is, eight ALA molecules synthesize one urogen III molecule. In E. coli and B. subtilis, PbgS, HmbS, and UroS are encoded by hemB, hemC, and hemD, respectively. Zhao et al. have constructed the expression plasmid pRSF-hemB-hemC-hemD to promote the conversion of ALA to urogen III, thereby promoting the synthesis of heme in E. coli (Zhao et al. 2018). Yang et al. have promoted heme synthesis by integrating hemC-hemD-hemB into the genome of B. subtilis (Yang et al. 2023).
Heme synthetic module
Depending on the species, there are three synthetic pathways from urogen III to heme, including protoporphyrin-dependent (PPD), coproporphyrin-dependent (CPD), and siroheme-dependent (SHD) pathways (Fig. 2) (Dailey et al. 2017; Layer 2021). The PPD pathway exists in both Gram-negative and Gram-positive bacteria, while the CPD pathway only exists in some Gram-positive bacteria, such as C. glutamicum and B. subtilis. The SHD pathway is considered the most ancient route for heme synthesis as it is present in archaea, sulfate-reducing bacteria. At present, there are no research reports on the heme synthesis through modifying the SHD pathway.
Urogen III decarboxylase (UroD) is a common enzyme in the PPD and CPD pathways, catalyzing the removal of four CO2 molecules from urogen III to generate coproporphyrinogen (coprogen) III. In the PPD pathway, coprogen III is oxidized to protoporphyrinogen IX by oxygen-dependent coprogen III decarboxylase (CgdC) or oxygen-independent coprogen III dehydrogenase (CgdH). Then, protoporphyrinogen IX is oxidized to form protoporphyrin IX, catalyzed by protoporphyrinogen oxidase (PgoX) or protoporphyrinogen dehydrogenase (PgdH1/PgdH2). Ultimately, protoporphyrin ferrochelatase (PpfC) catalyzes the insertion of Fe2+ into protoporphyrin IX to produce heme. In E. coli, genes hemE, hemF, hemG, and hemH encode UroD, CgdC, PgdH1, and PpfC, respectively. Zhao et al. (2018) constructed the plasmid pET-hemE-hemF-hemG-hemH to increase the expression levels of four endogenous enzymes, i.e., UroD, CgdC, PgdH1, and PpfC, thereby promoting the conversion of urogen III to heme in E. coli. In the CPD pathway, coprogen III reacts with three O2 molecules to form coproporphyrin III catalyzed by coprogen III oxidase (CgoX). In B. subtilis, CgoX can also oxidize protoporphyrinogen IX to generate protoporphyrin IX, with lower oxidization rate than that of oxidization of coprogen III to form coproporphyrin III (Corrigall et al. 1998; Hansson and Hederstedt 1994). Then, coproporphyrin ferrochelatase (CpfC) catalyzes the insertion of Fe2+ into coproporphyrin III to produce Fe-coproheme III. Finally, coproheme is decarboxylated to heme by coproheme decarboxylase (ChdC). In C. glutamicum, UroD, CgoX, CpfC, and ChdC are encoded by hemE, hemY, hemH, and hemQ, respectively. Ko et al. have reinforced the endogenous CPD pathway by overexpressing hemE, hemY, hemH, and hemQ (pX2-hemAM-hemL-alaS-dtxR-hemE and pMTC-hemY-hemH-hemQ-hrtBA) to promote the synthesis of urogen III to heme in C. glutamicum (Ko et al. 2021).
In microorganisms, excessive accumulation of heme (> 1 µM) (Sassa 2004) is highly cytotoxic due to its Lewis acidity, redox activity, and hydrophobicity (Nishinaga et al. 2021), causing deleterious effects, such as membrane disorder, oxidative stress damage, lipid oxidation, and DNA damage (Choby and Skaar 2016). To maintain the intracellular homeostasis of heme, heme oxygenase catalyzes the degradation of heme to produce bilirubin (Park et al. 2014; Shibahara 1988).
Fermentation production of free heme with engineered bacteria
To increase the production of heme, many researchers have engineered a synthetic heme pathway. Table 1 shows an overview of previous reports on heme production by engineered microbial strains.
Fermentation by engineered E. coli
Among numerous microbial cell factories, E. coli is the most popular and user-friendly workhorse primarily due to its genetic amenability and well-developed bioprocessing strategies. The native biosynthetic pathway of heme in E. coli includes the C5, urogen III, and PPD pathways. Kwon et al. were the first to have used E. coli as the chassis and constructed three expression plasmids to overexpress AlaS from R. capsulatus, endogenous PbgS, HmbS, UroS (pACmod-hemA-hemB-hemC-hemD), and UroD from Synechocystis sp., and endogenous CgdC (pBBR-hemE-hemF), and CpfC from B. subtilis (pUCmod-hemH). By introducing the heterogeneous C4 pathway and strengthening the urogen III and PPD pathways, the heme yield of the final engineered strain was 3.3 ± 0.3 μmol/L after 48 h of fermentation (Kwon et al. 2003). Pranawidjaja et al. have constructed plasmid pTrc-hemA-coaA to co-express AlaS from R. sphaeroides and endogenous pantothenate kinase CoaA in E. coli W3110, where heme production reached 9.1 μmol/g dry cell weight (DCW) (Pranawidjaja et al. 2015). Ge et al. have constructed three plasmids to overexpress ferredoxin-dependent hemeoxygenase gene hox1, phycocyanobilin:ferredoxinoxidoreductase gene pcyA from Synechocystis sp., endogenous hemB, hemG, and hemH (pETDuet-hox1-pcyA, pET-28a-hemB, and pCDFDuet-hemG-hemH). The constructed E. coli S5 strain produced 10.30 ± 0.39 μmol/L heme (Ge et al. 2018).
Zhao et al. have constructed four expression plasmids (pCDF-hemA-hemL, pRSF-hemB-hemC-hemD, pET-hemE-hemF-hemG-hemH, and pACYC-ccmABC) to strengthen the endogenous C5, urogen III, and PPD pathways and overexpress heme exporter CcmABC. Furthermore, phosphate acetyl transferase gene pta, lactate dehydrogenase gene ldhA, and putative heme-degrading enzyme gene yfeX were also knocked out. The cells were harvested by centrifugation with the supernatant colected to detect the production of extracellular free heme. The cell pellet was resuspended into 1 M NaOH, and the cell suspension was ultrasonicated for 10 min in an ice bath and centrifuged again; the supernatant of the cell lysate was collected to examine the production of intracellular free heme. The amount of heme was quantified using high performance liquid chromatography (HPLC) equipped with a reverse-phase C18 column and a UV-detector set at 400 nm. The mobile phase was 30% methanol with 0.1% trifluoroacetic acid and a gradient elution was performed at 0.4 mL/min (Zhao et al. 2018). The engineered E. coli produced 239.2 ± 7.2 mg/L of total heme with 151.4 ± 5.6 mg/L of extracellular heme during fed-batch fermentation in a 6.6-L fermenter (Zhao et al. 2018). Furthermore, its heme production reached up to 1,034 mg/L by increasing cell density, regular iron supplementation, and supply of excess feeding solution (Choi et al. 2022). However, the release of endotoxin from the cell walls of E. coli precipitates an acute inflammatory response that often leads to shock and death (Epstein et al. 1999; Kilbourn et al. 1993). Thus, heme produced by the engineered E. coli needs to be purified to remove endotoxin prior to its use in the healthcare, food, and pharmaceutical industries.
Fermentation by engineered C. glutamicum
Corynebacterium glutamicum is a Gram-positive actinobacterium with the advantages of stable fermentation performance and no endotoxins. It is generally recognized as a safe (GRAS) microorganism, which has become a model organism in industrial biotechnology due to its use in large-scale amino acid production (Lei et al. 2021; Ogata and Hirasawa 2021). The native biosynthetic heme pathways in C. glutamicum include the C5, urogen III, PPD and CPD pathways. Yu et al. were the first to have used C. glutamicum as the chassis, with the heme yield of the constructed recombinant strain of 4.22 ± 0.62 mg/L via co-expression of hemAM (GtrRM) from S. arizona, hemL (GsaM) from E. coli, and endogenous gltX (GluRS) (Yu et al. 2015). Choi et al. have expressed hemA (AlaS) from R. sphaeroides alone (pSL360-hemA) in C. glutamicum, with the heme yield of 1.2 ± 0.2 μmoL/g DCW (Choi et al. 2017). Ko et al. have constructed C. glutamicum CGPB07 strain by co-overexpressing hemAM (GtrRM) from S. typhimurium, hemL (GsaM) from E. coli, and endogenous dtxR encoding the transcriptional regulator diphtheria toxin repressor. As a result, heme concentration reached 29.57 ± 2.46 mg/L (Ko et al. 2018).
Subsequently, Ko et al. have used a two-plasmid expression system to overexpress the C5 pathway (hemAM derived from Salmonella typhimurium, and hemL derived from E. coli), the heterologous C4 pathway (alaS derived from R. capsulatus), the diphtheria toxin repressor protein DtxR, the endogenous CPD pathway (hemE, hemY, hemH, and hemQ) and the heme transporter HrtBA, and knocked out heme-binding protein genes (hrrS, htaA, and hmuT). The C. glutamicum recombinant strain (pX2-hemAM-hemL-alaS-dtxR-hemE, pMTC-hemY-hemH-hemQ-hrtBA, ΔhrrS, ΔhtaA, and ΔhmuT) resulted in 309.18 ± 16.43 mg/L of total heme with 242.95 ± 11.45 mg/L of extracellular heme in 2-L fed-batch fermentation using modified CGXII medium supplemented with ethambutol for changing the lipid component (Ko et al. 2021). Ethambutol is a first-line drug for treating tuberculosis (Blumberg et al. 2003) that has been reported to cause Leber's hereditary optic neuropathy in patients carrying mitochondrial DNA mutations (Kim et al. 2010). Hence, the purification process of heme produced by engineered C. glutamicum should be performed prior to its application in the food industry (Ko et al. 2021).
Fermentation by engineered B. subtilis
Bacillus subtilis is an attractive alternative due to its status as a GRAS strain commonly used for efficiently synthesizing various high-value added compounds, such as pyrimidine nucleosides (Fan et al. 2018; Zhu et al. 2015) and vitamins (Liao et al. 2021; Yang et al. 2019). It is widely used in food, feed, medicine, agriculture, and industry (Su et al. 2020; Xiang et al. 2020). In B. subtilis, the native biosynthetic pathway of heme also includes the C5, urogen III, PPD, and CPD pathways, where the CPD pathway is the main pathway for synthesizing heme (Dailey et al. 2017).
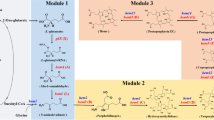
Yang et al. were the first to have used B. subtilis as the chassis and engineered its heme synthetic pathway through genome modification (Fig. 3). Strengthening the endogenous C5 pathway by sequentially knocking out the negative regulatory protein gene hemX, overexpressing the endogenous glutamyl-tRNA reductase gene hemA, and knocking out the glutamate dehydrogenase gene rocG, increased the heme production by 427%. However, introducing the heterologous C4 pathway by expressing four different heterologous AlaSs had no significant effect on the heme synthesis. It is possible that ALA synthesized by increasing the metabolic flux of the endogenous C5 pathway was sufficient for heme synthesis, and the metabolic flux of urogen III pathway was low, which limited the positive effect of the C4 pathway on heme synthesis. Strengthening the urogen III pathway was reinforced by overexpressing endogenous hemC (encoding HmbS)-hemD (encoding UroS)-hemB (encoding PbgS), while the heme yield was increased by 39%. In addition, methyltransferase gene nasF was knocked out to block the competitive consumption of urogen III, while monooxygenase hemes hmoA and hmoB were knocked out to block the intracellular degradation of heme, where heme production was increased by 11%, 26%, and 11%, respectively. The final recombinant strain BSH11 secreted 221.83 ± 4.71 mg/L of heme, which was 89% of total heme (248.26 ± 6.97 mg/L) produced during fed-batch fermentation in a 10-L fermenter (Yang et al. 2023). Notably, although the CPD pathway in strain BSH11 was not further modified, its extracellular heme production was comparable to that of C. glutamicum, which secreted the highest level of free heme (242.95 ± 11.45 mg/L) (Ko et al. 2021). In addition, B. subtilis strains constructed via genome editing (Liu et al. 2008) instead of using multi-plasmid expression systems (Ko et al. 2021; Zhao et al. 2018) avoided the addition of multiple antibiotics, simplifying the heme purification process and improving the genetic stability of engineered strains (Bu et al. 2021), demonstrating the advantages of B. subtilis in efficient heme production on a commercial scale. Although its yield was not high enough, using B. subtilis to produce heme from a food safety perspective is still worthwhile to explore.
Schematic diagram of heme biosynthetic pathway and overall engineering strategy in B. subtilis (Yang et al. 2023). rocG, encoding glutamate dehydrogenase; gltAB, encoding glutamate synthase; gltX, encoding glutamyl-tRNA synthase; hemA, encoding glutamyl-tRNA reductase; hemL, encoding glutamate-1-semialdehyde-2,1-aminomutase; gcvTP, encoding aminomethyltransferase (glycine cleavage system protein T) and glycine decarboxylase; alaS, 5-aminolevulinic acid synthase; hemB, encoding porphobilinogen synthase; hemC, encoding hydroxymethylbilane synthase; hemD, encoding urogen III synthase; hemE, encoding urogen III decarboxylase; hemY, encoding coprogen/protoporphyrinogen oxidase; hemH, encoding coproporphyrin/protoporphyrin ferrochelatase; hemQ, encoding coproheme decarboxylase; hemN and hemZ, encoding oxygen-independent coprogen III dehydrogenase; nasF, encoding uroporphyrinogen methyltransferase; hmoA and hmoB, encoding heme monooxygenase; ccmABC, encoding heme exporter from E. coli
Strategies for further improving microbial synthesis of free heme
Heme synthetic module is crucial for the heme synthesis, usually by strengthening overexpression of the PPD or CPD pathway to promote its biosynthesis. However, the efficient synthesis of heme might lead to excessive accumulation of heme in the cell, which would produce cytotoxicity, thus inhibiting the strain growth, which is counterproductive for heme synthesis. Therefore, heme secretion is a major drawback limiting its production by microbial fermentation. In addition, amplifying and optimizing the fermentation process can also improve microbial synthesis of heme.
Enhancement of heme synthetic module
In E. coli, the heme synthetic module only includes the PPD pathway, consisting of reactions catalyzed by UroD (hemE), CgdC (hemF), PgdH1 (hemG), and PpfC (hemH). Zhao et al. have investigated the impact of individual expression and co-expression of these four genes with different combination sequences on heme synthesis, and demonstrated that the expression of pET-hemE-hemF-hemG-hemH promoted heme synthesis (Zhao et al. 2018). In C. glutamicum, the heme synthetic module includes the PPD and CPD pathways, the latter consisting of reactions catalyzed by UroD (hemE), CgoX (hemY), CpfC (hemH), and ChdC (hemQ). Ko et al. overexpressed hemY, hemH, hemQ, hemH-hemQ, and hemY-hemH-hemQ, respectively, and found that both heme production and specific growth rate were decreased (Ko et al. 2021). However, engineered C. glutamicum they constructed still overexpressed four enzymes of the CPD pathway to promote heme synthesis. In B. subtilis, the CPD pathway consisting of reactions catalyzed by UroD, CgoX, CpfC, and ChdC is the main pathway for heme synthesis, which has not been modified in the engineered B. subtilis (Yang et al. 2023). They have only made engineering modifications to the ALA synthetic module and urogen III synthetic module. Therefore, if the metabolic flux of CPD pathway is further increased, it may significantly improve the heme synthesis ability of B. subtilis.
Overexpression of heme exporter
In E. coli, CcmABCDEFGH is responsible for the synthesis of cytochrome c, in which CcmABC has the function of heme transfer (Kranz et al. 2009; Sutherland et al. 2021). Zhao et al. have constructed plasmid pACYC-ccmABC to induce the expression of CcmABC and transferred it into recombinant E. coli HAEM6 to obtain strain HAEM7. Intracellular and extracellular heme titers of HAEM7 were 6.8 ± 0.2 mg/L and 1.4 ± 0.1 mg/L after 48 h of flask fermentation, respectively, which were slightly higher than those of HAEM6 (6.6 ± 0.2 mg/L and 1.3 ± 0.2 mg/L). However, its total heme titer reached 239.2 ± 7.2 mg/L, comprising 87.7 ± 1.7 mg/L of intracellular heme and 151.4 ± 5.6 mg/L (63.3%) of extracellular heme during the fed-batch fermentation (Zhao et al. 2018).
In C. glutamicum, the HrtBA protein is responsible for transporting heme from the inside to the outside. Ko et al. have constructed plasmid pMTC-hemY-hemH-hemQ-hrtBA to induce the expression of native HrtBA and transferred it into the recombinant C. glutamicum ALSdt-YHQ to obtain strain ALSdt-YHQBA. It produced 2.96 ± 0.22 mg/L of extracellular heme and 32.00 ± 0.37 mg/L of total heme, 2.78- and 1.12-fold increases compared to those of ALSdt-YHQ. The final recombinant strain ΔSAT:ALSdtE-YHQBA produced 111.87 ± 6.48 mg/L of total heme in ethambutol-treated fed-batch fermentation, including 102.08 ± 6.28 mg/L (91.25%) of extracellular heme (Ko et al. 2021).
In B. subtilis, the gene encoding heme exporter remains unknown. We integrated the ccmABC genes from E. coli under the control of constitutive promoter PlapS into the genome of B. subtilis BSH7, resulting in a 27% decrease in heme production. However, total heme titer of strain BSH11, which we constructed on the basis of BSH8, reached 248.26 ± 6.97 mg/L during fed-batch fermentation with 221.83 ± 4.71 mg/L (89%) of extracellular heme (Yang et al. 2023). Perhaps finding an endogenous heme exporter or dynamically regulating the expression of a heterologous heme exporter will be more effective in promoting the synthesis and secretion of heme in B. subtilis.
Optimization of fermentation processes
In the final step of the PPD pathway, PpfC catalyzes the binding of protoporphyrin IX with iron ions to form heme (Dailey et al. 2017). In the third step of the CPD pathway, CpfC catalyzes the binding of coproporphyrin III with iron ions to generate Fe-coproheme III (Dailey et al. 2017). Therefore, it indicates that iron is an important component of heme. Pranawidjaja et al. (2015) performed the batch culture of E. coli W3110 harboring pTrc-hemA-coaA with or without the addition of ferrous ion, and found that the addition of ferrous ion enabled the strain to produce 5.8% more heme than that of control. In addition, iron is also the cofactor of many enzymes involved in the respiration, tricarboxylic acid (TCA) cycle, and DNA biosynthesis (Andrews et al. 2003). However, excessive ferrous ions not only causes metabolic disorders, but also catalyzes the decomposition of hydrogen peroxide to generate the highly reactive hydroxyl radical, resulting in oxidative damage and ultimate cell death (Grass 2006). Choi et al. (2022) conducted fed-batch fermentation using strain E. coli HAEM7 with FeSO4∙7H2O of 0.5 g/L, 1 g/L, 2 g/L, 4 g/L, and 8 g/L in the feed solution, respectively, to achieve the total heme production of 201.8 mg/L, 362.6 mg/L, 330.9 mg/L, 353.9 mg/L, and 317.4 mg/L, respectively. Therefore, optimizing the supplementation concentration of ferrous ions is crucial for improving heme synthesis. Furthermore, in both E. coli and B. subtilis, the iron acquisition and storage is controlled by the ferric uptake regulator (Fur) for maintaining iron homeostasis (Baez et al. 2022; Steingard et al. 2023). Alternatively, the improved expression of Fur could further enhance the heme synthesis.
In addition, the production and secretion ratio of heme change when the composition of fermentation medium is optimized. For example, heme titer of engineered E. coli HAEM7 up to 1,034 mg/L, with a secretion ratio of 45.5%, by replacing carbon source glucose with glycerol, optimization of iron concentration in culture medium and feed solution, and optimization of the pH control, etc. (Choi et al. 2022). The engineered C. glutamicum ΔSAT:ALSdtE-YHQBA was used to conduct fed-batch fermentation in the modified CGXII (mCGXII) medium, heme titer was 177.79 ± 14.20 mg/L, including 129.81 ± 10.18 mg/L of secreted heme (73.01%) (Ko et al. 2021). However, its heme titer was 111.87 ± 6.48 mg/L and secretion ratio was 91.25% in mCGXII medium supplemented with 10 mg/L ethambutol, with a maximum titer of 309.18 ± 16.43 mg/L, including 242.95 ± 11.45 mg/L of secreted heme (78.58%) in mCGXII-Tr (mCGXII + 10 g/L tryptone) medium (Ko et al. 2021). When the engineered B. subtilis strain BSH11 was used to conduct fed-batch fermentation in a 2-L fermenter, the heme yield was 150.78 ± 0.59 mg/L and the secretion ratio was 92% (Yang et al. 2023). However, when sucrose was replaced with glucose of the same concentration, the heme yield was 87.77 ± 0.32 mg/L, with the secretion ratio of 88% (Yang et al. 2023). When the fed-batch fermentation was carried out in a 10-L fermenter, glucose served as the carbon source in the early and middle stages of fermentation, and was replaced with sucrose in the later stage. The maximum heme yield reached 248.26 ± 6.97 mg/L, and its secretion ratio was 89% (Yang et al. 2023). Since only the fermentation carbon source was optimized so far, optimizing fermentation parameters, such as iron supply and composition of feeding medium, will further improve heme synthesis in B. subtilis.
Conclusions and perspectives
Heme has important physiological functions and many application values. The production of free heme by microbial fermentation is more advantageous and attractive than animal blood extraction. Currently, engineered E. coli constructed using four expression plasmids result in the highest production of free heme, accompanied by endotoxin secretion. Heme production by engineered C. glutamicum constructed using two expression plasmids is the second best method, where ethambutol needs to be added during fermentation. Hence, the produced heme is not suitable for use in the food industry. Instead of using multi-plasmid expression systems, engineered B. subtilis constructed via genome editing avoids the addition of antibiotics and inducers during fermentation and enhances the genetic stability of the strain. The produced heme can thus be used in the food industry, although its yield is relatively low.
Generally, excessive heme synthesis is achieved by strengthening its biosynthetic pathway, and heme synthetic module of engineered B. subtilis has not been further modified. In the microbial synthesis of heme, improving the secretion of heme and optimizing fermentation processes for scale-up fermentation are the key to further increasing heme production. Inducing the expression of endogenous heme exporter in E. coli and C. glutamicum can promote heme secretion and synthesis. Although the constitutive expression of E. coli's heme exporter in B. subtilis can also promote heme secretion, finding endogenous heme exporter or dynamically regulating the expression of heterologous heme exporter will further improve heme secretion and synthesis. In addition, optimizing the carbon nitrogen ratio of fed medium, DO, and other fermentation parameters to achieve high-density fermentation will greatly improve heme synthesis. In summary, engineered B. subtilis is expected to become an attractive cell factory for the production of free heme on a commercial scale.
Data availability
Not applicable.
References
Abbas AM, Elhalwagy MM, Afifi K, Ibrahim K, Sweed MS (2020) Single vs. double dose iron supplementation for prevention of iron deficiency anemia in twin pregnancy: a randomized controlled clinical trial. Open J Obstet Gynecol 10:1788–1802. https://doi.org/10.4236/ojog.2020.10120161
Ahmada M, Qureshi S, Akbar MH, Siddiqui SA, Gani A, Mushtaq M, Hassan I, Dhull SB (2022) Plant-based meat alternatives: compositional analysis, current development and challenges. Appl Food Res 2:100154. https://doi.org/10.1016/j.afres.2022.100154
Andrews SC, Robinson AK, Rodríguez-Quiones F (2003) Bacterial iron homeostasis. FEMS Microbiol Rev 27:215–237. https://doi.org/10.1016/S0168-6445(03)00055-X
Baez A, Sharma AK, Bryukhanov A, Anderson ED, Rudack L, Olivares-Hernandez R, Quan D, Shiloach J (2022) Iron availability enhances the cellular energetics of aerobic Escherichia coli cultures while upregulating anaerobic respiratory chains. New Biotechnol 71:11–20. https://doi.org/10.1016/j.nbt.2022.06.004
Beas JZ, Videira MAM, Saraiva LM (2022) Regulation of bacterial haem biosynthesis. Coordin Chem Rev 452:214286. https://doi.org/10.1016/j.ccr.2021.214286
Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, Fujiwara P, Grzemska M, Hopewell PC, Iseman MD, Jasmer RM, Koppaka V, Menzies RI, O’Brien RJ, Reves RR, Reichman LB, Simone PM, Starke JR, Vernon AA (2003) American Thoracic Society/Centers for disease control and prevention/infectious diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med 167:603–662. https://doi.org/10.1164/rccm.167.4.603
Bu QT, Li YP, Xie H, Li JF, Lv ZY, Su YT, Li YQ (2021) Rational engineering strategies for achieving high-yield, high-quality and high-stability of natural product production in actinomycetes. Metab Eng 67:198–215. https://doi.org/10.1016/j.ymben.2021.06.003
Cannon JB (1993) Pharmaceutics and drug delivery aspects of heme and porphyrin therapy. J Pharm Sci 82:435–446. https://doi.org/10.1002/jps.2600820502
Choby JE, Skaar EP (2016) Heme synthesis and acquisition in bacterial pathogens. J Mol Biol 428:3408–3428. https://doi.org/10.1016/j.jmb.2016.03.018
Choi KR, Yu HE, Lee H, Lee SY (2022) Improved production of heme using metabolically engineered Escherichia coli. Biotechnol Bioeng 119:3178–3193. https://doi.org/10.1002/bit.28194
Choi SI, Park J, Kim P (2017) Heme derived from Corynebacterium glutamicum: a potential iron additive for swine and an electron carrier additive for lactic acid bacterial culture. J Microbiol Biotechnol 27:500–506. https://doi.org/10.4014/jmb.1611.11010
Corrigall AV, Siziba KB, Maneli MH, Shephard EG, Ziman M, Dailey TA, Dailey HA, Kirsch RE, Meissner PN (1998) Purification of and kinetic studies on a cloned protoporphyrinogen oxidase from the aerobic bacterium Bacillus subtilis. Arch Biochem Biophys 358:251–256. https://doi.org/10.1006/abbi.1998.0834
Dailey HA, Dailey TA, Gerdes S, Jahn D, Jahn M, Brian MRO, Warren J (2017) Prokaryotic heme biosynthesis: multiple pathways to a common essential product. Microbiol Mol Biol Rev 81:e00048–e00016. https://doi.org/10.1128/MMBR.00048-16
Epstein FH, Gabay C, Kushner I (1999) Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340:448–454. https://doi.org/10.1056/nejm199902113400607
Fan XG, Wu HY, Jia ZF, Li GL, Li Q, Chen N, Xie XX (2018) Metabolic engineering of Bacillus subtilis for the co-production of uridine and acetoin. Appl Microbiol Biotechnol 102:8753–8762. https://doi.org/10.1007/s00253-018-9316-7
Fraser RZ, Shitut M, Agrawal P, Mendes O, Klapholz S (2018) Safety evaluation of soy leghemoglobin protein preparation derived from Pichia pastoris, intended for use as a flavor catalyst in plant-based meat. Int J Toxicol 37:241–262. https://doi.org/10.1177/1091581818766318
Gallio AE, Fung SP, Cammack-Najera A, Hudson AJ, Raven EL (2021) Understanding the logistics for the distribution of heme in cells. JACS Au 1:1541–1555. https://doi.org/10.1021/jacsau.1c00288
Ge BS, Chen Y, Yu Q, Lin XJ, Li JQ, Qin S (2018) Regulation of the heme biosynthetic pathway for combinational biosynthesis of phycocyanobilin in Escherichia coli. Process Biochem 71:23–30. https://doi.org/10.1016/j.procbio.2018.05.011
Grass G (2006) Iron transport in Escherichia coli: all has not been said and done. Biometals 19:159–172. https://doi.org/10.1007/s10534-005-4341-2
Hansson M, Hederstedt L (1994) Bacillus subtilis HemY is a peripheral membrane protein essential for protoheme IX synthesis which can oxidize coproporphyrinogen III and protoporphyrinogen IX. J Bacteriol 176:5962–5970. https://doi.org/10.1128/jb.176.19.5962-5970.1994
Hansson M, Rutberg L, Schröder I, Hederstedt L (1991) The Bacillus subtilis hemAXCDBL gene cluster, which encodes enzymes of the biosynthetic pathway from glutamate to uroporphyrinogen III. J Bacteriol 173:2590–2599. https://doi.org/10.1128/jb.173.8.2590-2599.1991
Jia Y, Xu XX, Chen L, Xu ZR (2017) Solid-phase extraction of hemoglobin from human whole blood with a coordination-polymer-derived composite material based on ZnO and mesoporous carbon. Chem-Eur J 23:16026–16033. https://doi.org/10.1002/chem.201703232
Johansson P, Hederstedt L (1999) Organization of genes for tetrapyrrole biosynthesis in gram-positive bacteria. Microbiol 145:529–538. https://doi.org/10.1099/13500872-145-3-529
Kilbourn RG, Griffith OW, Gross SS (1993) Pathogenetic mechanisms of septic shock. N Engl J Med 329:1427–1428. https://doi.org/10.1056/NEJM199311043291916
Kim JK, Fahimi A, Fink W, Nazemi PP, Nguyen D, Sadun AA (2010) Characterizing ethambutol-induced optic neuropathy with a 3D computer-automated threshold Amsler grid test. Clin Exp Ophthalmol 36:484–488. https://doi.org/10.1111/j.1442-9071.2008.01807.x
Ko YJ, Joo YC, Hyeon JE, Lee E, Lee ME, Seok J, Kim SW, Park C, Han SO (2018) Biosynthesis of organic photosensitizer Zn-porphyrin by diphtheria toxin repressor (DtxR)-mediated global upregulation of engineered heme biosynthesis pathway in Corynebacterium glutamicum. Sci Rep 8:14460. https://doi.org/10.1038/s41598-018-32854-9
Ko YJ, Kim M, You SK, Sang KS, Chang J, Choi HJ, Jeong WY, Lee ME, Hwang DH, Han SO (2021) Animal-free heme production for artificial meat in Corynebacterium glutamicum via systems metabolic and membrane engineering. Metab Eng 66:217–228. https://doi.org/10.1016/j.ymben.2021.04.013
Kranz RG, Richard-Fogal C, Taylor JS, Frawley ER (2009) Cytochrome c biogenesis: mechanisms for covalent modifications and trafficking of heme and for heme-iron redox control. Microbiol Mol Biol Rev 73:510–528. https://doi.org/10.1128/mmbr.00001-09
Kwon SJ, de Boer AL, Petri R, Schmidt-Dannert C (2003) High-level production of porphyrins in metabolically engineered Escherichia coli: systematic extension of a pathway assembled from overexpressed genes involved in heme biosynthesis. Appl Environ Microb 69:4875–4883. https://doi.org/10.1128/AEM.69.8.4875-4883.2003
Layer G (2021) Heme biosynthesis in prokaryotes. BBA - Mol Cell Res 1868:118861. https://doi.org/10.1016/j.bbamcr.2020.118861
Lei M, Peng XW, Sun WJ, Zhang D, Wang ZU, Yang ZJ, Zhang C, Yu B, Niu HQ, Ying HJ, Ouyang PK, Liu D, Chen Y (2021) Nonsterile L-lysine fermentation using engineered phosphite-grown Corynebacterium glutamicum. ACS Omega 6:10160–10167. https://doi.org/10.1021/acsomega.1c00226
Liao CY, Ayansola H, Ma YB, Ito K, Guo YM, Zhang BK (2021) Advances in enhanced menaquinone-7 production from Bacillus subtilis. Front Bioeng Biotech 9:695526. https://doi.org/10.3389/fbioe.2021.695526
Liu SH, Endo K, Ara K, Ozaki K, Ogasawara N (2008) Introduction of marker-free deletions in Bacillus subtilis using the AraR repressor and the ara promoter. Microbiol 154:2562–2570. https://doi.org/10.1099/mic.0.2008/016881-0
Lu QD, Ma JM, Rong H, Fan J, Yuan Y, Li K, Gao YX, Zhang X, Teng MK, Niu LW (2010) Cloning, expression, purification, crystallization and preliminary crystallographic analysis of 5-aminolaevulinic acid dehydratase from Bacillus subtilis. Acta Crystallogr F 66:1053–1055. https://doi.org/10.1107/s1744309110027582
Nishinaga M, Sugimoto H, Nishitani Y, Nagai S, Nagatoishi S, Muraki N, Tosha T, Tsumoto K, Aono S, Shiro Y, Sawai H (2021) Heme controls the structural rearrangement of its sensor protein mediating the hemolytic bacterial survival. Commun Biol 4:467. https://doi.org/10.1038/s42003-021-01987-5
Ogata S, Hirasawa T (2021) Induction of glutamic acid production by copper in Corynebacterium glutamicum. Appl Microbiol Biot 1015:6909–6920. https://doi.org/10.1007/s00253-021-11516-3
Park S, Kim D, Jang I, Oh HB, Choe J (2014) Structural and biochemical study of Bacillus subtilis HmoB in complex with heme. Biochem Biophys Res Commun 446:286–291. https://doi.org/10.1016/j.bbrc.2014.02.092
Pranawidjaja S, Choi SI, Lay BW, Kim P (2015) Analysis of heme biosynthetic pathways in a recombinant Escherichia coli. J Microbiol Biotechnol 25:880–886. https://doi.org/10.4014/jmb.1411.11050
Sassa S (2004) Why heme needs to be degraded to iron, biliverdin IXα, and carbon monoxide? Antioxidants 6:819–824. https://doi.org/10.1089/ars.2004.6.819
Schroder I, Johansson P, Rutberg L, Hederstedt L (1994) The hemX gene of the Bacillus subtilis hemAXCDBL operon encodes a membrane protein, negatively affecting the steady-state cellular concentration of HemA (glutamyl-tRNA reductase). Microbiology 140:731–740. https://doi.org/10.1099/00221287-140-4-731
Shao YR, Xue CL, Liu WQ, Zuo SQ, Wei PL, Huang L, Lian JZ, Xu ZN (2022) High-level secretory production of leghemoglobin in Pichia pastoris through enhanced globin expression and heme biosynthesis. Bioresour Technol 363:127884. https://doi.org/10.1016/j.biortech.2022.127884
Shibahara S (1988) Regulation of heme oxygenase gene expression. Semin Hematol 25:370–376
Stamford NPJ, Capretta A, Battersby AR (1995) Expression, purification and characterisation of the product from the Bacillus Subtilis hemD gene, uroporphyrinogen III synthase. Eur J Biochem 231:236–241. https://doi.org/10.1111/j.1432-1033.1995.0236f.x
Steingard CH, Pinochet-Barros A, Wendel BM, Helmann JD (2023) Iron homeostasis in Bacillus subtilis relies on three differentially expressed efflux systems. Microbiology 169:001289. https://doi.org/10.1099/mic.0.001289
Stojanovski BM, Hunter GA, Na I, Uversky VN, Ferreira GC (2019) 5-aminolevulinate synthase catalysis: the catcher in heme biosynthesis. Mol Genet Metab 128:178–189. https://doi.org/10.1016/j.ymgme.2019.06.003
Su Y, Liu C, Fang H, Zhang DW (2020) Bacillus subtilis: a universal cell factory for industry, agriculture, biomaterials and medicine. Microb Cell Fact 19:173. https://doi.org/10.1186/s12934-020-01436-8
Sutherland MC, Mendez DL, Babbitt SE, Tillman DE, Melnikov O, Tran NL, Prizant NT, Collier AL, Kranz RG (2021) In vitro reconstitution reveals major differences between human and bacterial cytochrome c synthases. eLife 10:e64891. https://doi.org/10.7554/eLife.64891
Weinstein JD, Beale SI (1983) Separate physiological roles and subcellular compartments for two tetrapyrrole biosynthetic pathways in Euglena gracilis. J Biol Chem 258:6799–6807. https://doi.org/10.1016/S0021-9258(18)32293-2
Xiang MJ, Kang Q, Zhang DW (2020) Advances on systems metabolic engineering of Bacillus subtilis as a chassis cell. Syn Syst Biotechno 5:245–251. https://doi.org/10.1016/j.synbio.2020.07.005
Yang SM, Wang AL, Li JC, Shao YH, Sun FJ, Li SC, Cao K, Liu HL, Xiong P, Gao ZQ (2023) Improved biosynthesis of heme in Bacillus subtilis through metabolic engineering assisted fed-batch fermentation. Microb Cell Fact 22:102. https://doi.org/10.1186/s12934-023-02077-3
Yang SM, Cao YX, Sun LM, Li CF, Lin X, Cai ZG, Zhang GY, Song H (2019) Modular pathway engineering of Bacillus subtilis to promote de novo biosynthesis of menaquinone-7. ACS Synth Biol 8:70–81. https://doi.org/10.1021/acssynbio.8b00258
Yarra P, Faust D, Bennett M, Rudnick S, Bonkovsky HL (2019) Benefits of prophylactic heme therapy in severe acute intermittent porphyria. Mol Genet Metab Rep 19:100450. https://doi.org/10.1016/j.ymgmr.2019.01.002
Yu XL, Jin HY, Liu WJ, Wang Q, Qi QS (2015) Engineering Corynebacterium glutamicum to produce 5-aminolevulinic acid from glucose. Microb Cell Fact 14:183. https://doi.org/10.1186/s12934-015-0364-8
Zhao XR, Zhou JW, Du GC, Chen J (2021) Recent advances in the microbial synthesis of hemoglobin. Trends Biotechnol 39:286–297. https://doi.org/10.1016/j.tibtech.2020.08.004
Zhao XR, Choi KR, Lee SY (2018) Metabolic engineering of Escherichia coli for secretory production of free haem. Nat Catal 1:720–728. https://doi.org/10.1038/s41929-018-0126-1
Zhu H, Yang SM, Yuan ZM, Ban R (2015) Metabolic and genetic factors affecting the productivity of pyrimidine nucleoside in Bacillus subtilis. Microb Cell Fact 14:54. https://doi.org/10.1186/s12934-015-0237-1
Funding
This study was funded by the Natural Science Foundation of Shandong Province (grant no. ZR2022QC078).
Author information
Authors and Affiliations
Contributions
SY and XG conceived the idea of the review; SY, ZG, JS and JW surveyed the literature; SY drafted the manuscript; SY, XG and QM revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, S., Guo, Z., Sun, J. et al. Recent advances in microbial synthesis of free heme. Appl Microbiol Biotechnol 108, 68 (2024). https://doi.org/10.1007/s00253-023-12968-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-023-12968-5