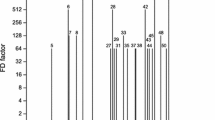
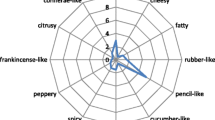
Abstract
Aldehydes represent a versatile and favored class of flavoring substances. A biocatalytic access to odor-active aldehydes was developed by conversion of fatty acids with two enzymes of the α-dioxygenase pathway. The recombinant enzymes α-dioxygenase (α-DOX) originating from Crocosphaera subtropica and fatty aldehyde dehydrogenase (FALDH) from Vibrio harveyi were heterologously expressed in E. coli, purified, and applied in a coupled (tandem) repetitive reaction. The concept was optimized in terms of number of reaction cycles and production yields. Up to five cycles and aldehyde yields of up to 26% were achieved. Afterward, the approach was applied to sea buckthorn pulp oil as raw material for the enzyme catalyzed production of flavoring/fragrance ingredients based on complex aldehyde mixtures. The most abundant fatty acids in sea buckthorn pulp oil, namely palmitic, palmitoleic, oleic, and linoleic acid, were used as substrates for further biotransformation experiments. Various aldehydes were identified, semi-quantified, and sensorially characterized by means of headspace–solid phase microextraction–gas chromatography–mass spectrometry–olfactometry (HS–SPME–GC–MS–O). Structural validation of unsaturated aldehydes in terms of double-bond positions was performed by multidimensional high-resolution mass spectrometry experiments of their Paternò–Büchi (PB) photoproducts. Retention indices and odor impressions of inter alia (Z,Z)-5,8-tetradecadienal (Z,Z)-6,9-pentadecadienal, (Z)-8-pentadecenal, (Z)-4-tridecenal, (Z)-6-pentadecenal, and (Z)-8-heptadecenal were determined for the first time.
Key points
• Coupled reaction of Csα-DOX and VhFALDH yields chain-shortened fatty aldehydes.
• Odors of several Z-unsaturated fatty aldehydes are described for the first time.
• Potential for industrial production of aldehyde-based odorants from natural sources.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Medium- and long-chain fatty aldehydes represent an important class of substances, widely applied for flavor and fragrance applications (Truong et al. 2017; Kim et al. 2022a). Saturated and unsaturated fatty aldehydes with carbon chain lengths between 11 and 18 exhibit floral, soapy, citrus-like, and waxy odors (Guadagni et al. 1963; Buttery et al. 1988). Even though fatty aldehydes are found in a wide variety of organisms, their concentrations are typically rather low and extraction from, e.g., citrus peel lacks economic efficiency. Besides, due to their similar physicochemical properties, fractionation and separation of saturated and unsaturated fatty aldehydes requires costly and process-intensive techniques. On the other hand, chemical synthesis, as a rather traditional and convenient industrial method, increasingly fades from the spotlight due to several aspects. One of the main drawbacks is the trend toward natural ingredients caused by the rising consumer awareness for sustainability, ecology, and health issues. Apart from considerable skepticism for chemically synthesized food and cosmetic ingredients, such production methods still typically require augmented amounts of chemicals, often originating from petroleum and its derivatives (Burger et al. 2019).
In order to find alternative ways for the synthesis of fatty aldehydes, several approaches in the field of biotechnology have aroused. However, they mostly showed limited yields or restricted substrate specificity (Buchhaupt et al. 2012; Kerler et al. 2005). Among them, multiple enzymatic systems were applied. Lipoxygenase (LOX), which is commonly present in plant tissue, catalyzes the conversion of fatty acids to short- to medium-chained aldehydes. Kerler et al. (2005) applied a soy-derived LOX for biotransformation of hydrolyzed triglycerides or free fatty acids to short-chain aldehydes via hydroperoxides and thermal treatment under acidic conditions. Zhu et al. (2018) applied a multifunctional LOX from the algae Pyropia haitanensis expressed in E. coli for the production of C5–C9 aldehydes. Buchhaupt et al. (2012) obtained ~ 60 mg/L of C6-aldehydes from biotransformation of fatty acids via co-expression of a recombinant LOX and hydroperoxide lyase in Saccharomyces cerevisiae. Another approach makes use of direct reduction of fatty acids to the corresponding aldehydes by means of carboxylic acid reductase (CAR) (Fraatz et al. 2018; Horvat and Winkler 2020; Hammer et al. 2021). The opposite reaction from alcohols to aldehydes catalyzed by alcohol dehydrogenases has been established as well (Berger 1995). Alcohol dehydrogenases are dependent on the cofactor NAD+ and CARs require NADPH and additionally ATP. Therefore, cofactor regeneration is essential for large-scale biotechnological applications. In contrast, α-dioxygenase solely requires molecular oxygen for the catalytic α-oxidation of fatty acids. The resulting 2-hydroperoxy fatty acid either reacts to a 2-hydroxy fatty acid (Cn) or spontaneously decarboxylates, forming a Cn-1 aldehyde (Hamberg et al. 2002; Kim et al. 2022a). Several plant-derived α-dioxygenases have been described and applied for the production of aliphatic aldehydes, e.g., from cucumber (Galliard and Matthew 1976), tobacco (Kawasaki et al. 1998; Hamberg et al. 1999), rice (Koeduka et al. 2002; Kaehne et al. 2011), Arabidopsis thaliana (Hamberg et al. 1999; Liu et al. 2006), and algae (Kajiwara et al. 1989; Akakabe et al. 1999). More recently, α-dioxygenases were identified in the cyanobacteriae Crocosphaera subtropica (Hammer et al. 2020), Calothrix parietina, and Leptolyngbya sp. (Kim et al. 2022b).
The herein described α-dioxygenase reaction is naturally linked to a further oxidation of the aldehyde to the corresponding fatty acid by an aldehyde dehydrogenase (Fig. 1) (Hamberg et al. 2005). This reaction cycle was already assumed to be present in plants by Shine and Stumpf (1974) and is known to act as a defense mechanism against environmental stress and pathogen infections (Hamberg et al. 2002). The α-dioxygenase reaction needs oxygen as a co-substrate, while aldehyde dehydrogenases are usually dependent on NAD(P)+ (Buchhaupt et al. 2013). In the context of industrial biotechnology, there is a high demand for readily available and inexpensive substrates to raise profitability. Thus, naturally abundant materials with valuable contents are of significant interest. For the purpose of aldehyde biosynthesis, organisms rich in lipids might prove beneficial for the generation of complex aldehyde mixtures applying the biotechnological methods described.
Sea buckthorn (Hippophae rhamnoides) is a deciduous shrub distributed across Eurasia (Wang et al. 2014). The lipid fraction of sea buckthorn could serve as a promising candidate for aldehyde synthesis, since the pulp and seeds of the berries are relatively rich in lipids. The seeds are reported to contain up to 15% and the pulp up to 34% lipids in dry matter (Yang and Kallio 2002). Sea buckthorn seed oil mainly consists of polyunsaturated linoleic [18:2(9Z,12Z)] and α-linolenic acid [18:3(9Z,12Z, 15Z)], while the pulp’s predominant fatty acids are palmitic [16:0], palmitoleic [16:1(9Z)], oleic [18:1(9Z)], and linoleic acid [18:2(9Z,12Z)] (Yang and Kallio 2001). Sea buckthorn stands out for its high lipid contents and interesting fatty acid profile, wide distribution in nature, extreme temperature tolerance of –43 °C to + 40 °C, and drought resistance (Koskovac et al. 2017). These features make sea buckthorn oil an interesting candidate for industrial applications.
In this study, a repetitive tandem reaction of an α-dioxygenase from Crocosphaera subtropica (Csα-DOX) (Hammer et al. 2020) in combination with a fatty aldehyde dehydrogenase from Vibrio harveyi (VhFALDH) (Buchhaupt et al. 2013) was developed to produce odorous carbon chain shortened fatty aldehydes from the corresponding fatty acids (Fig. 1). By using a combination of these two enzymes, it was possible to produce multiple different aldehydes with carbon chains shortened by one C-atom per reaction cycle in a one-pot reaction. The enzymatic tandem reaction demonstrated in the present work was optimized to obtain higher quantities of odor-active fatty aldehydes. Application of a lipid extract from sea buckthorn was chosen as an exemplary natural substrate for the reaction cycle. Upon lipid hydrolysis, the free fatty acids serve as substrates for the generation of complex odorous aldehyde mixtures that could be used as natural flavoring/fragrance ingredients. To identify the major aldehydes formed unambiguously, analytical standards of the most predominant fatty acids of sea buckthorn pulp oil were biotransformed and sensorially characterized. Molecular structures of the generated aldehydes in terms of the double-bond positions were verified, and the efficiency and substrate specificity of the process were estimated by semi-quantitation.
Materials and methods
Chemicals
Acetone (99.8%) and palmitoleic acid [16:1(9Z)] (99%) were obtained from Acros (Fair Lawn, NJ, USA). Nitrogen was purchased from Air Liquide (Düsseldorf, Germany). Decanal (96%), dodecanal (95%), and tridecanal (90%) were supplied by Alfa Aesar (Ward Hill, MA, USA). Coomassie Brilliant Blue R250 and sodium dodecyl sulfate (99%) were purchased from AppliChem (Darmstadt, Germany). Disodium hydrogen phosphate (99.5%), glycine (99%), imidazole (99.5%), lysogeny broth (LB) medium, potassium dihydrogen phosphate (98%), tris(hydroxymethyl)aminomethane (TRIS) (99%), and Triton X-100 were obtained from Carl Roth (Karlsruhe, Germany) and iso-octane from Merck (Darmstadt, Germany). Helium (5.0) was supplied by Praxair (Düsseldorf, Germany). Isopropyl β-D-1-thiogalactopyranoside (IPTG) (99%) and kanamycin sulfate (> 750 I.U./mg) were purchased from Serva (Heidelberg, Germany). 3-Acetylpyridine (98%), (Z)-7-decenal (97%), nicotinamide adenine dinucleotide (NAD+), oleic acid [18:1(9Z)] (99%), palmitic acid [16:0] (99%), and linoleic acid [18:1(9Z, 12Z)] (99%) were obtained from Sigma Aldrich (St. Louis, MO, USA). Heptadecanal (97%), hexadecanal (97%), (Z)-11-hexadecenal (95%), pentadecanal (97%), tetradecanal (95%), undecanal (97%), and decanal (97%) were purchased from TCI (Tokyo, Japan). Hydrochloric acid 25% (HCl) was obtained from Th. Geyer (Renningen, Germany). Acetonitrile (99.9%) was supplied by VWR Chemicals (Radnor, PA, USA).
Enzymes, heterologous expression, and purification
A pETDuet vector with a codon-optimized gene insert encoding for VhFALDH was produced by GENEART (Regensburg, Germany) (GenBank accession number: ON677428) (Buchhaupt et al. 2013). This gene was transferred to a pET28a-vector to add an N-terminal HIS-Tag using restriction enzymes NdeI and XhoI (Thermo Scientific) and T4-Ligase (Thermo Scientific). The construct was validated by sequencing (Eurofins, Luxemburg) using a T7-primer. E. coli W3110 (DE3) cells were transformed with this vector. Preparation of Csα-DOX was performed as described by Hammer et al. (2020) (GenBank accession number: ON711410). Both E. coli strains were cultivated in LB medium with 30 µg/mL kanamycin to an OD600 of 1.4–1.6 at 37 °C in baffled shake flasks. Induction was initiated by addition of isopropyl β-D-1-thiogalactopyranoside (IPTG) (0.5 mM). Expression was conducted at 21 °C (Csα-DOX) and 18 °C (VhFALDH) overnight, after which the cells were harvested by centrifugation.
For enzyme purification, the cells were mixed with extraction buffer (25% (w/v), cell wet weight, 50 mM phosphate buffer (pH 7.5), 20 mM imidazole), and lyzed by a sonifier (Bandelin Sonopuls, Berlin, Germany). One percent of Triton X-100 was added to the buffer to enhance extraction performance. After centrifugation, the enzymes were purified by means of a nickel loaded nitrilotriacetic acid column (Ni–NTA) (Macherey–Nagel, Düren, Germany). The His-tagged enzymes were eluted with 50 mM phosphate buffer (pH 7.5) containing 250 mM imidazole, concentrated by centrifugal filter devices (Merck, Darmstadt, Germany) with molecular mass cutoff of 30 kDa for VhFALDH and 50 kDa for Csα-DOX, and desalted using a PD-10 column (GE Healthcare, Buckinghamshire, UK).
Enzyme concentrations were determined after purification by photospectroscopy using an Implen NanoPhotometer© P300 (Munich, Germany) with 5 µL sample volume. The specific ε values used were 34,295 L/(mol ∙ cm) for VhFALDH (MW 56,649.95) and 96,433 L/(mol ∙ cm) for Csα-DOX (MW 69,894.92). The values were calculated based on the respective amino acid sequences using the ProtParam calculator of the Swiss Institute of Bioinformatics (Walker 2005).
Enzyme expression was checked by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (Laemmli 1970) with 4% stacking and 12% resolving gel and Coomassie R250 staining.
Enzyme activity
To determine Csα-DOX activity, the consumption of oxygen in the reaction mixture was measured by use of an optical oxygen probe (Microx TX3, PreSens, Regensburg, Germany). The total reaction volume in the microtiter plate was 300 µL containing 5 mM dodecanoic acid as a fatty acid standard substrate and 10 µg/mL of Csα-DOX. The probe was calibrated with saturated sodium dithionite solution and with double distilled water, saturated with compressed air for at least 5 min. The measurements were performed in microtiter plates at 25 °C and 250 rpm stirrer speed every 10 s. The values from 20–120 s after addition of the enzyme solution were used for calculation. Fivefold determinations were conducted to calculate enzyme activity.
Since the reaction of VhFALDH is NAD(P)+-dependent, enzyme activity was measured spectrophotometrically via absorbance of NAD(P)H. The measurements were performed in microtiter plates at 340 nm and 25 °C. The total volume per well was 200 µL containing 50 µM NAD+, 1 µg/mL VhFALDH, and 50 µM undecanal were used. NAD+ was applied instead of NADP+ due to a higher enzyme activity observed in the presence of the former (supplementary Fig. S1). Triplicates were measured in each case. The extinction coefficient used was determined as 3328 L/(mol ∙ cm). The measurement was immediately started after addition of NAD+, and activity was determined within the first 60 s.
Application of the coupled enzyme reaction
Method optimization with model substrate oleic acid
Biotransformation experiments were performed in 20 mL headspace vials (Th. Geyer, Renningen, Germany) containing 5 mg oleic acid, dispersed in 2 mL phosphate buffer (50 mM, pH 7.5) by means of an ultrasonic bath (Bandelin Sonorex, Berlin, Germany) for 5 min. Two hundred µL cofactor NAD+ (5 mM) and purified Csα-DOX and VhFALDH enzyme solutions were added to a final reaction volume of 4 mL. ~ 20 glass beads (Ø 3 mm) were added to increase dispersion. Incubation was performed in a rotary shaker (40 rpm, Stuart Rotator SB3, Merck, Darmstadt, Germany) at 24 °C in the dark. Incubation time (1 h, 4 h, 8 h), enzyme ratios of Csα-DOX to VhFALDH (4:1, 8:1, 12:1, and 16:1, where VhFALDH was kept constant at a concentration corresponding to an activity of 12.5 U/L), and total enzyme activity with constant relative enzyme ratios of 8:1 (100:12.5 U/L, 50:6.25 U/L, 150:18.75 U/L) were compared. After incubation, the reaction mixtures were cooled in an ice bath and the reaction was stopped by addition of 200 µL of 4 M HCl.
Instrumental analysis was performed by means of HS–SPME–GC–MS–O. SPME extraction was executed with a polydimethylsiloxane/divinylbenzene (PDMS/DVB) coated fiber (1 cm length, 65 µm) (Merck, Darmstadt, Germany). Samples were incubated for 10 min at 60 °C and extracted for 30 min at 60 °C at 250 rpm agitation rate using a GERSTEL MPS2 XL autosampler (Mülheim/Ruhr, Germany). Analytes were desorbed in an SPME liner within the inlet of an Agilent (Waldbronn, Germany) A7890 GC system, equipped with an Agilent VF-WAXms column (30 m L × 0.25 mm ID × 0.25 µm film thickness) at 250 °C for 90 s and injected with a split ratio of 10:1. Helium (5.0) was used as a carrier gas with a constant flow rate of 1.56 mL/min. The oven was programmed with an initial temperature of 40 °C (3 min) and heating with 5 °C/min to 240 °C (12 min). Detection was performed with an Agilent 7000B triple quadrupole tandem mass spectrometer with the following parameters applied: electron ionization energy, 70 eV; ion source temp., 230 °C; scan range, m/z 33–300; quadrupoles temp., 150 °C. The GC system was equipped with a GERSTEL olfactory detection port ODP 3 (transfer line temp., 250 °C; mixing chamber temp., 150 °C; makeup gas, N2).
Biotransformation of sea buckthorn oil and standard fatty acids
Sea buckthorn pulp oil (obtained from Henry Lamotte Oils GmbH, Bremen, Germany) was enzymatically hydrolyzed. Therefore, 5 mg oil was dispersed in 2 mL phosphate buffer (50 mM, pH 7.5) by means of an ultrasonic bath for 5 min. Lipase (6 U, E.C. 3.1.1.3) from Candida rugosa (Sigma Aldrich) was added and the mixture was incubated in a rotary shaker (40 rpm) at 24 °C for 3 h in the dark.
Besides the hydrolyzed lipid extract, standard fatty acids palmitic, palmitoleic, oleic, and linoleic acid were prepared as indicated above. Biotransformation experiments were executed under optimized conditions. 150 U/L Csα-DOX and 18.75 U/L VhFALDH were added to the substrate-cofactor dispersion. After incubation for 4 h, the reaction was stopped by addition of HCl as described above, and the samples were stored at –20 °C until analysis.
Compound identification and GC–olfactometry
HS–SPME–GC–MS analysis for determination of retention indices (van den Dool and Kratz 1963) and GC–MS–olfactometry were carried out as described under “Method optimization with model substrate oleic acid.” Olfactometric assessment was executed by two trained assessors.
Retention indices on a nonpolar GC column were determined after SPME extraction on an Agilent 7890B GC system equipped with an Agilent DB-5 ms column (30 m L × 0.25 mm ID × 0.25 µm film thickness). The flow rate of the carrier gas helium (5.0) was set to 1.2 mL/min (constant flow). The initial temperature was held at 40 °C for 3 min, heated to 320 °C with 5 °C/min and held for 12 min. Detection was performed with an Agilent 5977B mass spectrometer (electron ionization energy, 70 eV; ion source temp., 230 °C; quadrupole temp., 150 °C).
The reaction products were identified by comparison of mass spectra to those of the NIST MS library (NIST MS Search 2011, National Institute of Standards and Technology, Gaithersburg, MD, USA) and of retention indices calculated from nonpolar (DB-5 ms) and polar (VF-WAXms) GC columns with published retention indices or analyzed authentic standards.
For determination of double-bond positions, enzymatic reaction mixtures of palmitoleic, oleic, and linoleic acid as well as incubated blanks (either without substrates or enzymes) were extracted three times with 4 mL of n-pentane. The extracts were concentrated under a gentle stream of nitrogen to a volume of approximately 10 µL and diluted with 500 µL of acetonitrile. Five µL of the resulting solutions was mixed with 93 µL of acetonitrile and 1 µL of acetylpyridine and 1 µL of formic acid to allow for nano-electrospray ionization–online PB functionalization–tandem mass spectrometric experiments (nanoESI–online–PB–MS/MS). Nanospray capillaries (2 µm ID, in-house pulled; P-97, Sutter Instruments, Novato, CA, USA) were loaded with 10 µL of sample. A home-built nanoESI source with a voltage of 700 V between nanospray capillary and mass spectrometer inlet was applied. The emitting sample was exposed to the light of a low-pressure mercury UV lamp (254 nm emission maximum; UVP, Upland, CA, USA) as described previously (Esch and Heiles 2018). All measurements were performed with an orbital trapping mass spectrometer in positive-ion mode (Q Exactive™ HF-X, Thermo Scientific, San Jose, CA, USA), and higher energy collisional dissociation (HCD) experiments by employing 25 to 30 normalized collision energy (NCE) values.
Semi-quantitation
Saturated aldehydes with corresponding carbon chain lengths were used for semi-quantitation of not commercially available unsaturated fatty aldehydes. As a proof of concept for this approach, signal intensities of two saturated fatty aldehydes and available authentic unsaturated counterparts were compared by calculating relative response factors (supplementary Fig. S2).
Stock solutions of analytical standards of C11–C18 saturated aldehydes (~ 1 g/L dissolved in acetone) were diluted to a final concentration of 100 µg/L in 50 mM phosphate buffer (pH 7.5). Biotransformation samples were diluted 1:500 (linoleic acid: 1:100) in phosphate buffer (50 mM, pH 7.5) to a final volume of 4 mL and spiked with 200 µg/L (Z)-7-decenal as internal standard.
Instrumental analysis was performed by means of HS–SPME–GC–MS with nonpolar DB-5 ms column as stated above. Semi-quantitation of fatty aldehydes from biotransformation experiments was carried out with biological duplicates and triplicate analytical measurements.
Results
Establishment of the coupled enzyme reaction
Both enzymes were successfully expressed and purified to electrophoretic homogeneity. The enzymatic tandem reaction was successfully applied for the biotransformation of oleic acid as a model substrate. In order to increase the yield of the target aldehydes and extend the number of reaction cycles, the parameters incubation time, enzyme ratio, and concentration were optimized.
Biotransformation mixtures incubated for 1 h showed the highest signal intensity for the primary reaction product (Z)-8-heptadecenal. However, metabolites of further reaction cycles were less abundant in comparison with longer incubation times (Fig. 2a). While incubation for 1 h and 8 h resulted in 3 reaction cycles, however samples incubated for 4 h showed a 4th cycle exhibiting a remarkably high signal intensity for (Z)-5-tetradecenal and even minor amounts of (Z)-4-tridecenal resulting from a 5th reaction cycle. With prolonged incubation, the activity of the Csα-DOX decreased compared to that of VhFALDH, leading to reduced aldehyde concentrations.
A Csα-DOX to VhFALDH ratio of 8:1 (corresponding to 100:12.5 U/L) was found to be most efficient (Fig. 2b). With this ratio, 5 reaction cycles were observed, while other ratios resulted in a maximum of 4 cycles and significantly lower signal intensities of the aldehydes (Z)-6-pentadecenal (3rd cycle) and (Z)-5-tetradecenal (4th cycle). With the optimized parameters of 8:1 enzyme ratio and 4 h incubation time, total enzyme activity was varied to investigate effects on cycle number and aldehyde yields (Fig. 2c). Reduced enzyme concentrations, corresponding to 50 U/L Csα-DOX and 6.25 U/L VhFALDH, showed an insufficient efficacy regarding the reaction cycle numbers. Conversely, the concentration of the 1st cycle reaction product (Z)-7-heptadecenal was higher in comparison with higher doses applied. An increase of enzyme concentration by 50% (150:18.75 U/L) resulted in twofold abundance of 4th cycle product (Z)-5-tetradecenal in comparison with initial concentration.
Production of fatty aldehydes
Biotransformation of sea buckthorn oil
The biotransformation of hydrolyzed sea buckthorn oil resulted in the generation of numerous fatty aldehydes (Table 1, supplementary Fig. S3). In order to investigate the substrate spectrum in correlation to the aldehydes generated, the fatty acid profile of sea buckthorn oil was determined (Table S1). The most abundant fatty acids were palmitic, palmitoleic, and oleic acid. In smaller amounts, vaccenic, linoleic, linolenic, and stearic acid were detected. GC–MS–O analyses demonstrated that most of the aldehydes formed by biotransformation of hydrolyzed sea buckthorn oil were olfactorily perceived with manifold odor impressions (Table 1). The most abundant odor attributes were soapy, waxy, and green. Furthermore, citrus-like, metallic, and fatty odors were detected. Upon biotransformation, a total yield of ~ 203 mg aldehydes per gram employed oil was obtained. Pentadecanal and (Z)-8-pentadecenal were the most abundant fatty aldehydes, followed by (Z)-8-heptadecenal, tetradecanal, and (Z)-7-tetradecenal.
Biotransformation of single fatty acids
GC–MS–O analyses of biotransformed standard fatty acids revealed a wide variety of odor impressions within the formed fatty aldehydes (Table 2, 3, 4 and 5). Odors described as soapy, waxy, and green were most abundant and not clearly limited to a specific structural feature. However, a tendency of fatty odor perception with increase in chain length could be observed. Results of semi-quantitation (Fig. 3) clearly demonstrated a substrate specificity toward saturated fatty acid palmitic acid, which showed the highest total yield with roughly 430 mg aldehyde per gram substrate. The biotransformation of unsaturated fatty acids resulted in significantly lower yields, with oleic acid yielding the highest aldehyde concentrations of ~ 260 mg/g, followed by palmitoleic acid with ~ 67 mg/g. The conversion of polyunsaturated linoleic acid was even lower with a yield of ~ 30 mg/g.
Compound identification
Fatty aldehydes formed during biotransformation via the proposed enzyme tandem reaction were tentatively identified by means of MS data and retention indices, calculated from analyses on polar and nonpolar GC columns and comparison to published data. As several compounds had not been reported previously, structure elucidation was performed via PB reaction (Fig. 4). The observed MS signals indicated the successful generation of adducts of 3-acetylpyridine and a series of expected unsaturated fatty acids and aldehydes for all three samples. The reaction mixture of oleic acid showed m/z values of 404, 390, 376, 374, 362, 360, 348, 346, 334, 332, and 318. For instance, m/z 374 resulted from the two isomeric oxetanes (PB products) of heptadecanal and 3-acetylpyridine. Retro-PB reaction, initiated during HCD experiments, revealed the double-bond position between C8 and C9, as characteristic fragments of m/z 232.1696 (α-ion) and 232.2060 (ω-ion) were detected. On the other hand, PB adducts with m/z 376, 362, 348, 346, 334, 332, 320, 318, and 304 were detected in the palmitoleic acid, m/z 402, 388, 374, 372, 360, 358, 346, 344, and 330 in the linoleic acid sample. Diagnostic fragmentation signals of all PB products are reported in supplementary Tables S2–S4. While ω-ions were always detectable, signals of α-ions were found only in some cases. Apart from not transformed substrate acids, blanks contained no or more than 1000-fold lower signal intensities of the relevant m/z values.
Discussion
In the current study, a biotechnological approach yielding numerous targeted odor-active fatty aldehydes with promising efficiency was developed. Enzyme activity and resulting coupled reaction of Csα-DOX and VhFALDH were shown to be well suitable for the efficient production of targeted fatty aldehydes from various long-chain fatty acids.
The efficacy of fatty aldehyde generation was optimized by varying reaction parameters by use of oleic acid as a model substrate. One of the main challenges when dealing with coupled enzyme reactions in a one-pot bioprocess is to assure the quasi co-working of enzymes in order to maximize the yield of target compounds. Since enzyme activities of the applied enzymes are likely to differ from each other over time, the generation of aldehydes was investigated for varying incubation times. The highest signal intensities were obtained after 4 h. Shorter incubation resulted in the highest concentration of the Cn-1 aldehyde (Z)-7-heptadecenal, whereas product yields of the following reaction cycles were considerably lower. For targeted applications in industrial processes, the incubation time might serve as powerful tool for controlling the product pattern, and thus overall odor impression of the resulting aldehyde mixture. However, with prolonged incubation times of more than 4 h, a decrease of total aldehyde yield was observed. A lower enzyme stability of Csα-DOX might well explain this observation. As a result, an excess VhFALDH activity catalyzes the oxidation of aldehydes as the terminal reaction step. The optimal enzyme activity ratio of Csα-DOX and VhFALDH was thus crucial for the purpose of fatty aldehyde generation. Throughout biotransformation, Csα-DOX should exceed VhFALDH activity to avoid augmented oxidation of aldehydes to acids. An activity ratio of 8:1 (Csα-DOX:VhFALDH) was identified as most suitable for generating an excess of fatty aldehydes. The effect of total enzyme concentration in the reaction mixture on aldehyde production is an important parameter, especially when it comes to bioprocess upscaling to an industrial scale. Hence, varying total enzyme concentrations were applied for biotransformation trials. The results of laboratory scale experiments revealed that increased enzyme concentrations resulted in higher aldehyde yields, thus no needless excess of enzyme introduction was observed. Nevertheless, results indicated that varying enzyme concentrations could serve as a tool for regulation of aldehyde distribution in the resulting reaction mixture, e.g., if Cn-1 aldehyde was aimed as predominant target compound, a lower enzyme concentration would be most suitable.
The optimized enzymatic tandem reaction was successfully applied for biotransformation of a hydrolyzed lipid extract from sea buckthorn pulp and single aliphatic fatty acids yielding odor-active fatty aldehydes. The fatty acid profile of sea buckthorn oil was in accordance with published data (Yang and Kallio 2001; Zielińska and Nowak 2017). The predominant fatty acids palmitoleic, oleic, and linoleic acid are of particular interest, since their chain-shortened fatty aldehydes produced via the coupled enzyme reaction have been scarcely described as aroma ingredients and their chemical synthesis is highly complex. GC–MS–O analyses revealed most of them being odor-active, exhibiting even auspicious smells. Most of the Z-unsaturated aldehydes have not yet been described in terms of their odorant properties.
Biotransformation of the most abundant fatty acids present in sea buckthorn pulp oil indicated a clear substrate preference toward the saturated fatty acid palmitic acid in comparison with unsaturated substrates. Earlier studies on the substrate specificity of the well described α-dioxygenase from Oryza sativa (Osα-DOX) did not show a clear preference for palmitic acid compared to unsaturated oleic or palmitoleic acids (Koszelak-Rosenblum et al. 2008). Furthermore, investigations on Arabidopsis thaliana α-dioxygenase (Athα-DOX) even showed opposite results with substrate preference toward unsaturated oleic and palmitoleic acid compared to saturated stearic and palmitic acid (Liu et al. 2006; Koszelak-Rosenblum et al. 2008). Surprisingly, the biotransformation of the polyunsaturated linoleic acid resulted in lowest yields of aldehydes. This likely results from the steric hindrance caused by the two Z-configured double bonds, which might prevent a proper docking of the ligand into the active site. However, this seems to be quite specific for the herein applied Csα-DOX since earlier studies on α-dioxygenases of various other organisms showed a high acceptance toward linoleic acid (Kajiwara et al. 1989; Koeduka et al. 2002; Koszelak-Rosenblum et al. 2008; Bannenberg et al. 2009). Recently, a study on the substrate specificity of a cyanobacterial α-dioxygenase (Kim et al. 2022b) showed results that are in good agreement with our findings. Thus, low substrate acceptance for polyunsaturated fatty acids is possibly a cyanobacteria-specific trait. Interestingly, palmitoleic acid gave lower aldehyde yields in comparison with oleic acid even though the double bond of both is located at C9. The coupled enzyme reaction terminated when reaching the cycle with products exhibiting a double bond at C5. Nevertheless, for oleic acid a 5th reaction cycle, yielding a product with the unsaturated bond at C4, could be observed. The termination at these positions could be a result of steric hindrance within the binding site of the enzymes. This would be in accordance with findings that indicated a drastic decrease of Osα-DOX activity for substrates with unsaturated bonds within the first 6 carbon atoms (Koszelak-Rosenblum et al. 2008). Further investigations suggest that the first 7 carbon atoms are pivotal for substrate binding within the active site of Osα-DOX and related Athα-DOX, and therefore for an efficient catalysis (Goulah et al. 2013).
To verify the structural properties of reaction products, mass spectrometric fragmentation analyses of PB photoproducts of unsaturated compounds enabled the determination of their double-bond positions. This targeted approach renders elaborate purification procedures unnecessary and allows for an investigation of different compounds in parallel. Typically, it is employed for the characterization of different types of lipids (Esch and Heiles 2018; Wäldchen et al. 2019), but was already applied for structure elucidation in flavor research as well (Birk et al. 2019). By means of nanoESI–online–PB–MS/MS experiments, unsaturated positions of all fatty aldehydes, assigned in (Tables 3, 4, and 5), and their intermediate fatty acids were verified (see Supplementary Tables S2–S4 for assignments of all m/z signals and fragmentation). While the applied SPME–GC–MS technique was well suitable for the analysis of volatile fatty aldehydes, the corresponding fatty acids could not be analyzed simultaneously due to their high boiling points as well as inefficient desorption resulting in persistent memory effects on the SPME fibers. Thus, PB-MS/MS was also employed to unambiguously prove the presence of fatty acids.
In the literature, Z-unsaturated fatty aldehydes are predominantly discussed in the context of insect pheromones (Swedenborg and Jones 1992; Teal et al. 1995; Silva et al. 2016; Becher et al. 2018) and to a lesser extent in the field of flavors and fragrances (Shi et al. 2013; Lorber et al. 2018). Odor impressions of numerous unsaturated fatty aldehydes like (Z)-6-tridecenal, (Z)-7-tetradecenal, and (Z)-8-heptadecenal or dienals like (Z,Z)-5,8-tetradecadienal, (Z,Z)-6,9-pentadecadienal, and (Z,Z)-7,10-hexadecadienal are described here for the first time (Tables 3, 4, and 5). Only few of the produced aldehydes have already been sensorily characterized (Kajiwara et al. 1989; Wakamura et al. 1999; Chisholm et al. 2003; Choi 2005; Eyres et al. 2005). The majority of the aldehydes showed pleasant odor impressions with fresh, green, and soapy notes, which are of great interest to the flavor and fragrance industry. By subjecting naturally occurring oils and fats—such as the herein applied buckthorn-derived oil—to the developed biotechnological approach, the production of appealing aroma mixtures seems possible. Moreover, this approach includes solely NAD+ as a cofactor, which is relatively inexpensive, in comparison with cofactors such as NADH or NADPH. However, addition of stoichiometric amounts of cofactors to the reaction lacks economic efficiency. Hence, to increase the total turnover number, a suitable cofactor recycling system would be crucial for an efficient large-scale production approach. Therefore, the introduction of a NADH oxidase (E.C 1.6.99), e.g., isolated from Lactobacillus brevis as proposed by Geueke et al. (2003) or applications of advanced enzyme immobilization techniques (Twala et al. 2012) are likely suitable for the herein described approach due to similar reaction conditions. In particular, immobilized enzymes would be of special interest for industrial processes due to their higher stability, re-usability and easy handling (Sheldon 2007). However, since NADH oxidases require molecular oxygen as electron acceptor, additional gassing would have to be considered in the experimental design.
The coupled one-pot enzyme reaction process with Csα-DOX and VhFALDH was shown to be highly efficient to produce various homologous series of fatty aldehydes from the corresponding long-chain fatty acids. Gas chromatography–olfactometric analyses provided deeper insights into previously not reported odor impressions of Z-unsaturated fatty aldehydes. Aldehyde mixtures obtained from the biotransformation of buckthorn pulp or other oils could be of great interest to the food and cosmetic industry as flavorings or fragrances and represent a promising start for further screening of natural lipid extracts as substrates for bioprocess developments.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Akakabe Y, Matsui K, Kajiwara T (1999) Enantioselective α-hydroperoxylation of long-chain fatty acids with crude enzyme of marine green alga Ulva pertusa. Tetrahedron Lett 40(6):1137–1140
Bannenberg G, Martínez M, Rodríguez MJ, López MA, Ponce de León I, Hamberg M, Castresana C (2009) Functional analysis of α-DOX2, an active α-dioxygenase critical for normal development in tomato plants. Plant Physiol 151(3):1421–1432
Becher PG, Lebreton S, Wallin EA, Hedenström E, Borrero F, Bengtsson M, Jörger V, Witzgall P (2018) The scent of the fly. J Chem Ecol 44:431–435
Berger RG (1995) Aroma Biotechnology. Springer, Berlin-Heidelberg
Birk F, Fraatz MA, Esch P, Heiles S, Pelzer R, Zorn H (2019) Industrial riboflavin fermentation broths represent a diverse source of natural saturated and unsaturated lactones. J Agric Food Chem 67(49):13460–13469
Buchhaupt M, Guder J, Sporleder F, Paetzold M, Schrader J (2013) Oxidation of fatty aldehydes to fatty acids by Escherichia coli cells expressing the Vibrio harveyi fatty aldehyde dehydrogenase (FALDH). World J Microbiol Biotechnol 29(3):569–575
Buchhaupt M, Guder JC, Etschmann MMW, Schrader J (2012) Synthesis of green note aroma compounds by biotransformation of fatty acids using yeast cells coexpressing lipoxygenase and hydroperoxide lyase. Appl Microbiol Biotechnol 93(1):159–168
Burger P, Plainfosse H, Brochet X, Chemat F, Fernandez X (2019) Extraction of natural fragrance ingredients: History overview and future Trends. Chem Biodivers 16(10):e1900424
Buttery RG, Turnbaugh JG, Ling LC (1988) Contribution of volatiles to rice aroma. J Agric Food Chem 36(5):1006–1009
Chisholm MG, Jell JA, Cass DM (2003) Characterization of the major odorants found in the peel oil of Citrus reticulata Blanco cv. Clementine using gas chromatography-olfactometry. Flavour Frag J 18(4):275–281
Choi H-S (2005) Characteristic odor components of kumquat (Fortunella japonica Swingle) peel oil. J Agric Food Chem 53(5):1642–1647
Choi H-S (2006) Headspace analyses of fresh leaves and stems of Angelica gigas Nakai, a Korean medicinal herb. Flavour Frag J 21(4):604–608
Esch P, Heiles S (2018) Charging and charge switching of unsaturated lipids and apolar compounds using Paternò-Büchi Reactions. J Am Soc Mass Spectrom 29:1971–1980
Eyres G, Dufour J-P, Hallifax G, Sotheeswaran S, Marriott PJ (2005) Identification of character-impact odorants in coriander and wild coriander leaves using gas chromatography-olfactometry (GCO) and comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry (GC×GC–TOFMS). J Sep Sci 28(9–10):1061–1074
Fraatz MA, Goldmann M, Geissler T, Gross E, Backes M, Hilmer J-M, Ley J, Rost J, Francke A, Zorn H (2018) Biotechnological production of methyl-branched aldehydes. J Agric Food Chem 66(10):2387–2392
Galliard T, Matthew JA (1976) The enzymic formation of long chain aldehydes and alcohols by α-oxidation of fatty acids in extracts of cucumber fruit (Cucumis sativus). Biochim Biophys Acta 424(1):26–35
Geueke B, Riebel B, Hummel W (2003) NADH oxidase from Lactobacillus brevis: a new catalyst for the regeneration of NAD. Enzyme Microb Technol 32(2):205–211
Goulah CC, Zhu G, Koszelak-Rosenblum M, Malkowski MG (2013) The crystal structure of α-Dioxygenase provides insight into diversity in the cyclooxygenase-peroxidase superfamily. Biochem 52(8):1364–1372
Guadagni DG, Buttery RG, Okano S (1963) Odor thresholds of some organic compounds associated with food flavors. J Sci Food Agric 14(10):761–765
Hamberg M, de Leon IP, Rodriguez MJ, Castresana C (2005) α-Dioxygenases. Biochem Biophys Res Commun 338(1):169–174
Hamberg M, de León IP, Sanz A, Castresana C (2002) Fatty acid α-dioxygenases. Prostaglandins Other Lipid Mediat 68–69:363–374
Hamberg M, Sanz A, Castresana C (1999) α-oxidation of fatty acids in higher plants. Identification of a pathogen-inducible oxygenase (piox) as an α-dioxygenase and biosynthesis of 2-hydroperoxylinolenic acid. J Biol Chem 274(35):24503–24513
Hammer AK, Albrecht F, Hahne F, Jordan P, Fraatz MA, Ley J, Geissler T, Schrader J, Zorn H, Buchhaupt M (2020) Biotechnological production of odor-active methyl-branched aldehydes by a novel α-dioxygenase from Crocosphaera subtropica. J Agric Food Chem 68(38):10432–10440
Hammer AK, Emrich NO, Ott J, Birk F, Fraatz MA, Ley JP, Geissler T, Bornscheuer UT, Zorn H (2021) Biotechnological production and sensory evaluation of ω1-unsaturated aldehydes. J Agric Food Chem 69(1):345–353
Horvat M, Winkler M (2020) In vivo reduction of medium-to long-chain fatty acids by carboxylic acid reductase (CAR) enzymes: limitations and solutions. ChemCatChem 12(20):5076–5090
Kaehne F, Buchhaupt M, Schrader J (2011) A recombinant α-dioxygenase from rice to produce fatty aldehydes using E. coli. Appl Microbiol Biotechnol 90(3):989–995
Kajiwara T, Yoshikawa H, Matsui K, Hatanaka A, Kawai T (1989) Specificity of the enzyme system producing long chain aldehydes in the green algaUlvaPertusa. Phytochem 28(2):407–409
Kawasaki W, Matsui K, Akakabe Y, Itai N, Kajiwara T (1998) Long-chain aldehyde-forming activity in tobacco leaves. Phytochem 49(6):1565–1568
Kerler J, Kohlen E, Van Der VA, Fitz W, Winkel C (2005) Method for the enzymatical preparation of flavors rich in C6–C10 aldehydes. US Patent 6864072
Kim IJ, Bayer T, Terholsen H, Bornscheuer UT (2022a) α-Dioxygenases (α-DOXs): Promising biocatalysts for the environmentally friendly production of aroma compounds. Chembiochem:e202100693
Kim IJ, Brack Y, Bayer T, Bornscheuer UT (2022) Two novel cyanobacterial α-dioxygenases for the biosynthesis of fatty aldehydes. Appl Microbiol Biotechnol 106(1):197–210
Koeduka T, Matsui K, Akakabe Y, Kajiwara T (2002) Catalytic properties of rice α-oxygenase A comparison with mammalian prostaglandin H synthases. J Biol Chem 277(25):22648–22655
Koskovac M, Cupara S, Kipic M, Barjaktarevic A, Milovanovic O, Kojicic K, Markovic M (2017) Sea buckthorn oil. A valuable source for cosmeceuticals. Cosmetics 4(4):40
Koszelak-Rosenblum M, Krol AC, Simmons DM, Goulah CC, Wroblewski L, Malkowski MG (2008) His-311 and Arg-559 are key residues involved in fatty acid oxygenation in pathogen-inducible oxygenase. J Biol Chem 283(36):24962–24971
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Liu W, Wang L-H, Fabian P, Hayashi Y, McGinley CM, van der Donk WA, Kulmacz RJ (2006) Arabidopsis thaliana fatty acid α-dioxygenase-1: evaluation of substrates, inhibitors and amino-terminal function. Plant Physiol Biochem 44(5–6):284–293
Lorber K, Zeh G, Regler J, Büttner A (2018) Structure-odor relationships of (Z)-3-Alken-1-ols, (Z)-3-Alkenals, and (Z)-3-Alkenoic Acids. J Agric Food Chem 66(10):2334–2343
Mahattanatawee K, Rouseff R, Valim MF, Naim M (2005) Identification and aroma impact of norisoprenoids in orange juice. J Agric Food Chem 53(2):393–397
Marques FDA, McElfresh JS, Millar JG (2000) Kováts retention indexes of monounsaturated C12, C14, and C16 alcohols, acetates and aldehydes commonly found in lepidopteran pheromone blends. J Braz Chem Soc 11(6):592–599
Miyazawa M, Horiuchi E, Kawata J (2007) Components of the essential oil from Matteuccia struthiopteris. J Oleo Sci 56(9):457–461
Sheldon RA (2007) Enzyme immobilization: the quest for optimum performance. Adv Synth Cat 349(8–9):1289–1307
Shi X, Zhang X, Song S, Tan C, Jia C, Xia S (2013) Identification of characteristic flavour precursors from enzymatic hydrolysis-mild thermal oxidation tallow by descriptive sensory analysis and gas chromatography–olfactometry and partial least squares regression. J Chromatogr B 913–914:69–76
Shine WE, Stumpf PK (1974) Fat metabolism in higher plants: recent studies on plant α-oxidation systems. Arch Biochem Biophys 162(1):147–157
Silva WD, Millar JG, Hanks LM, Bento JMS (2016) (6E,8Z)-6,8-Pentadecadienal, a novel attractant pheromone produced by males of the cerambycid beetles Chlorida festiva and Chlorida costata. J Chem Ecol 42(10):1082–1085
Sukhonthara S, Theerakulkait C, Miyazawa M (2009) Characterization of volatile aroma compounds from red and black rice bran. J Oleo Sci 58(3):155–161
Swedenborg PD, Jones RL (1992) Z)-4-Tridecenal, a pheromonally active air oxidation product from a series of (Z,Z)-9, 13 dienes in Macrocentrus grandii Goidanich(Hymenoptera: Braconidae. J Chem Ecol 18(11):1913–1931
Teal PEA, Heath RR, Dueben BD, Coffelt JA, Vick KW (1995) Production and release of (Z,E)-9, 12-tetradecadienal by sex pheromone glands of females of Plodia interpunctella (Lepidoptera:Pyralidae). J Chem Ecol 21(6):787–799
Truong K-N, Weger LB, Stahl W, Mouhib H (2017) Favored conformations of carbonyl compounds: A structural study of n-octanal. ChemPhysChem 18(19):2631–2636
Twala BV, Sewell BT, Jordaan J (2012) Immobilisation and characterisation of biocatalytic co-factor recycling enzymes, glucose dehydrogenase and NADH oxidase, on aldehyde functional ReSyn™ polymer microspheres. Enzyme Microb Technol 50(6–7):331–336
van den Dool H, Kratz PD (1963) A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J Chromatogr A 11:463–471
Wakamura S, Hattori M, Igita K, Yasuda K (1999) Sex pheromone of Etiella behrii, a pod borer of soybean in Indonesia: identification and field attraction. Entomol Exp Appl 91(3):413–420
Wäldchen F, Spengler B, Heiles S (2019) Reactive matrix-assisted laser desorption/ionization mass spectrometry imaging using an intrinsically photoreactive Paternò-Büchi matrix for double-bond localization in isomeric phospholipids. J Am Chem Soc 141(30):11816–11820
Wang R, Zong S-X, Yu L-F, Lu P-F, Luo Y-Q (2014) Rhythms of volatile release from female and male sea buckthorn plants and electrophysiological response of sea buckthorn carpenter moths. J Plant Interact 9(1):763–774
Xu M, Jin Z, Gu Z, Rao J, Chen B (2020) Changes in odor characteristics of pulse protein isolates from germinated chickpea, lentil, and yellow pea: Role of lipoxygenase and free radicals. Food Chem 314(1):126184
Yang B, Kallio H (2002) Composition and physiological effects of sea buckthorn (Hippophaë) lipids. Trends Food Sci Technol 13(5):160–167
Yang B, Kallio HP (2001) Fatty acid composition of lipids in sea buckthorn (Hippophaë rhamnoides L.) berries of different origins. J Agric Food Chem 49(4):1939–1947
Zhu ZJ, Chen HM, Chen JJ, Yang R, Yan XJ (2018) One-step bioconversion of fatty acids into C8–C9 volatile aroma compounds by a multifunctional lipoxygenase cloned from Pyropia haitanensis. J Agric Food Chem 66(5):1233–1241
Zielińska A, Nowak I (2017) Abundance of active ingredients in sea-buckthorn oil. Lipids Health Dis 16(1):95
Acknowledgements
The authors thank Sina Bruns for scientific input.
Funding
Open Access funding enabled and organized by Projekt DEAL. Parts of the study have been funded by the German Ministry of Environment, Nature Conservation and Nuclear Safety via the Fachagentur Nachwachsende Rohstoffe (FNR, Agency of Renewable Resources, funding code 22001617) and by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, funding code 463380894). Financial support for MAF and HZ by LOEWE-Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz-AromaPlus (State Offensive for the Development of Scientific and Economic Excellence) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
JPK, PJH, and DL conducted experiments, JPK and DL analyzed data. The manuscript was written by JPK, PJH, and AKH. PJH, JPK, MAF, HZ, and AKH conceived and designed research. JPL, SH, BS, and CH gave intellectual input. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with animals performed by any of the authors.
Conflicts of interest
Authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kanter, JP., Honold, P.J., Lüke, D. et al. An enzymatic tandem reaction to produce odor-active fatty aldehydes. Appl Microbiol Biotechnol 106, 6095–6107 (2022). https://doi.org/10.1007/s00253-022-12134-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-12134-3