Abstract
Extracellular RNAs are an emerging research topic in fungal-plant interactions. Fungal plant pathogens and symbionts release small RNAs that enter host cells to manipulate plant physiology and immunity. This communication via extracellular RNAs between fungi and plants is bidirectional. On the one hand, plants release RNAs encapsulated inside extracellular vesicles as a defense response as well as for intercellular and inter-organismal communication. On the other hand, recent reports suggest that also full-length mRNAs are transported within fungal EVs into plants, and these fungal mRNAs might get translated inside host cells. In this review article, we summarize the current views and fundamental concepts of extracellular RNAs released by plant-associated fungi, and we discuss new strategies to apply extracellular RNAs in crop protection against fungal pathogens.
Key points
• Extracellular RNAs are an emerging topic in plant-fungal communication.
• Fungi utilize RNAs to manipulate host plants for colonization.
• Extracellular RNAs can be engineered to protect plants against fungal pathogens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fungal-plant interactions can have beneficial, detrimental, or neutral effects on plant hosts. Pathogenic fungi pose serious threats to agronomic yield and ecosystems (Fisher et al. 2020), and innovative strategies for controlling these notorious pathogens are needed. Decades of research have been spent to unravel the function of fungal extracellular proteins, effectors, and toxins and their contribution to fungal pathogenesis and disease (Giraldo and Valent 2013; Lo Presti et al. 2015).
Small RNAs are known players in the gene regulatory mechanism often referred to as RNA interference (RNAi) that is largely conserved between fungi and plants. Key factors of RNAi, namely RNA-dependent RNA polymerase (RDR), Dicer-like (DCL), and Argonaute (AGO) proteins, are highly conserved in both plants and fungi (Bologna and Voinnet 2014; Chang et al. 2012). RDR-produced double-stranded (ds)RNAs are cleaved by type-III RNA endonucleases DCL, resulting in mature small interfering RNA (siRNA) duplexes of 21–25 nucleotides in length. DCLs also produce microRNA (miRNA) from hairpin-structured RNA precursors in an RDR-independent fashion. The guide strand of siRNA/miRNA duplexes is loaded onto AGO proteins to form the RNA-induced silencing complex (RISC). This complex can silence RNAs with sequences complementary to small RNAs at the transcriptional or post-transcriptional level. The latter occurs through cleavage of target mRNAs by the AGO endonuclease activity. Shared functions of RNAi in fungi and plants are antiviral immunity, transposon, and transgene silencing, as well as endogenous gene regulation. Among these roles, small RNAs are recognized to impart significant contributions in regulating plant immunity and are proposed to also play crucial roles in fungal pathogenesis (Huang et al. 2019; Qiao et al. 2021b; Weiberg et al. 2014). A fascinating phenomenon is that small RNAs can move between cells and tissues to induce systemic RNAi in plants (Maizel et al. 2020), while extracellular small RNAs produced by fungi mediate cross-kingdom RNAi in plants during host colonization.
In recent years, research on extracellular RNA communication between fungi and plants has emerged as a new topic in plant–microbe interaction (Wang and Dean 2020; Weiberg et al. 2015, 2014). Extracellular small RNAs are secreted by pathogenic as well as beneficial fungi that can enter cells of respective plant hosts to induce cross-kingdom RNAi. Fungal small RNAs silence genes in trans within an interacting organism of a different kingdom to promote infection. One potential mechanism of RNA transport from fungi into plants is via extracellular vesicles (EVs). EVs are nanoparticles encasing cytoplasmic molecules including proteins and RNAs in a lipid bilayer, which are secreted into the extracellular space (Colombo et al. 2014). It became evident that cell wall–containing organisms such as bacteria, fungi, and plants release diverse types of EVs. While EV-packaged RNAs have been already associated with plant immunity during fungal and bacterial infections (de la Canal and Pinedo 2018; Rutter and Innes 2018; Rybak and Robatzek 2019), we are only beginning to understand that plant-associated fungi also release EVs, but their function in host infection is not understood. EVs released by animal-associated fungi were reported to play a positive role for pathogenesis (Bielska and May 2019; Zamith-Miranda et al. 2018). Moreover, an increasing number of studies analyzing EVs released by both plant- and animal-associated fungal species led to the detection of not only small RNAs, which are presumed to induce cross-kingdom RNAi, but also full-length mRNAs as cargo, which might get translated in the recipient host cell (Kwon et al. 2021).
Gaining a better understanding of the molecular functions and the roles of fungal extracellular RNAs and EVs in plant infection has a great potential of opening new avenues to invent novel plant protection strategies. While previous reviews have focused on plant-derived extracellular RNAs and EVs in host-microbe interactions (Cai et al. 2021, 2019; Ruf et al. 2022; Stotz et al. 2022), this review highlights the recent discoveries and concepts of extracellular RNAs and EVs released by plant-associated fungi and the potential of utilizing this information to design innovative biotechnological applications for crop protection.
Fungal small RNAs and cross-kingdom RNA interference
A current model of fungal-plant RNA communication is shown in Fig. 1A. The concept of cross-kingdom RNAi has been established based on host-induced gene silencing (HIGS). Expression of antifungal dsRNAs in barley could induce gene silencing in the powdery mildew pathogen Blumeria graminis (Nowara et al. 2010). Since then, the HIGS strategy has been consolidated in diverse fungal-plant interactions, limited to not only in the pathogenic but also in the symbiotic arbuscular mycorrhiza interaction between Rhizophagus irregularis and its host plant Medicago truncatula (Hartmann et al. 2020).
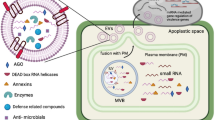
A current model of fungal-plant RNA communication and its implication on RNA spray application. A Fungal small RNAs and mRNAs are packaged into extracellular vesicles as potential means of transport into plant host cells. Fungal small RNAs bind to the plant’s own Argonaute/RNA-induced silencing complex to silence plant mRNAs. Fungal mRNAs might load into the plant’s own translational machinery to outsource fungal protein production into plant host cells. RNA communication between fungi and plants is bidirectional, as plants deliver small RNAs and potentially mRNAs into interacting fungi. B Current RNA spray applications are based on dsRNA precursors and mature small RNAs for spray-induced gene silencing against fungal pathogens. Future applications may be extended to mRNAs to produce inhibiting peptides inside fungi. C RNA formulations have been developed to increase sprayed RNA stability and target delivery
Another milestone was the discovery of the natural occurrence of cross-kingdom RNAi. Botrytis cinerea delivers small RNAs into plant cells that bind to the plant’s own AGO1 to silence host genes that are vital for plant immunity (Weiberg et al. 2013). Five B. cinerea small RNAs that induce cross-kingdom RNAi have been functionally characterized so far (Wang et al. 2017; Weiberg et al. 2013). Remarkably, cross-kingdom RNAi is a common natural phenomenon in diverse plant-biotic interactions not only restricted to fungal pathogens (Weiberg et al. 2015) but also exists in oomycetes (Dunker et al. 2020), parasitic plants (Shahid et al. 2018), and fungal as well as bacterial symbionts (Ren et al. 2019; Wong-Bajracharya et al. 2022). These cases of cross-kingdom RNAi were reported in highly diverse biotic interactors of plants comprising different lifestyles and interacting with different host plant species. Typically, these mutualistic and parasitic small RNAs manipulate host gene expression by exploiting the plant AGO proteins, seemingly being an Achilles’ heel that cannot differentiate between self and nonself small RNAs (Dunker et al. 2020; Ji et al. 2021; Ren et al. 2019; Weiberg et al. 2013).
Most of the B. cinerea small RNAs inducing cross-kingdom RNAi are derived from retrotransposons that became pathogenicity factors in this fungus (Porquier et al. 2021). Transposons are general hot spots of small RNA production in fungal pathogens (Raman et al. 2013), and their high sequence variation provides an ideal playground to target multiple plant mRNAs in diverse host species. This random gene targeting mechanism by pathogen small RNAs has been proposed as a “shotgun strategy” (Hudzik et al. 2020) that would be beneficial for multitrophic pathogens such as B. cinerea to infect diverse plant species.
The fungal vascular pathogen Fusarium oxysporum induces cross-kingdom RNAi to achieve tomato root infection. For this, F. oxysporum small RNAs bind to the tomato Sl-AGO4a, the ortholog of the Arabidopsis thaliana AGO4 (Ji et al. 2021). A. thaliana AGO4 mainly associates with 24-nt long heterochromatic siRNAs to silence transposons via RNA-directed DNA methylation (RdDM) (Matzke and Mosher 2014), a unique RNAi pathway existing in plants but not in fungi (Freitag et al. 2004). Whether fungal small RNAs associated with plant AGO4 orthologs can enter the plant nucleus to induce de novo DNA methylation in the host was not examined in the original work. Therefore, both post-transcriptional mRNA cleavage and RdDM are two plausible pathways of target silencing. The oomycete pathogen Hyaloperonospora arabidopsidis employs small RNAs that associate with the A. thaliana AGO1 to induce cross-kingdom RNAi (Dunker et al. 2020), a striking similarity to B. cinerea–induced cross-kingdom RNAi. Oomycetes belong to the phylogenetic group of Chromista, a eukaryotic kingdom that diverged from fungi over 1.5 billion years ago (Parfrey et al. 2011). Moreover, H. arabidopsidis is an obligate biotrophic pathogen that is highly adapted to its sole host plant A. thaliana. A long co-evolutionary history of the H. arabidopsidis–A. thaliana relationship is illustrated by an ongoing arm-race (Baxter et al. 2010). One would expect that co-evolution is reflected in cross-kingdom RNAi, in which target gene variation to escape silencing should be followed by pathogen small RNA sequence adaptation. Such co-evolutionary race in trans-species RNAi has been suggested in the parasitic plant genus Cuscuta (Johnson et al. 2019). The species Cuscuta campestris silences host immunity genes with a subset of 22-nt long miRNAs (Shahid et al. 2018). These miRNAs are conserved in several parasitic Cuscuta species, in which they have evolved to generate larger miRNA families comprising compensatory sequence variations according to the binding sites in the host target genes (Johnson et al. 2019). Owing to this compensatory sequence variation, Cuscuta might be able to quickly adapt miRNAs to keep functionality in trans-species RNAi by matching with host target allelic variants. The ectomycorrhizal fungus Pisolithus microcarpus delivers miRNA-like RNAs (milRNAs) into the root cortex of its host plant Eucalyptus grandis. Treatment of roots with synthetic Pisolithus milRNAs mimicked regulation of Eucalyptus target genes and strengthened formation of deep Hartig net during root colonization (Wong-Bajracharya et al. 2022). Cross-kingdom RNAi is a common strategy among the distinct plant root colonizing microbes, encompassing not only eukaryotic pathogens and symbionts but also prokaryotes. The gram-negative bacterium Bradyrhizobium japonicum delivers small RNAs into soybean (Glycine max) roots in order to establish root nodule symbiosis (Ren et al. 2019). Bacteria lack a canonical RNAi pathway and do not possess DCL type-III RNA nucleases. Nevertheless, B. japonicum delivers transfer RNA-derived small RNAs (tRFs) into the soybean AGO1b to induce cross-kingdom RNAi of nodule-repressive plant genes. Interestingly, both cases of cross-kingdom RNAi help to establish distinct forms of root symbiosis, in which microbial small RNAs seem to act as early stage interaction signals, because RNA delivery into plant cells occurs before the formation of fungal Hartig net and bacterial nodules.
It is important to note that not all fungal plant pathogens rely on extracellular small RNAs, as lack of cross-kingdom RNAi and HIGS was reported in the fungal wheat pathogen Zymoseptoria tritici (Kettles et al. 2019; Ma et al. 2019). Also, the model smut fungus Ustilago maydis lost DCL and AGO over evolution, which are key components for small RNA biogenesis and RNAi (Laurie et al. 2008); however, U. maydis might use DCL-independent small RNAs for cross-kingdom RNA communication.
Fungal extracellular vesicles as carriers of RNA
A key question in exRNA-mediated communication between fungi and plants is how RNAs are transported between interacting organisms. EVs represent one of the potential mechanisms of exRNA transport. The existence of fungal and plant EVs has been reported over the last two decades, although this has been controversially discussed due to questions of how EVs might traverse via the plasma membrane and cell wall. While the origin and the identity of fungal EVs had been discussed (Coelho and Casadevall 2019; McMillan and Kuehn 2021), suitable protocols for EV isolation and analysis of their molecular cargo are now available to address these points. Regarding the cell wall as a barrier, the fungal cell wall is considered to be a highly dynamic structure with pore sizes up to hundreds of nanometers wide that could allow passage of EVs (Brown et al. 2015; Ebrahimi et al. 2023). Liposomes, which are comparable to natural EVs, can pass through the fungal cell wall due to their viscoelastic properties (Walker et al. 2018). Moreover, cell wall remodeling enzymes have been consistently detected in fungal EV proteomes and may mediate local loosening of the cell wall to allow passage of EVs (Zhao et al. 2019).
Intimate contact sites where fungal hyphae or feeding structures are encased by the host plant plasma membrane are likely spots for EV-mediated RNA exchange. EV-like structures from both plants (An et al. 2006) and plant-colonizing fungi (Ivanov et al. 2019; Ludwig et al. 2021; Roth et al. 2019) have been observed accumulating at such contact sites. The maize smut fungus, Ustilago maydis, produces both paramural vesicles contained within the fungal cell wall (Roth et al. 2019), as well as membrane protrusions beyond the fungal cell wall, surrounded by the maize plasma membrane (Ludwig et al. 2021). These fungal membrane protrusions harbor a protein complex, which not only mediates effector delivery but also interacts with various proteins in the maize plasma membrane, including aquaporins. In A. thaliana, aquaporins are endocytosed upon salicylic acid–induced ROS stress (Boursiac et al. 2008). Borrowing from the model of bacterial effector translocation via endocytosis with plant aquaporins (Zhang et al. 2019), fungal EVs and RNAs may also target plant aquaporins for uptake. Furthermore, clathrin-mediated endocytosis is a major route of uptake for filamentous pathogen effectors targeted to the plant cytosol (Oliveira-Garcia et al. 2023; Wang et al. 2023), and co-uptake of conventionally secreted fungal effectors and EVs may be possible. Preliminary data support the notion that RNAs loaded into fungal EVs might enter into A. thaliana cells via clathrin-mediated endocytosis (He et al. 2023). While endocytosis is emerging as a probable mode of EV and RNA uptake into plant cells, EV cargo release and delivery to the host cytosol would require fusion with the limiting membrane of the endosomes. Factors required for endosomal escape of EV cargos remain to be elucidated.
As mRNAs are recognized as common, bona fide cargos of EVs (O’Brien et al. 2020), it is hypothesized that plants and microbes exchange mRNAs that may be translated into functional proteins in recipient cells. Reads from coding transcripts had long been detected in sequencing of fungal EV-associated small RNAs (Peres da Silva et al. 2015). mRNAs are more recently being analyzed in earnest as fungal EV cargos, although the biological purpose of their secretion remains unclear (Alves et al. 2019; Kwon et al. 2021; Peres da Silva et al. 2019; Zamith-Miranda et al. 2018). While current studies on fungal EV-associated mRNAs are descriptive, they provide a glimpse into potential biological functions and mechanisms of mRNA loading into EVs. Among phytopathogenic fungi, EV-associated mRNAs were first extensively cataloged in the maize smut pathogen, Ustilago maydis (Kwon et al. 2021). The presence of intact, spliced, and poly(A)-tailed mRNAs in U. maydis EVs was evident, albeit with lower integrity overall, compared to intracellular transcripts, as reported in mammalian systems (Hinger et al. 2018). Comparable to findings in human cell lines (Hinger et al. 2018; O’Grady et al. 2022), shorter mRNAs were relatively enriched in U. maydis EVs, with a median ORF length of ~ 1 kb, while longer transcripts were relatively underrepresented in EVs. Given that the EV-associated transcript profiles remained similar regardless of external RNase treatment (Kwon et al. 2021), it is likely that mRNAs are protected within the EV lumen, although EV-independent modes of RNA secretion and delivery cannot be ruled out.
While a vast majority of mRNAs transcribed in U. maydis cells could be detected in the heterogeneous EV population, a subset of transcripts was relatively enriched in EVs. For example, transcripts encoding cytosolic metabolic enzymes were particularly overrepresented in EVs; these may bring about amplifiable physiological changes to the plant host when translated in the recipient cells. Furthermore, this reflects the capacity of the smut fungus to reprogram host plant metabolism during infection (Doehlemann et al. 2008). Subcellular localization of the mRNAs may also influence their loading into EVs. mRNAs are often transported and locally translated where the protein products are required, as previously reviewed (Das et al. 2021; Muntjes et al. 2021). Based on the data from U. maydis EVs, mRNAs encoding endosomal or cytosolic proteins were more likely to be overrepresented in EVs than those that must be targeted to the ER (Kwon et al. 2021). Thus, proximity of an mRNA to limiting membranes of maturing endosomes or the cell periphery could increase their chances of being incorporated into exosomes or microvesicles, respectively.
The process of selective RNA loading and secretion via EVs is not understood in detail, but as is common for intracellular RNA transport, entails RNA-binding proteins (RBPs) and mRNAs with cognate motifs for targeting them to sites of EV biogenesis. In mammalian systems, multiple RBPs have been implicated in RNA loading into EVs (Fabbiano et al. 2020). For example, in EVs from human umbilical vein endothelial cells, enriched mRNAs harbor structural features linked to increased stability, as well as motifs for HNRNPA2B1-binding (O’Grady et al. 2022). Interestingly, retrotransposon-derived ARC proteins, convergently co-opted in human and fruit fly, form virus-like capsids, bind their own mRNA, and are secreted from neurons via EVs (Ashley et al. 2018; Pastuzyn et al. 2018). Such virus-like mechanisms of exRNA transport await discovery in plants and fungi. An extensive RBP correlation footprinting analysis based on eCLIP data of 150 human RBPs with exRNA reads has found sequences from at least 30% of all human protein-coding genes (LaPlante et al. 2023). Moreover, mRNA-derived sequences were significantly enriched with EV-associated RBPs compared to other EV-independent RBPs, supporting that EVs are the major means of mRNA secretion. The presence of various canonical and non-canonical mRNA-binding proteins in EV proteomes of mammalian cell lines further supports the role of RBPs in mRNA loading into EVs (Castello et al. 2012; Pathan et al. 2019). In A. thaliana, selective sorting of small RNAs that induce cross-kingdom RNAi into EVs is facilitated by AGO1 and the two DEAD-box RNA helicases (RH)11 and RH37, which all specifically bind to the EV small RNAs, as well as the two non-specific RBPs annexins (ANN)1 and ANN2 (He et al. 2021). It is probable that orthologs of these proteins may be responsible for selective RNA loading into fungal EVs. Given that annexins are mRNA-binding proteins in mammalian cells (Strand et al. 2021), they might also mediate mRNA cargo selection into fungal EVs.
EVs may be a mechanism for delivering proteins lacking signal peptides for conventional secretion, in the form of either protein or mRNA to be translated in planta. Median translation rate, estimated in mammalian cells, can be over 100 protein molecules per mRNA per hour (Schwanhausser et al. 2011), and a single mRNA can yield from a few hundred to hundreds of thousands of protein molecules (Edfors et al. 2016). If fungal mRNAs are translated into effector proteins in host plant cells, which in turn produce amplifiable physiological effects, it could be a highly cost-effective strategy for the pathogen. In the clinically important fungus Paracoccidioides brasiliensis, the presence of intact, translation-competent, EV-associated mRNAs was demonstrated by in vitro translation of the extracted RNA, followed by proteomic analysis (Peres da Silva et al. 2019). While this approach has led to detection of only a handful of proteins, it was a proof of concept that the EV-associated mRNAs can be translated using a heterologous system. It remains to be determined whether compatibility of factors such as codon usage preference, untranslated regions, and RBPs would allow sufficient translation efficiency to yield a physiologically relevant level of fungal protein in the host. Delivery of pathogen mRNAs and their translation in host plant cells still must be demonstrated, and a clear biological function has yet to be attributed to candidate mRNA effectors. Nonetheless, effector delivery in the form of mRNAs is a fascinating and theoretically probable hypothesis (Kwon et al. 2021).
RNA communication from plants to fungi
Extracellular RNAs and EVs are produced by both fungi and plants. Effective silencing of fungal genes by HIGS emphasizes that cross-kingdom RNAi is bidirectional in fungal-plant interactions (Wang et al. 2016). As a natural defense mechanism, cotton plants transfer miRNAs into the vascular pathogen Verticillium dahliae that cleave V. dahliae mRNA targets (Zhang et al. 2016). The cotton miRNAs were detectable in the mycelium up to 20 days post re-isolation from infected cotton tissue, indicating a potential amplification loop of exogenous plant small RNAs after intruding into the fungal cells. The relevance of fungal RNAi components, such as RDRs, DCLs, and AGOs, in plant-induced cross-kingdom RNAi still needs to be examined. A. thaliana delivers miRNAs and trans-acting (ta)siRNAs into infecting B. cinerea. These A. thaliana small RNAs are suggested to be transported via plant EVs (Cai et al. 2018), together with the plant AGO1 and two RNA helicases (He et al. 2021), suggesting that RBPs are important factors in small RNA secretion, extracellular RNA stability, and function. Furthermore, enrichment of N6-methyladenine (m6A) RNAs was found in the plant extracellular fraction (Karimi et al. 2022), which hints to RNA modification as another mechanism to direct RNA secretion and extracellular stability. It is worth to mention that m6A RNA profiles were recorded on exRNAs of non-infected plants, while EV-encapsulated exRNAs might be predominantly released upon infectious stress. The discovery of full-length mRNAs in U. maydis EVs, as well as bidirectional exchange of mRNAs between A. thaliana and the parasitic plant species C. campestris (David-Schwartz et al. 2008), suggests plausible bidirectional transfer of mRNAs between fungi and plants, too.
Applying extracellular RNAs for crop protection
To date, agronomic control of fungal pathogens strongly relies on the application of chemical pesticides. Besides their crop protective effects, some pesticides have harmful side effects on human health, pollute the environment, and force selection for pesticide-resistant pathogen variants (Pathak et al. 2022). New RNA-based pesticide strategies, aka RNA spray, has been developed over the last years (Fig. 1B) that promise to overcome these obstacles.
Since the discovery of cross-kingdom RNAi and its technological implementation into HIGS application, RNAs have been engineered to confer resistance in plants against diverse pathogenic organisms with significant success (Hou and Ma 2019; Koch and Wassenegger 2021; Nunes and Dean 2012). Nevertheless, HIGS is a transgenic approach, which still faces hurdles to gain broader societal acceptance and approval for large-scale application. As a non-GMO approach, spray-induced gene silencing (SIGS) has now been tested in several plant pathology laboratories (Koch et al. 2019). Like the HIGS approach, a dsRNA is directed against an essential gene of a pathogen or pest. A first market-ready product called Calantha™ with the active RNAi compound “ledprona” has been released by the GreenLight Biosciences company, which protects potato plants against the Colorado potato beetle (Leptinotarsa decemlineata). Accordingly, essential field trials in the USA are proceeding to pave the way for final approval. Such development of successful SIGS application keeps high hopes that RNA spray also becomes conceivable for plant protection against fungal pathogens in the near future.
In order to develop SIGS-based fungicides, at least three goals need to be conceived. First, a suitable fungal target gene needs to be identified that is effectively downregulated by the RNAi spray and stop pathogen infection. First candidate genes have been approved, such as the fungal CYP51s (essential for ergosterol biosynthesis) and DCLs (RNAi pathway) (Koch et al. 2016; Wang et al. 2016), which were before successfully targeted by HIGS to confer plant resistance. However, suppressing conserved fungal genes by SIGS may co-inhibit related fungal species comprising target sequence overlaps, too, which may have impacts on the natural fungal microbiome of plants. A strategy to exclusively target genes in pathogenic species could be a next logical step. These genes could be identified in large-scale genome comparisons utilizing the rapidly growing numbers of high-quality genome sequencing data becoming available.
Second, sprayed RNA onto plants must be sustained active against a fungal pathogen over a period of time. Application of “naked” RNA onto leaf and fruit surfaces was capable to suppress fungal infection for few days under controlled condition. In this context, it is still not clear if RNA molecules take a path through the plant tissue, vasculature, or even plant cells before being taken up by the infecting fungus. Using fluorescently labeled RNA molecules, circulation of fluorescence was observed in the plant vasculature (Koch et al. 2016). Moreover, first experiments supported the idea of long-distance RNA transport that could provide systemic protection against fungi. There are also a couple of concomitant challenges for SIGS to achieve lab-to-field transition that has been previously reviewed in detail (Rank and Koch 2021). These challenges are related to RNA formulation and application which includes aspects of RNA stability in the field, methods, and timing of RNA application and profitable costs.
Third, sprayed RNAs should be effectively delivered into target fungi. In a screening of naked RNA application, it turned out that RNA uptake efficiency varies among fungal plant-pathogenic species. While in the cases of B. cinerea, Sclerotinia sclerotiorum, Rhizoctonia solani, Aspergillus niger, and V. dahliae RNA was readily taken up, Colletotrichum gloeosporioides and Trichoderma virens exhibited poor RNA uptake efficiencies (Qiao et al. 2021a). Ultimately, RNA uptake as well as HIGS completely failed in the case of Zymoseptoria tritici (Kettles et al. 2019). These observations indicate that a potential RNA-based fungicide application needs to be always carefully evaluated. The RNA uptake mechanisms into fungal cells are not understood (Schlemmer et al. 2022), but small RNA transport from plants into fungi is mediated by EVs and EV-associated RBPs (Cai et al. 2018; He et al. 2021), which both might enhance efficiency of RNA uptake into fungal cells. Using such information of naturally occurring cross-kingdom RNAi in plant-fungal interactions seems to be valuable to indicate the suitability for an RNA fungicide application, as demonstrated for the species B. cinerea and V. dahliae that induce natural cross-kingdom RNAi and are sensitive the RNA spray and Z. tritici that does not induce cross-kingdom RNAi and does not take up RNA. With the discovery of full-length protein-coding mRNAs transported via EVs (Kwon et al. 2021), a potential application of mRNA spray for plant protection can be envisioned. Delivery of mRNAs that encode suitable inhibitors or toxins effective against fungal pathogens could expand the RNA portfolio for crop protection, which could be effective in fungi that have lost the capacity for RNAi, such as U. maydis.
Since extracellular RNA stability and delivery have been identified as the major challenges to bring SIGS into a success story against fungal pathogens (Hernandez-Soto and Chacon-Cerdas 2021; Rank and Koch 2021), nowadays, a lot of attention is paid on RNA formulations. These are mostly derived from biomedical RNA vaccine or therapeutic strategies and are currently tested in the plant context (Fig. 1C). In this regard, packaging layered clay nanoparticles, called BioClay™, can promote RNA stability for SIGS application. These RNA nanoparticles have been proven to be effective against the different developmental stages of the whitefly (Bemisia tabaci) on cotton (Jain et al. 2022) as well as against fungal B. cinerea infection in tomato and chickpea under controlled conditions (Nino-Sanchez et al. 2022). Recent discoveries on small RNA and mRNA exchange via EVs in fungal-plant interactions (Goehre and Weiberg 2023; Ruf et al. 2022) have inspired plant biotechnologists to explore liposome-based RNA applications. Indeed, artificial nanovesicles derived from cationic lipid formulations protected sprayed RNAs from rapid degradation and could prolong SIGS durability to protect plant surfaces from B. cinerea infection (Qiao et al. 2023). In addition to RNA nanocarriers, coupling RNAs to proteins to form a ribonucleoprotein complex (RNP) and RNA-lipid formulations is expected to further improve stability and delivery efficiencies of RNA molecules. RBPs such as AGOs, RNA helicases, and Annexins, which have been found to bind to extracellular RNAs (He et al. 2021), are promising candidates to form RNPs for improving SIGS application.
The SIGS approach stands for a more eco-friendly plant protection strategy that is already in transition into potential field application in first trails (Rank and Koch 2021; Schlemmer et al. 2022). RNA-based insect control currently spearheads the field. In the future, a range of SIGS-based products are expectable to control microbial pathogens of agronomic important crops, too. Extracellular RNA application is an emerging field not only in plant research but also in biomedicine. RNA therapeutics and vaccines are current and future strategies to combat infections and cure diseases. Before applying these innovative RNA solutions in agriculture, they need to meet safety regulatory requirements and, most importantly, broad societal acceptance (Fletcher et al. 2020; Taning et al. 2021). Since mRNA vaccines have now been widely accepted in biomedicine throughout the COVID-19 crisis, RNA-based plant protection strategies might benefit from this wind of change.
References
Alves LR, Peres da Silva R, Sanchez DA, Zamith-Miranda D, Rodrigues ML, Goldenberg S, Puccia R, Nosanchuk JD (2019) Extracellular vesicle-mediated RNA release in Histoplasma capsulatum. mSphere 4(2) https://doi.org/10.1128/mSphere.00176-19
An Q, Huckelhoven R, Kogel KH, van Bel AJ (2006) Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell Microbiol 8(6):1009–1019. https://doi.org/10.1111/j.1462-5822.2006.00683.x
Ashley J, Cordy B, Lucia D, Fradkin LG, Budnik V, Thomson T (2018) Retrovirus-like Gag protein Arc1 binds RNA and traffics across synaptic boutons. Cell 172(1–2):262-274.e11. https://doi.org/10.1016/j.cell.2017.12.022
Baxter L, Tripathy S, Ishaque N, Boot N, Cabral A, Kemen E, Thines M, Ah-Fong A, Anderson R, Badejoko W, Bittner-Eddy P, Boore JL, Chibucos MC, Coates M, Dehal P, Delehaunty K, Dong S, Downton P, Dumas B, Fabro G, Fronick C, Fuerstenberg SI, Fulton L, Gaulin E, Govers F, Hughes L, Humphray S, Jiang RHY, Judelson H, Kamoun S, Kyung K, Meijer H, Minx P, Morris P, Nelson J, Phuntumart V, Qutob D, Rehmany A, Rougon-Cardoso A, Ryden P, Torto-Alalibo T, Studholme D, Wang Y, Win J, Wood J, Clifton SW, Rogers J, Van den Ackerveken G, Jones JDG, McDowell JM, Beynon J, Tyler BM (2010) Signatures of adaptation to obligate biotrophy in the Hyaloperonospora arabidopsidis genome. Science 330(6010):1549–1551. https://doi.org/10.1126/science.1195203
Bielska E, May RC (2019) Extracellular vesicles of human pathogenic fungi. Curr Opin Microbiol 52:90–99. https://doi.org/10.1016/j.mib.2019.05.007
Bologna NG, Voinnet O (2014) The diversity, biogenesis, and activities of endogenous silencing small RNAs in Arabidopsis. Annu Rev Plant Biol 65:473–503. https://doi.org/10.1146/annurev-arplant-050213-035728
Boursiac Y, Prak S, Boudet J, Postaire O, Luu DT, Tournaire-Roux C, Santoni V, Maurel C (2008) The response of Arabidopsis root water transport to a challenging environment implicates reactive oxygen species- and phosphorylation-dependent internalization of aquaporins. Plant Signal Behav 3(12):1096–1098. https://doi.org/10.4161/psb.3.12.7002
Brown L, Wolf JM, Prados-Rosales R, Casadevall A (2015) Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol 13(10):620–630. https://doi.org/10.1038/nrmicro3480
Cai Q, Qiao L, Wang M, He B, Lin FM, Palmquist J, Huang SD, Jin H (2018) Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 360(6393):1126–1129. https://doi.org/10.1126/science.aar4142
Cai Q, He B, Weiberg A, Buck AH, Jin H (2019) Small RNAs and extracellular vesicles: new mechanisms of cross-species communication and innovative tools for disease control. PLoS Pathog 15(12):e1008090. https://doi.org/10.1371/journal.ppat.1008090
Cai Q, He B, Wang S, Fletcher S, Niu D, Mitter N, Birch PRJ, Jin H (2021) Message in a bubble: shuttling small RNAs and proteins between cells and interacting organisms using extracellular vesicles. Annu Rev Plant Biol 72:497–524. https://doi.org/10.1146/annurev-arplant-081720-010616
Castello A, Fischer B, Eichelbaum K, Horos R, Beckmann BM, Strein C, Davey NE, Humphreys DT, Preiss T, Steinmetz LM, Krijgsveld J, Hentze MW (2012) Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell 149(6):1393–1406. https://doi.org/10.1016/j.cell.2012.04.031
Chang SS, Zhang Z, Liu Y (2012) RNA interference pathways in fungi: mechanisms and functions. Annu Rev Microbiol 66:305–323. https://doi.org/10.1146/annurev-micro-092611-150138
Coelho C, Casadevall A (2019) Answers to naysayers regarding microbial extracellular vesicles. Biochem Soc Trans 47(4):1005–1012. https://doi.org/10.1042/BST20180252
Colombo M, Raposo G, Thery C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289. https://doi.org/10.1146/annurev-cellbio-101512-122326
Das S, Vera M, Gandin V, Singer RH, Tutucci E (2021) Intracellular mRNA transport and localized translation. Nat Rev Mol Cell Biol 22(7):483–504. https://doi.org/10.1038/s41580-021-00356-8
David-Schwartz R, Runo S, Townsley B, Machuka J, Sinha N (2008) Long-distance transport of mRNA via parenchyma cells and phloem across the host-parasite junction in Cuscuta. New Phytol 179(4):1133–1141. https://doi.org/10.1111/j.1469-8137.2008.02540.x
de la Canal L, Pinedo M (2018) Extracellular vesicles: a missing component in plant cell wall remodeling. J Exp Bot 69(20):4655–4658. https://doi.org/10.1093/jxb/ery255
Doehlemann G, Wahl R, Horst RJ, Voll LM, Usadel B, Poree F, Stitt M, Pons-Kuhnemann J, Sonnewald U, Kahmann R, Kamper J (2008) Reprogramming a maize plant: transcriptional and metabolic changes induced by the fungal biotroph Ustilago maydis. Plant J 56(2):181–195. https://doi.org/10.1111/j.1365-313X.2008.03590.x
Dunker F, Trutzenberg A, Rothenpieler JS, Kuhn S, Prols R, Schreiber T, Tissier A, Kemen A, Kemen E, Huckelhoven R, Weiberg A (2020) Oomycete small RNAs bind to the plant RNA-induced silencing complex for virulence. Elife 9:e56096. https://doi.org/10.7554/eLife.56096
Ebrahimi H, Siavoshi F, Jazayeri MH, Sarrafnejad A, Saniee P, Mobini M (2023) Physicochemical properties of intact fungal cell wall determine vesicles release and nanoparticles internalization. Heliyon 9(3):e13834. https://doi.org/10.1016/j.heliyon.2023.e13834
Edfors F, Danielsson F, Hallstrom BM, Kall L, Lundberg E, Ponten F, Forsstrom B, Uhlen M (2016) Gene-specific correlation of RNA and protein levels in human cells and tissues. Mol Syst Biol 12(10):883. https://doi.org/10.15252/msb.20167144
Fabbiano F, Corsi J, Gurrieri E, Trevisan C, Notarangelo M, D’Agostino VG (2020) RNA packaging into extracellular vesicles: an orchestra of RNA-binding proteins? J Extracell Vesicles 10(2):e12043. https://doi.org/10.1002/jev2.12043
Fisher MC, Gurr SJ, Cuomo CA, Blehert DS, Jin H, Stukenbrock EH, Stajich JE, Kahmann R, Boone C, Denning DW, Gow NAR, Klein BS, Kronstad JW, Sheppard DC, Taylor JW, Wright GD, Heitman J, Casadevall A, Cowen LE (2020) Threats posed by the fungal kingdom to humans, wildlife, and agriculture. mBio 11(3) https://doi.org/10.1128/mBio.00449-20
Fletcher SJ, Reeves PT, Hoang BT, Mitter N (2020) A perspective on RNAi-based biopesticides. Front Plant Sci 11:51. https://doi.org/10.3389/fpls.2020.00051
Freitag M, Lee DW, Kothe GO, Pratt RJ, Aramayo R, Selker EU (2004) DNA methylation is independent of RNA interference in Neurospora. Science 304(5679):1939. https://doi.org/10.1126/science.1099709
Giraldo MC, Valent B (2013) Filamentous plant pathogen effectors in action. Nat Rev Microbiol 11(11):800–814. https://doi.org/10.1038/nrmicro3119
Goehre V, Weiberg A (2023) RNA dialogues in fungal-plant relationships. The Mycota - Plant Relationships 5:31–51
Hartmann M, Voss S, Requena N (2020) Host-induced gene silencing of arbuscular mycorrhizal fungal genes via Agrobacterium rhizogenes-mediated root transformation in Medicago truncatula. Methods Mol Biol 2146:239–248. https://doi.org/10.1007/978-1-0716-0603-2_18
He B, Cai Q, Qiao L, Huang CY, Wang S, Miao W, Ha T, Wang Y, Jin H (2021) RNA-binding proteins contribute to small RNA loading in plant extracellular vesicles. Nat Plants 7(3):342–352. https://doi.org/10.1038/s41477-021-00863-8
He B, Wang H, Liu G, Chen A, Calvo A, Cai Q, Jin H (2023) Fungal small RNAs ride in extracellular vesicles to enter plant cells through clathrin-mediated endocytosis. BioRxiv. https://doi.org/10.1101/2023.06.15.545159
Hernandez-Soto A, Chacon-Cerdas R (2021) RNAi crop protection advances. Int J Mol Sci 22(22) https://doi.org/10.3390/ijms222212148
Hinger SA, Cha DJ, Franklin JL, Higginbotham JN, Dou Y, Ping J, Shu L, Prasad N, Levy S, Zhang B, Liu Q, Weaver AM, Coffey RJ, Patton JG (2018) Diverse long RNAs are differentially sorted into extracellular vesicles secreted by colorectal cancer cells. Cell Rep 25(3):715-725.e4. https://doi.org/10.1016/j.celrep.2018.09.054
Hou Y, Ma W (2019) Natural host-induced gene silencing offers new opportunities to engineer disease resistance. Trends Microbiol. https://doi.org/10.1016/j.tim.2019.08.009
Huang CY, Wang H, Hu P, Hamby R, Jin H (2019) Small RNAs - big players in plant-microbe interactions. Cell Host Microbe 26(2):173–182. https://doi.org/10.1016/j.chom.2019.07.021
Hudzik C, Hou Y, Ma W, Axtell MJ (2020) Exchange of small regulatory RNAs between plants and their pests. Plant Physiol 182(1):51–62. https://doi.org/10.1104/pp.19.00931
Ivanov S, Austin J 2nd, Berg RH, Harrison MJ (2019) Extensive membrane systems at the host-arbuscular mycorrhizal fungus interface. Nat Plants 5(2):194–203. https://doi.org/10.1038/s41477-019-0364-5
Jain RG, Fletcher SJ, Manzie N, Robinson KE, Li P, Lu E, Brosnan CA, Xu ZP, Mitter N (2022) Foliar application of clay-delivered RNA interference for whitefly control. Nat Plants 8(5):535–548. https://doi.org/10.1038/s41477-022-01152-8
Ji HM, Mao HY, Li SJ, Feng T, Zhang ZY, Cheng L, Luo SJ, Borkovich KA, Ouyang SQ (2021) Fol-milR1, a pathogenicity factor of Fusarium oxysporum, confers tomato wilt disease resistance by impairing host immune responses. New Phytol 232(2):705–718. https://doi.org/10.1111/nph.17436
Johnson NR, dePamphilis CW, Axtell MJ (2019) Compensatory sequence variation between trans-species small RNAs and their target sites. Elife 8 https://doi.org/10.7554/eLife.49750
Karimi HZ, Baldrich P, Rutter BD, Borniego L, Zajt KK, Meyers BC, Innes RW (2022) Arabidopsis apoplastic fluid contains sRNA- and circular RNA-protein complexes that are located outside extracellular vesicles. Plant Cell. https://doi.org/10.1093/plcell/koac043
Kettles GJ, Hofinger BJ, Hu P, Bayon C, Rudd JJ, Balmer D, Courbot M, Hammond-Kosack KE, Scalliet G, Kanyuka K (2019) sRNA profiling combined with gene function analysis reveals a lack of evidence for cross-kingdom RNAi in the wheat - Zymoseptoria tritici pathosystem. Front Plant Sci 10:892. https://doi.org/10.3389/fpls.2019.00892
Koch A, Wassenegger M (2021) Host-induced gene silencing - mechanisms and applications. New Phytol 231(1):54–59. https://doi.org/10.1111/nph.17364
Koch A, Biedenkopf D, Furch A, Weber L, Rossbach O, Abdellatef E, Linicus L, Johannsmeier J, Jelonek L, Goesmann A, Cardoza V, McMillan J, Mentzel T, Kogel KH (2016) An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog 12(10):e1005901. https://doi.org/10.1371/journal.ppat.1005901
Koch A, Hofle L, Werner BT, Imani J, Schmidt A, Jelonek L, Kogel KH (2019) SIGS vs HIGS: a study on the efficacy of two dsRNA delivery strategies to silence Fusarium FgCYP51 genes in infected host and non-host plants. Mol Plant Pathol. https://doi.org/10.1111/mpp.12866
Kwon S, Rupp O, Brachmann A, Blum CF, Kraege A, Goesmann A, Feldbrugge M (2021) mRNA inventory of extracellular vesicles from Ustilago maydis. J Fungi (Basel) 7(7) https://doi.org/10.3390/jof7070562
LaPlante EL, Stuerchler A, Fullem R, Chen D, Starner AC, Esquivel E, Alsop E, Jackson AR, Ghiran I, Pereira G, Rozowsky J, Chang J, Gerstein MB, Alexander RP, Roth ME, Franklin JL, Coffey RJ, Raffai RL, Mansuy IM, Stavrakis S, deMello AJ, Laurent LC, Wang YT, Tsai CF, Liu T, Jones J, van Keuren-Jensen K, van Nostrand E, Mateescu B, Milosavljevic A (2023) exRNA-eCLIP intersection analysis reveals a map of extracellular RNA binding proteins and associated RNAs acrossmajor human biofluids and carriers. Cell Genomics 3(5): https://doi.org/10.1016/j.xgen.2023.100303
Laurie JD, Linning R, Bakkeren G (2008) Hallmarks of RNA silencing are found in the smut fungus Ustilago hordei but not in its close relative Ustilago maydis. Curr Genet 53(1):49–58. https://doi.org/10.1007/s00294-007-0165-7
Lo Presti L, Lanver D, Schweizer G, Tanaka S, Liang L, Tollot M, Zuccaro A, Reissmann S, Kahmann R (2015) Fungal effectors and plant susceptibility. Annu Rev Plant Biol 66:513–545. https://doi.org/10.1146/annurev-arplant-043014-114623
Ludwig N, Reissmann S, Schipper K, Gonzalez C, Assmann D, Glatter T, Moretti M, Ma LS, Rexer KH, Snetselaar K, Kahmann R (2021) A cell surface-exposed protein complex with an essential virulence function in Ustilago maydis. Nat Microbiol 6(6):722–730. https://doi.org/10.1038/s41564-021-00896-x
Ma X, Wiedmer J, Palma-Guerrero J (2019) Small RNA bidirectional crosstalk during the interaction between wheat and Zymoseptoria tritici. Front Plant Sci 10:1669. https://doi.org/10.3389/fpls.2019.01669
Maizel A, Markmann K, Timmermans M, Wachter A (2020) To move or not to move: roles and specificity of plant RNA mobility. Curr Opin Plant Biol 57:52–60. https://doi.org/10.1016/j.pbi.2020.05.005
Matzke MA, Mosher RA (2014) RNA-directed DNA methylation: an epigenetic pathway of increasing complexity. Nat Rev Genet 15(6):394–408. https://doi.org/10.1038/nrg3683
McMillan HM, Kuehn MJ (2021) The extracellular vesicle generation paradox: a bacterial point of view. EMBO J 40(21):e108174. https://doi.org/10.15252/embj.2021108174
Muntjes K, Devan SK, Reichert AS, Feldbrugge M (2021) Linking transport and translation of mRNAs with endosomes and mitochondria. EMBO Rep 22(10):e52445. https://doi.org/10.15252/embr.202152445
Nino-Sanchez J, Sambasivam PT, Sawyer A, Hamby R, Chen A, Czislowski E, Li P, Manzie N, Gardiner DM, Ford R, Xu ZP, Mitter N, Jin H (2022) BioClay prolongs RNA interference-mediated crop protection against Botrytis cinerea. J Integr Plant Biol 64(11):2187–2198. https://doi.org/10.1111/jipb.13353
Nowara D, Gay A, Lacomme C, Shaw J, Ridout C, Douchkov D, Hensel G, Kumlehn J, Schweizer P (2010) HIGS: host-induced gene silencing in the obligate biotrophic fungal pathogen Blumeria graminis. Plant Cell 22(9):3130–3141. https://doi.org/10.1105/tpc.110.077040
Nunes CC, Dean RA (2012) Host-induced gene silencing: a tool for understanding fungal host interaction and for developing novel disease control strategies. Mol Plant Pathol 13(5):519–529. https://doi.org/10.1111/j.1364-3703.2011.00766.x
O’Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO (2020) RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol 21(10):585–606. https://doi.org/10.1038/s41580-020-0251-y
O’Grady T, Njock MS, Lion M, Bruyr J, Mariavelle E, Galvan B, Boeckx A, Struman I, Dequiedt F (2022) Sorting and packaging of RNA into extracellular vesicles shape intracellular transcript levels. BMC Biol 20(1):72. https://doi.org/10.1186/s12915-022-01277-4
Oliveira-Garcia E, Tamang TM, Park J, Dalby M, Martin-Urdiroz M, Rodriguez Herrero C, Vu AH, Park S, Talbot NJ, Valent B (2023) Clathrin-mediated endocytosis facilitates the internalization of Magnaporthe oryzae effectors into rice cells. Plant Cell. https://doi.org/10.1093/plcell/koad094
Parfrey LW, Lahr DJ, Knoll AH, Katz LA (2011) Estimating the timing of early eukaryotic diversification with multigene molecular clocks. Proc Natl Acad Sci U S A 108(33):13624–13629. https://doi.org/10.1073/pnas.1110633108
Pastuzyn ED, Day CE, Kearns RB, Kyrke-Smith M, Taibi AV, McCormick J, Yoder N, Belnap DM, Erlendsson S, Morado DR, Briggs JAG, Feschotte C, Shepherd JD (2018) The neuronal gene arc encodes a repurposed retrotransposon Gag protein that mediates intercellular RNA transfer. Cell 173(1):275. https://doi.org/10.1016/j.cell.2018.03.024
Pathak VM, Verma VK, Rawat BS, Kaur B, Babu N, Sharma A, Dewali S, Yadav M, Kumari R, Singh S, Mohapatra A, Pandey V, Rana N, Cunill JM (2022) Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: a comprehensive review. Front Microbiol 13:962619. https://doi.org/10.3389/fmicb.2022.962619
Pathan M, Fonseka P, Chitti SV, Kang T, Sanwlani R, Van Deun J, Hendrix A, Mathivanan S (2019) Vesiclepedia 2019: a compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res 47(D1):D516–D519. https://doi.org/10.1093/nar/gky1029
Peres da Silva R, Puccia R, Rodrigues ML, Oliveira DL, Joffe LS, Cesar GV, Nimrichter L, Goldenberg S, Alves LR (2015) Extracellular vesicle-mediated export of fungal RNA. Sci Rep 5:7763. https://doi.org/10.1038/srep07763
Peres da Silva R, Longo LGV, Cunha J, Sobreira TJP, Rodrigues ML, Faoro H, Goldenberg S, Alves LR, Puccia R (2019) Comparison of the RNA content of extracellular vesicles derived from Paracoccidioides brasiliensis and Paracoccidioides lutzii. Cells 8(7) https://doi.org/10.3390/cells8070765
Porquier A, Tisserant C, Salinas F, Glassl C, Wange L, Enard W, Hauser A, Hahn M, Weiberg A (2021) Retrotransposons as pathogenicity factors of the plant pathogenic fungus Botrytis cinerea. Genome Biol 22(1):225. https://doi.org/10.1186/s13059-021-02446-4
Qiao L, Lan C, Capriotti L, Ah-Fong A, Nino Sanchez J, Hamby R, Heller J, Zhao H, Louise Glass N, Judelson HS, Mezzetti B, Niu D, Jin H (2021a) Spray-induced gene silencing for disease control is dependent on the efficiency of pathogen RNA uptake. Plant Biotechnol J. https://doi.org/10.1111/pbi.13589
Qiao Y, Xia R, Zhai J, Hou Y, Feng L, Zhai Y, Ma W (2021b) Small RNAs in plant immunity and virulence of filamentous pathogens. Annu Rev Phytopathol 59:265–288. https://doi.org/10.1146/annurev-phyto-121520-023514
Qiao L, Nino-Sanchez J, Hamby R, Capriotti L, Chen A, Mezzetti B, Jin H (2023) Artificial nanovesicles for dsRNA delivery in spray-induced gene silencing for crop protection. Plant Biotechnol J 21(4):854–865. https://doi.org/10.1111/pbi.14001
Raman V, Simon SA, Romag A, Demirci F, Mathioni SM, Zhai J, Meyers BC, Donofrio NM (2013) Physiological stressors and invasive plant infections alter the small RNA transcriptome of the rice blast fungus. Magnaporthe Oryzae BMC Genomics 14:326. https://doi.org/10.1186/1471-2164-14-326
Rank AP, Koch A (2021) Lab-to-field transition of RNA spray applications - how far are we? Front Plant Sci 12:755203. https://doi.org/10.3389/fpls.2021.755203
Ren B, Wang X, Duan J, Ma J (2019) Rhizobial tRNA-derived small RNAs are signal molecules regulating plant nodulation. Science 365(6456):919–922. https://doi.org/10.1126/science.aav8907
Roth R, Hillmer S, Funaya C, Chiapello M, Schumacher K, Lo Presti L, Kahmann R, Paszkowski U (2019) Arbuscular cell invasion coincides with extracellular vesicles and membrane tubules. Nat Plants 5(2):204–211. https://doi.org/10.1038/s41477-019-0365-4
Ruf A, Oberkofler L, Robatzek S, Weiberg A (2022) Spotlight on plant RNA-containing extracellular vesicles. Curr Opin Plant Biol 69:102272. https://doi.org/10.1016/j.pbi.2022.102272
Rutter BD, Innes RW (2018) Extracellular vesicles as key mediators of plant-microbe interactions. Curr Opin Plant Biol 44:16–22. https://doi.org/10.1016/j.pbi.2018.01.008
Rybak K, Robatzek S (2019) Functions of extracellular vesicles in immunity and virulence. Plant Physiol 179(4):1236–1247. https://doi.org/10.1104/pp.18.01557
Schlemmer T, Lischka R, Wegner L, Ehlers K, Biedenkopf D, Koch A (2022) Extracellular vesicles isolated from dsRNA-sprayed barley plants exhibit no growth inhibition or gene silencing in Fusarium graminearum. Fungal Biol Biotechnol 9(1):14. https://doi.org/10.1186/s40694-022-00143-w
Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M (2011) Global quantification of mammalian gene expression control. Nature 473(7347):337–342. https://doi.org/10.1038/nature10098
Shahid S, Kim G, Johnson NR, Wafula E, Wang F, Coruh C, Bernal-Galeano V, Phifer T, dePamphilis CW, Westwood JH, Axtell MJ (2018) MicroRNAs from the parasitic plant Cuscuta campestris target host messenger RNAs. Nature 553(7686):82–85. https://doi.org/10.1038/nature25027
Stotz HU, Brotherton D, Inal J (2022) Communication is key: extracellular vesicles as mediators of infection and defence during host-microbe interactions in animals and plants. Fems Microbiol Rev 46(1) ARTN fuab044 https://doi.org/10.1093/femsre/fuab044
Strand E, Hollas H, Sakya SA, Romanyuk S, Saraste MEV, Grindheim AK, Patil SS, Vedeler A (2021) Annexin A2 binds the internal ribosomal entry site of c-myc mRNA and regulates its translation. RNA Biol 18(sup1):337–354. https://doi.org/10.1080/15476286.2021.1947648
Taning CNT, Mezzetti B, Kleter G, Smagghe G, Baraldi E (2021) Does RNAi-based technology fit within EU sustainability goals? Trends Biotechnol 39(7):644–647. https://doi.org/10.1016/j.tibtech.2020.11.008
Walker L, Sood P, Lenardon MD, Milne G, Olson J, Jensen G, Wolf J, Casadevall A, Adler-Moore J, Gow NAR (2018) The viscoelastic properties of the fungal cell wall allow traffic of AmBisome as intact liposome vesicles. MBio 9(1) https://doi.org/10.1128/mBio.02383-17
Wang MY, Dean RA (2020) Movement of small RNAs in and between plants and fungi. Mol Plant Pathol 21(4):589–601. https://doi.org/10.1111/mpp.12911
Wang M, Weiberg A, Lin FM, Thomma BP, Huang HD, Jin H (2016) Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat Plants 2:16151. https://doi.org/10.1038/nplants.2016.151
Wang M, Weiberg A, Dellota E Jr, Yamane D, Jin H (2017) Botrytis small RNA Bc-siR37 suppresses plant defense genes by cross-kingdom RNAi. RNA Biol 14(4):421–428. https://doi.org/10.1080/15476286.2017.1291112
Wang H, Wang S, Wang W, Xu L, Welsh LRJ, Gierlinski M, Whisson SC, Hemsley PA, Boevink PC, Birch PRJ (2023) Uptake of oomycete RXLR effectors into host cells by clathrin-mediated endocytosis. Plant Cell. https://doi.org/10.1093/plcell/koad069
Weiberg A, Wang M, Lin FM, Zhao H, Zhang Z, Kaloshian I, Huang HD, Jin H (2013) Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 342(6154):118–123. https://doi.org/10.1126/science.1239705
Weiberg A, Wang M, Bellinger M, Jin H (2014) Small RNAs: a new paradigm in plant-microbe interactions. Annu Rev Phytopathol 52:495–516. https://doi.org/10.1146/annurev-phyto-102313-045933
Weiberg A, Bellinger M, Jin H (2015) Conversations between kingdoms: small RNAs. Curr Opin Biotechnol 32:207–215. https://doi.org/10.1016/j.copbio.2014.12.025
Wong-Bajracharya J, Singan VR, Monti R, Plett KL, Ng V, Grigoriev IV, Martin FM, Anderson IC, Plett JM (2022) The ectomycorrhizal fungus Pisolithus microcarpus encodes a microRNA involved in cross-kingdom gene silencing during symbiosis. Proc Natl Acad Sci U S A 119(3) https://doi.org/10.1073/pnas.2103527119
Zamith-Miranda D, Nimrichter L, Rodrigues ML, Nosanchuk JD (2018) Fungal extracellular vesicles: modulating host-pathogen interactions by both the fungus and the host. Microbes Infect 20(9–10):501–504. https://doi.org/10.1016/j.micinf.2018.01.011
Zhang T, Zhao YL, Zhao JH, Wang S, Jin Y, Chen ZQ, Fang YY, Hua CL, Ding SW, Guo HS (2016) Cotton plants export microRNAs to inhibit virulence gene expression in a fungal pathogen. Nat Plants 2(10):16153. https://doi.org/10.1038/nplants.2016.153
Zhang L, Chen L, Dong H (2019) Plant aquaporins in infection by and immunity against pathogens - a critical review. Front Plant Sci 10:632. https://doi.org/10.3389/fpls.2019.00632
Zhao K, Bleackley M, Chisanga D, Gangoda L, Fonseka P, Liem M, Kalra H, Al Saffar H, Keerthikumar S, Ang CS, Adda CG, Jiang L, Yap K, Poon IK, Lock P, Bulone V, Anderson M, Mathivanan S (2019) Extracellular vesicles secreted by Saccharomyces cerevisiae are involved in cell wall remodelling. Commun Biol 2:305. https://doi.org/10.1038/s42003-019-0538-8
Funding
Open Access funding enabled and organized by Projekt DEAL. APC and AW as well as SK and MF were supported by the German Research Foundation (DFG; Grant-ID WE 5707/2–1 and FE448/15–1, respectively) in the framework of the Research Unit FOR5116. Furthermore, research of MF and VG was funded by Germany’s Excellence Strategy DFG EXC-2048/1—Project ID 39068111. VG was supported by the DFG Grant-ID GO 2064/4–1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheng, AP., Kwon, S., Adeshara, T. et al. Extracellular RNAs released by plant-associated fungi: from fundamental mechanisms to biotechnological applications. Appl Microbiol Biotechnol 107, 5935–5945 (2023). https://doi.org/10.1007/s00253-023-12718-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-023-12718-7