Abstract
The term vitamin B6 is a designation for the vitamers pyridoxal, pyridoxamine, pyridoxine and the respective phosphate esters pyridoxal-5′-phosphate (PLP), pyridoxamine-5′-phosphate and pyridoxine-5′-phosphate. Animals and humans are unable to synthesise vitamin B6. These organisms have to take up vitamin B6 with their diet. Therefore, vitamin B6 is of commercial interest as a food additive and for applications in the pharmaceutical industry. As yet, two naturally occurring routes for de novo synthesis of PLP are known. Both routes have been genetically engineered to obtain bacteria overproducing vitamin B6. Still, major genetic engineering efforts using the existing pathways are required for developing fermentation processes that could outcompete the chemical synthesis of vitamin B6. Recent suppressor screens using mutants of the Gram-negative and Gram-positive model bacteria Escherichia coli and Bacillus subtilis, respectively, carrying mutations in the native pathways or heterologous genes uncovered novel routes for PLP biosynthesis. These pathways consist of promiscuous enzymes and enzymes that are already involved in vitamin B6 biosynthesis. Thus, E. coli and B. subtilis contain multiple promiscuous enzymes causing a so-called underground metabolism allowing the bacteria to bypass disrupted vitamin B6 biosynthetic pathways. The suppressor screens also show the genomic plasticity of the bacteria to suppress a genetic lesion. We discuss the potential of the serendipitous pathways to serve as a starting point for the development of bacteria overproducing vitamin B6.
Key points
• Known vitamin B6 routes have been genetically engineered.
• Underground metabolism facilitates the emergence of novel vitamin B6 biosynthetic pathways.
• These pathways may be suitable to engineer bacteria overproducing vitamin B6.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term vitamin B6 collectively designates the vitamers pyridoxal (PL), pyridoxamine (PM), pyridoxine (PN) and the respective phosphate esters pyridoxal-5′-phosphate (PLP), pyridoxamine-5′-phosphate (PMP) and pyridoxine-5′-phosphate (PNP) (Rosenberg 2012) (Fig. 1a). PLP is the most important B6 vitamer that is required by a variety of enzymes for catalysis (Parra et al. 2018; Hoffarth et al. 2020). Bioinformatic analyses revealed that more than 4% of the known enzymes, amongst them the majority involved in amino acid metabolism, depend on PLP (Percudani and Peracchi 2003, 2009). The importance of vitamin B6 for the physiology of the cell is highlighted by the fact that the Gram-positive model bacterium Bacillus subtilis is likely to have 65 PLP-dependent proteins (Richts et al. 2019). In fact, for 61 of the proteins, it has been shown that they require PLP for functioning. Moreover, in prokaryotes and eukaryotes, the activity of DNA-binding transcription factors, amongst them regulators controlling the expression of genes involved in de novo synthesis of PLP (Oka et al. 2001; Belitsky 2004a; Huq et al. 2007; Jochmann et al. 2011; El Qaidi et al. 2013; Belitsky 2014; Tramonti et al. 2015; Suvorova and Rodionov 2016; Frezzini et al. 2020). A well-studied representative of a PLP-dependent DNA-binding transcription regulator is GabR, which is responsible for regulating the expression of genes involved in the metabolism of γ-amino butyric acid (Belitsky and Sonenshein 2002; Frezzini et al. 2020). A recent proteomic approach uncovered that uncharacterized PLP-dependent enzymes can be identified even in well-studied bacteria like the Gram-positive human pathogen Staphylococcus aureus (Hoegl et al. 2018). Novel PLP-dependent enzymes will certainly be discovered because the number of available genome sequences is rapidly increasing.
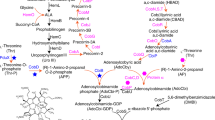
a The B6 vitamers pyridoxal (PL), pyridoxine (PN), pyridoxamine (PM) and the respective phosphate esters pyridoxal-5′-phosphate (PLP), pyridoxine-5′-phosphate (PNP) and pyridoxamine-5′-phosphate (PMP). b The deoxyxylulose 5-phosphate (DXP)-dependent and DXP-independent vitamin B6 biosynthetic routes and the salvage pathway for the interconversion of the B6 vitamers. Epd, erythrose 4-phosphate dehydrogenase; PdxB, 4-phosphoerythronate dehydrogenase; SerC, 3-phosphoserine aminotransferase; PdxA, 4-phosphohydroxy-l-threonine dehydrogenase; PdxJ, PNP synthase; Dxs, 1-deoxyxylulose 5-phosphate synthase; PdxH, PNP oxidase; PdxS (PLP synthase subunit) and PdxT (glutaminase subunit) form the PdxST PLP synthase complex; PdxK, PL kinase present in B. subtilis and E. coli; PdxY, PL kinase present in E. coli. PdxK from B. subtilis has PN, PL and PM kinase activity; PdxP, PNP and PLP phosphatase from S. meliloti; YbhA, PLP phosphatase from E. coli; PdxI, PL reductase from E. coli
Since animals and humans are unable to synthesise vitamin B6, these organisms have to take up vitamin B6 with their diet (Kraemer et al. 2012). Therefore, vitamin B6 is of commercial interest as a food additive and for applications in the pharmaceutical industry (Acevedo-Rocha et al. 2019). PN hypochloride, the commercial form of vitamin B6, is synthesised from PN and added usually in combination with other vitamins to food products like bakery products, cereals, baby nutrition, multivitamin juices and other beverages (Domke et al. 2005; Eggersdorfer et al. 2012). Moreover, PN is added to the food that is used for intensive animal farming to improve the yield (Johnson et al. 1950; Verbeek 1975; Eggersdorfer et al. 2012). Until today, the B6 vitamers are fully chemically synthesised using expensive and toxic chemicals (Eggersdorfer et al. 2012). Therefore, there is considerable interest in the biotech industry to develop a sustainable fermentation process for the production of vitamin B6.
This review intends to describe novel and hybrid vitamin B6 biosynthetic routes that have been identified via genetic suppressor screens using mutants of the Gram-negative and Gram-positive model bacteria Escherichia coli and B. subtilis, respectively, carrying mutations in the native genes required for synthesising the essential B6 vitamer PLP. We also discuss the potential of these pathways to serve as a starting point for the development of bacteria overproducing vitamin B6.
De novo synthesis of the B6 vitamer PLP
As described above, PLP is the most-important B6 vitamer that is required by a variety of proteins for functioning. As yet, two naturally occurring routes for de novo synthesis of PLP are known (Mittenhuber 2001; Fitzpatrick et al. 2007; Mukherjee et al. 2011; Rosenberg et al. 2017) (Fig. 1b). The long vitamin B6 biosynthetic pathway, which involves seven enzymes and depends on the phosphor sugar deoxy-xylulose-5′-phosphate (DXP), is present in α- and γ-proteobacteria and has been extensively studied in E. coli (Fitzpatrick et al. 2007; Rosenberg et al. 2017). In the first part of the DXP-dependent vitamin B6 pathway, the E4P dehydrogenase Epd, the 4-phospho-erythronate (4PE) dehydrogenase PdxB and the 3-phosphoserine (3PS) aminotransferase SerC convert E4P and glutamate to 4-hydroxy-threonine-phosphate (4HTP) (Zhao et al. 1995; Drewke et al. 1996; Tazoe et al. 2006; Rudolph et al. 2010). SerC is also present in organisms that synthesise PLP via the DXP-independent vitamin B6 pathway because the enzyme is essential for serine biosynthesis (Sakai et al. 2002a, 2002b; Lam and Winkler 1990). In the second part of the DXP-dependent pathway, the 4HTP dehydrogenase PdxA oxidises 4PHT to 2-amino-3-oxo-4-(phosphohydroxy)-butyric acid, which is spontaneously decarboxylated to 1-amino-propan-2-one-phosphate (APP) (Cane et al. 1998). The PNP synthase PdxJ converts APP and DXP to PNP (Cane et al. 1999). The final reaction, yielding the B6 vitamer PLP, is catalysed by the PNP oxidase PdxH (Zhao and Winkler 1995).
The PdxST PLP synthase complex, which consists of 12 PdxS and 12 PdxT subunits, produces vitamin B6 independent of DXP (Belitsky 2004b; Raschle et al. 2005; Burns et al. 2005). The glutaminase subunit PdxT converts glutamine to glutamate and ammonia of which the latter is delivered to the PdxS PLP synthase subunit in the PdxST enzyme complex via a transient channel (Belitsky 2004b; Strohmeier et al. 2006). Since PdxS is active as a triose and pentose isomerase, the enzyme is capable of converting ammonia with either ribulose-5-phosphate and glyceraldehyde-3-phosphate or ribose-5-phosphate and dihydroxyacetone phosphate to the B6 vitamer PLP (Burns et al. 2005). The individual subunits and the PdxST enzyme complex have been well studied, both biochemically and structurally (Bauer et al. 2004; Raschle et al. 2005; Zhu et al. 2005; Strohmeier et al. 2006; Guédez et al. 2012; Smith et al. 2015; Ullah et al. 2020). The DXP-independent vitamin B6 pathway is present in archaea, bacteria, fungi, plants, Plasmodium and in some sponges (Seack et al. 2001; Ehrenshaft and Daub 2001; Fitzpatrick et al. 2007; Guédez et al. 2012). The DXP-independent vitamin B6 pathway is phylogenetically older because in silico analyses revealed that it was originally also present in α- and γ-proteobacteria that nowadays rely on the DXP-dependent pathway for PLP synthesis (Mittenhuber 2001; Tanaka et al. 2005). At a first glance, the DXP-dependent and DXP-independent vitamin B6 biosynthetic pathways seem to be different with respect to the number of involved enzymes and the catalytic mechanism. However, a structural comparison of the key biosynthetic enzymes PdxS and PdxJ of both pathways revealed similarities in their structure and catalytic mechanisms (Fitzpatrick et al. 2007).
Salvage of B6 vitamers and proteins involved in vitamin B6 homeostasis
Most organisms, irrespective of whether they are able or unable to synthesise PLP de novo, possess a salvage pathway allowing the interconversion of the B6 vitamers PL, PN and PN and the respective phosphate esters PLP, PMP and PNP (Fitzpatrick et al. 2007; di Salvo et al. 2011). Those organisms carrying only the salvage pathway have to take up PN, PM or PL from the environment and phosphorylate them. Indeed, E. coli synthesises the vitamin B6 kinases PdxK and PdxY, which can convert PL into PLP (Yang et al. 1996, 1998). Recently, the PL reductase PdxI, a novel vitamin B6 salvage enzyme converting PL into PN, has been identified in E. coli (Ito and Downs 2020). It will be interesting to assess whether other bacteria are also endowed with this enzyme activity. The broadly conserved PLP-binding protein COG0325 is also involved in vitamin B6 homeostasis or metabolism (Prunetti et al. 2016; Tremino et al. 2017). The PLP-bound COG0325 protein from E. coli, YggS, has been structurally analysed (PDBid: 1W8G). Previous studies showed that the inactivation of the yggS gene in E. coli and Salmonella enterica causes pleiotropic phenotypes, amongst them the accumulation of PNP (Ito et al. 2019; Vu et al. 2020). Recently, it has been demonstrated that PNP interferes with glycine metabolism by competing with PLP for binding a subunit of the glycine cleavage system (Ito et al. 2020). However, the underlying molecular mechanism causing the YggS-dependent accumulation of PNP is still unclear. Interestingly, B. subtilis also contains a COG0325 protein, which is designated as YlmE and shares 33% overall sequence identify with YggS. In contrast to E. coli, B. subtilis relies on the DXP-independent pathway for PLP production. Therefore, it is tempting to assume that the COG0325 homologues are rather involved in modulating the salvage of B6 vitamers instead of controlling de novo synthesis of PLP. To conclude, even though vitamin B6 metabolism has already been well studied in bacteria, the functions of some proteins such as that of the conserved CO0325 proteins remain to be resolved.
Attempts to overproduce PLP via the DXP-dependent and -independent pathways
Over the past years, several attempts have been made to engineer microorganisms for producing vitamin B6 (Rosenberg et al. 2017; Acevedo-Rocha et al. 2019). For instance, E. coli has been genetically engineered either by overexpressing the native genes of the DXP-dependent vitamin B6 or by overexpressing the pdxST genes from B. subtilis (Rosenberg et al. 2017). Moreover, natural overproducers of vitamin B6 such as Sinorhizobium meliloti have been isolated and genetically engineered for vitamin B6 production (Hoshino et al. 2006a, b). B. subtilis was also subjected to genetic modification for overproducing the B6 vitamer PN (Commichau et al. 2014). For this purpose, a non-native DXP-dependent pathway derived from E. coli and S. meliloti was implemented in B. subtilis. Growth experiments revealed that the pathway was fully functional because it relieved the PLP auxotrophy of a pdxST mutant (Commichau et al. 2014). B. subtilis was also engineered to convert the toxic metabolite 4-hydroxythreonine (4HT) to PN (Commichau et al. 2015; Rosenberg et al. 2016). The latter study revealed that B. subtilis possesses 4HT uptake systems and that the promiscuous homoserine kinase ThrB can convert 4HT to 4HTP, which is the substrate of the dehydrogenase PdxA. In both cases, the engineered bacteria produced significant amounts of PN. Moreover, the presence of PN in the fermentation broth suggests that B. subtilis must possess a phosphatase that is capable of dephosphorylating PNP and an export system for PN (Commichau et al. 2014, 2015). However, both enzyme activities remain to be identified in this organism. For a detailed summary of previous attempts to develop a sustainable fermentation process for the production of B6 vitamers, we ask the reader to consult the comprehensive review by Rosenberg and co-workers (Rosenberg et al. 2017). However, it can be stated that all microorganisms that have been genetically engineered so far did not exceed production levels of 10 g/L in 48 h that are required for outcompeting chemical synthesis processes (Acevedo-Rocha et al. 2019). Thus, major genetic engineering efforts are required for developing commercially relevant fermentation processes.
Alternative metabolic routes for PLP biosynthesis in E. coli
Previously, it has been demonstrated that the overexpression of seven different native genes in E. coli (aroA, hisB, nudL, pdxA, php, thrB and yjbQ) relives the PLP auxotrophy of a pdxB mutant strain lacking the 4PE dehydrogenase PdxB (Cooper 2010; Kim et al. 2010) (Fig. 2). It is interesting to note that none of the encoded proteins acts as an enzymatic replacer for PdxB. Thus, the overproduced proteins must possess promiscuous activities that are not required for their primary function in the metabolic network of the “wild type” E. coli cell. Moreover, these so-called serendipitous pathways divert intermediates from other metabolic pathways and convert it to a metabolite that feeds downstream of PdxB into the DXP-dependent vitamin B6 pathway. Indeed, for one of the serendipitous pathways consisting of NudL, LtaE, SerA and ThrB it has been shown that the four enzymes connect serine to PLP biosynthesis by converting 3-phospho-glycerate to 4HTP via 3-phospho-hydroxypyruvate, 3-hydroxypyruvate, glycolaldehyde and 4HT (Fig. 2) (Kim et al. 2010; Kim and Copley 2012). Moreover, it has been suggested that two other serendipitous pathways consisting of at least AroA and either HisB, Php or YjbQ feed into the DXP-dependent vitamin B6 pathway upstream of SerC and PdxJ (Fig. 2). However, the reaction sequences of the latter pathways remain to be elucidated.
Serendipitous pathways for vitamin B6 synthesis and enzymes that feed into the DXP-dependent pathway in E. coli. Epd, erythrose 4-phosphate dehydrogenase; PdxB, 4-phosphoerythronatedehydrogenase; SerC, 3-phosphoserine aminotransferase; PdxA, 4-phosphohydroxy-l-threonine dehydrogenase; PdxJ, pyridoxine 5′-phosphate synthase; Dxs, 1-deoxyxylulose 5-phosphate synthase; PdxH, pyridoxine 5′-phosphate oxidase; SerA, phosphoglycerate dehydrogenase; NudL, putative NUDIX hydrolase; LtaE, l-allo-threonine aldolase; ThrB (and DUF1537), homoserine kinase; DUF2257, l-threonine dioxygenase; AroB, 3-dehydroquinate synthase; HisB, imidazoleglycerolphosphate dehydratase and histidinol phosphatase; Php, unknown function; YjbQ, unknown function; ThiG, thiazole synthase. RsgA is a GTPase involved in ribosome maturation in E. coli (Campbell and Brown 2008). RsgA shares 38% overall sequence identify with the B. subtilis CpgA protein, which was shown to dephosphorylate 4-phosphoerythronate (Sachla and Helmann 2019). It is tempting to speculate that RsgA is also capable of dephosphorylating 4-phosphoerythronate
Recently, parallel lineages of the E. coli pdxB mutant strain have been evolved for up to 150 generations (Kim et al. 2019). This adaptive laboratory evolution experiment led to the identification of a novel serendipitous pathway, which consist of the three promiscuous enzymes, an unknown phosphatase required for dephosphorylating 4PE, the 3-phospho-glycerate (3PG) dehydrogenase SerA and the homoserine kinase ThrB.
The four-step serendipitous pathway feeds downstream of PdxB into the disrupted pathway and enable the bacteria to produce wild type levels of PLP (Fig. 2) (Kim et al. 2019). The detailed characterisation of the suppressor mutants revealed that some strains mutations improved the oxidation of erythronate to 3,4-dihydroxy-2-oxobutyrate by the 3PG dehydrogenase SerA, which is usually active in serine biosynthesis (Fig. 2). One mutation caused a decrease of the cellular concentrations of 3PG, the natural substrate of SerA. Another mutation decreased the cellular levels of serine, thereby preventing feedback inhibition of SerA by serine. Furthermore, several evolved pdxB suppressor carried mutations in the ybhA gene encoding a PLP phosphatase, an enzyme destroying PLP (Kuznetsova et al. 2006; Sugimoto et al. 2018; Kim et al. 2019). Thus, the so-called underground metabolism caused by promiscuous enzymes allows E. coli can assemble a new pathway for synthesising an essential cofactor by patching together native promiscuous enzymes (D'Ari and Casadesus 1998; Notebaart et al. 2014, 2018; Rosenberg and Commichau 2019; Copley 2020).
E. coli has another promiscuous enzyme, which may replace the entire DXP-dependent vitamin B6 pathway. A system-wide in silico analyses predicted that ThiG could be active in PLP synthesis in E. coli (Oberhardt et al. 2016). Usually, ThiG participates in the synthesis of the thiazole moiety of thiamine (Vander Horn et al. 1993). By overexpressing the thiG gene, it could be demonstrated that ThiG indeed restores the PLP auxotrophy of an E. coli pdxB mutant (Oberhardt et al. 2016). A structural model of ThiG using the structure of the B. subtilis PLP synthase PdxS revealed that the proteins share the same fold and potentially overlapping residues in the active site (Oberhardt et al. 2016). However, by using E. coli pdxH mutant lacking the pdxH PNP oxidase gene, it has to be experimentally validated that ThiG fulfils the same function as the PdxS PLP synthase subunit in vivo (Fig. 2).
It has also been shown that members of the DUF2257 and DUF1537 protein families can convert threonine to 4HT and 4HT to 4HTP, respectively, of which the latter is the substrate of the 4HTP dehydrogenase PdxA in the DXP-dependent vitamin B6 pathway (Figs. 1 and 2) (Smirnov et al. 2012; Thiaville et al. 2016). Another study uncovered that the DUF1537 kinase family enzymes are rather involved in the catabolism of four-carbon acid sugars such as d-erythronate and l-threonate (Zhang et al. 2016). However, the promiscuous activity of the members of the DUF2257 and DUF1537 protein families could serve as a starting point for enhancing the dioxygenase and 4HT kinase activities of the enzymes by directed evolution. These enzyme variants could be interesting to enhance the production of PNP or PLP via serendipitous pathways, which feed into the DXP-dependent vitamin B6 upstream of PdxA in E. coli. Indeed, in many cases, novel enzymes for practical applications are generated by directed evolution, usually starting from promiscuous functions (Yang et al. 2019; Copley 2020).
Adaptive laboratory evolution establishes a non-native vitamin B6 pathway in B. subtilis
As described above, B. subtilis relies on the PdxST PLP enzyme complex for the synthesis of the B6 vitamer PLP (Belitsky 2004b). Recently, it has assessed whether B. subtilis has the potential to evolve a non-native DXP-dependent vitamin B6 pathway consisting of parts of the pathway (Rosenberg et al. 2018). For this purpose, the genes encoding the enzymes of the DXP-dependent vitamin B6 pathway from E. coli were introduced step by step starting with the last gene of the pathway in a B. subtilis ΔpdxST mutant strain. Surprisingly, the B. subtilis ΔpdxST mutant carrying only the pdxJ and pdxH genes encoding the PNP synthase PdxJ and the PNP oxidase PdxH, respectively, formed suppressor mutants in the absence of exogenous PL (Rosenberg et al. 2018). Genome sequencing of the suppressor mutants uncovered that all strains had inactivated genes involved in the biosynthesis of bacillithiol. bacillithiol is a thiol compound, which is involved in maintaining the cellular redox balance and in the resistance to the antibiotic fosfomycin (Newton et al. 2009; Gaballa et al. 2010). It has been shown that bacillithiol may substitute for glutathione, which is a common intracellular thiol in eukaryotes and some bacteria (Newton et al. 2009). The reason why the loss of bacillithiol biosynthesis allows a B. subtilis ΔpdxST mutant carrying the pdxJ and pdxH genes to employ the DXP-dependent vitamin B6 pathway for PLP synthesis remains to be resolved (see below). However, independent lineages of the B. subtilis suppressor mutants were subjected to an adaptive laboratory evolution experiment to isolate variants with enhanced growth rates (Rosenberg et al. 2018). After several passages, faster-growing variants could indeed be isolated. The following genome sequencing analyses revealed that the overexpression of the ytoQ gene of unknown function is required to promote rapid growth of the B. subtilis ΔpdxST pdxJH mutants lacking the bacillithiol biosynthetic genes (Rosenberg et al. 2018). Thus, only two genes, encoding the enzymes that catalyse the last steps in the non-native vitamin B6 pathway, and two genomic alterations are sufficient to restore growth to wild type levels (Fig. 3). Despite the fact that the novel vitamin B6 pathway has to be fully characterised, the study shows that the underground metabolism existing in B. subtilis facilitates the emergence of a pathway for synthesis of the essential cofactor PLP using parts of a non-native metabolic pathway (Rosenberg et al. 2018; Rosenberg and Commichau 2019).
Putative serindipitous pathways for vitamin B6 synthesis in B. subtilis. GapA, glyceraldehyde 3-phosphate dehydrogenase; CpgA, phosphatase; SerA, phosphoglycerate dehydrogenase; SerC, 3-phosphoserine aminotransferase; ThrB, homoserine kinase; PdxJ, pyridoxine 5′-phosphate synthase; Dxs, 1-deoxyxylulose 5-phosphate synthase; PdxH, pyridoxine 5′-phosphate oxidase; PdxS (PLP synthase subunit) and PdxT (glutaminase subunit) form the PdxST PLP synthase complex; YtoQ, a protein of unknown function
How could the new vitamin B6 pathway be structured? Certainly, there must be intermediates either generated by non-enzymatic catalysis of by promiscuous enzymes that feed into the truncated non-native pathway to allow synthesis of PLP. Similar to the serendipitous pathway, which connects serine biosynthesis with PLP synthesis in E. coli, the novel vitamin B6 pathway in the evolved B. subtilis ΔpdxST pdxJH suppressor mutants could also contain enzymes of the serine metabolic pathway (Fig. 3). Recently, it has been observed that the CpgA protein, which was previously known to be required for ribosome maturation, acts as a phosphatase dephosphorylating the toxic metabolite 4PE that inhibits the 6-phosphogluconate dehydrogenase GndA of the pentose phosphate pathway in B. subtilis (Sachla and Helmann 2019). As described above, 4PE is the natural substrate of PdxB of the DXP-dependent pathway in E. coli (Fig. 1b). Thus, α- and γ-proteobacteria either keep the cellular concentration of 4PE low by its rapid conversion via PdxB or the bacteria contain 6-phosphogluconate dehydrogenase variants that are insensitive to 4PE. However, B. subtilis produces an intermediate of the DXP-dependent vitamin B6 pathway that could serve as a precursor for the PLP synthesis of the truncated pathway that was implemented in the ΔpdxST mutant. Similar to E. coli, the generated erythronate could be converted via 3,4-dihydroxy-2-oxobutyrate and 4HT to 4HTP by the promiscuous activities of the 3PG dehydrogenase SerA, the serine aminotransferase SerC and the homoserine kinase ThrB (Fig. 3). This hypothesis is likely to be correct because it has been shown that the native SerC and ThrB enzymes were shown to be active in a complete non-native DXP-dependent vitamin B6 pathway and ThrB is able to convert 4HT to 4HTP, (Commichau et al. 2014, 2015). The remaining enzyme activity that would be missing would be the activity of PdxA, which does not exist in B. subtilis. However, several native enzymes are present in B. subtilis that could be part of the novel serendipitous DXP-dependent vitamin B6 pathway (Fig. 3). While it is rather unclear how the YtoQ protein of unknown function enables the evolved B. subtilis to employ the PdxH and PdxJ enzyme to produce PLP, the lack of bacillithiol could be beneficial because the thiol was shown to inactivate the 3PG dehydrogenase SerA by binding to the cysteine residue 410 in the active site (Chi et al. 2011; Chi et al. 2013) Thus, the loss of bacillithiol biosynthesis could enhance the promiscuous activity of SerA in the bacteria. However, this idea has to be experimentally validated.
Conclusion and future perspectives
Previous attempts to engineer bacteria, including the Gram-negative and Gram-positive model bacteria E. coli and B. subtilis, for overproducing the B6 vitamers PN and PL were unsuccessful. In fact, major genetic engineering efforts seem to be required for developing fermentation processes that could outcompete the chemical synthesis of vitamin B6. Moreover, the suppressor analyses using E. coli and B. subtilis mutants carrying mutations in the native pathways or heterologous genes uncovered novel routes for PLP biosynthesis consisting of promiscuous enzymes and enzymes that are already involved in vitamin B6 biosynthesis. These serendipitous pathways could serve as a promising starting point for engineering the bacteria for overproducing vitamin B6 at commercially attractive levels.
References
Acevedo-Rocha CG, Gronenberg LS, Mack M, Commichau FM, Genee HJ (2019) Microbial cell factories for the sustainable manufacturing of B vitamins. Curr Opin Biotechnol 56:18–29
Bauer JA, Bennett EM, Begley TP, Ealick SE (2004) Three-dimensional structure of YaaE from Bacillus subtilis, a glutaminase implicated in pyridoxal-5’-phosphate biosynthesis. J Biol Chem 279:2704–2711
Belitsky BR (2004a) Bacillus subtilis GabR, a protein with DNA-binding and aminotransferase domains, is a PLP-dependent transcriptional regulator. J Mol Biol 340:655–664
Belitsky BR (2004b) Physiological and enzymological interaction of Bacillus subtilis proteins required for de novo pyridoxal 5’-phosphate biosynthesis. J Bacteriol 186:1191–1196
Belitsky BR (2014) Role of PdxR in the activation of vitamin B6 biosynthesis in Listeria monocytogenes. Mol Microbiol 92:1113–1128
Belitsky BR, Sonenshein AL (2002) GabR, a member of a novel protein family, regulates the utilization of γ-aminobutyrate in Bacillus subtilis. Mol Microbiol 45:569–583
Burns KE, Xiang Y, Kinsland CL, McLafferty FW, Begley TP (2005) Reconstitution and biochemical characterization of a new pyridoxal5 ′ -phosphate biosynthetic pathway. J Am Chem Soc 127:3682–3683
Campbell TL, Brown ED (2008) Genetic interaction screens with ordered overexpression and deletion clone sets implicate the Escherichia coli GTPase YjeQ in late ribosome biogenesis. J Bacteriol 190:2537–2545
Cane DE, Hsiung Y, Cornish JA, Robinson JK, Spenser ID (1998) Biosynthesis of vitamin B6: the oxidation of 4-(phosphohydroxy)-L-threonine by PdxA. J Am Chem Soc 120:1936–1937
Cane DE, Du S, Robinson JK, Hsiung Y, Spenser ID (1999) Biosynthesis of vitamin B6: enzymatic conversion of 1-deoxy-D-xylulose-5-phosphate to pyridoxol phosphate. J Am Chem Soc 121:7722–7723
Chi BK, Gronau K, Mäder U, Hessling B, Becher D, Antelmann H (2011) S-bacillithiolation protects against hypochlorite stress in Bacillus subtilis as revealed by transcriptomics and redox proteomics. Mol Cell Proteomics 10:M111.009506
Chi BK, Roberts AA, Huyen TTT, Bäsell K, Becher D, Albrecht D, Hamilton CJ, Antelmann H (2013) S-bacillithiolation protects conserved and essential proteins against hypochlorite stress in firmicutes. Antioxid Redox Signal 18:1273–1295
Commichau FM, Alzinger A, Sande R, Bretzel W, Meyer FM, Chevreux B, Wyss M, Hohmann HP, Prágai Z (2014) Overexpression of a non-native deoxyxylulose-dependent vitamin B6 pathway in Bacillus subtilis for the production of pyridoxine. Metab Eng 25:38–49
Commichau FM, Alzinger A, Sande R, Bretzel W, Reuß DR, Dormeyer M, Chevreux B, Schuldes J, Daniel R, Akeroyd M, Wyss M, Hohmann HP, Prágai Z (2015) Engineering Bacillus subtilis for the conversion of the antimetabolite 4-hydroxy-L-threonine to pyridoxine. Metab Eng 29:196–207
Cooper TF (2010) Metabolism gets lucky. Mol Syst Biol 6:439
Copley SD (2020) The physical basis and practical consequences of biological promiscuity. Phys Biol 7. https://doi.org/10.1088/1478-3975/ab8697
D'Ari R, Casadesus J (1998) Underground metabolism. Bioessays 20:181–186
di Salvo ML, Contestabile R, Safo MK (2011) Vitamin B6 salvage enzymes: mechanism, structure and regulation. Biochim Biophys Acta 1814:1597–1608
Domke A, Großklaus R, Niemann B, Pryrzembel H, Richter K, Schmidt E, Weißenborn A, Wörner B, Ziegenhagen R (2005) Use of vitamins in foods – toxicological and nutritional-physiological aspects. BfR-Wissenschaft (ISSN 1614 – 3795, ISBN 3-938163-10-0.0)
Drewke C, Klein M, Clade D, Arenz A, Müller R, Leistner E (1996) 4-O-phosphoryl-L-threonine, a substrate of the pdxC(serC) gene product involved in vitamin B6 biosynthesis. FEBS Lett 390:179–182
Eggersdorfer M, Laudert D, Létinois U, McClymont T, Medlock J, Netscher T, Bonrath W (2012) One hundred years of vitamins - a success story of natural sciences. Angew Chem Int Ed Eng 51:12960–12990
Ehrenshaft M, Daub ME (2001) Isolation of PDX2, a second novel gene in the pyridoxine biosynthesis pathway of eukaryotes, archaebacteria, and a subset of eubacteria. J Bacteriol 183:3383–3390
El Qaidi S, Yang J, Zhang JR, Metzger DW, Bai G (2013) The vitamin B6 pathway in Streptococcus pneumoniae is controlled by pyridoxal 5’-phosphate and the transcription factor PdxR and has an impact on ear infection. J Bacteriol 195:2187–2196
Fitzpatrick T, Amrhein N, Kappes B, Macheroux P, Tews I, Raschle T (2007) Two independent routes for de novo vitamin B6 biosynthesis: not that different after all. Biochem J 407:1–13
Frezzini M, Narzi D, Sciolari AM, Guidoni L, Pascarella S (2020) Molecular dynamics of an asymmetric form of GabR, a bacterial transcriptional regulator. Biophys Chem 262:106380
Gaballa A, Newton GL, Antelmann H, Parsonage D, Upton H, Rawat M, Claiborne A, Fahey RC, Helmann JD (2010) Biosynthesis and functions of bacillithiol, a major low-molecular-weight thiol in Bacilli. Proc Natl Acad Sci U S A 107:6482–6486
Guédez G, Hipp K, Windeisen V, Derrer B, Gengenbacher M, Böttcher B, Sinning I, Kappes B, Tews I (2012) Assembly of the eukaryotic PLP-synthase complex from Plasmodium and activation of the Pdx1 enzyme. Structure 20:172–184
Hoegl A, Nodwell MB, Kirsch VC, Bach NC, Pfanzelt M, Stahl M, Schneider S, Sieber SA (2018) Mining the cellular inventory of pyridoxal phosphate-dependent enzymes with functionalized cofactor mimics. Nat Chem 10:1234–1245
Hoffarth ER, Rothchild KW, Ryan KS (2020) Emergence of oxygen- and pyridoxal-dependent reactions. FEBS J 287:1403–1428
Hoshino T, Ichikawa, K, Tazoe M (2006a) Recombinant microorganism for the production of vitamin B6. US Patent Application US2006/0228785 A1.
Hoshino T, Ischikawa K, Nagahashi K, Tazoe M (2006b) Microorganism and process for preparing vitamin B6. US Patent Application US2006/0127992 A1.
Huq MD, Tsai NP, Lin YP, Higgins I, Wei LN (2007) Vitamin B6 conjugation to nuclear corepressor RIP140 and its role in gene regulation. Nat Chem Biol 3:161–165
Ito T, Downs DM (2020) Pyridoxal reductase, PdxI, is critical for salvage of pyridoxal in Escherichia coli. J Bacteriol 202:e00056–e00020
Ito T, Yamamoto K, Hori R, Yamauchi A, Downs DM, Hemmi H, Yoshimura T (2019) Comserved pyridoxal 5’-phosphate-binding protein YggS impacts amino acid metabolism through pyridoxine 5’-phosphate in Escherichia coli. Appl Environ Microbiol 85:e00430–e00419
Ito T, Hori R, Hemmi H, Downs DM, Yoshimura T (2020) Inhibition of glycine cleavage system by pyridoxine 5’-phosphate causes synthetic lethality in glyA yggS and serA yggS in Escherichia coli. Mol Microbiol 113:270–284
Jochmann N, Götker S, Tauch A (2011) Positive control of the pyridoxal phosphate biosynthesis genes pdxST by the MocR-type regulator PdxR of Corynebacterium glutamicum. Microbiology 157:77–88
Johnson BC, Pinkos JA, Burke KA (1950) Pyridoxine deficiency in the calf. J Nutr 40:309–322
Kim J, Copley SD (2012) Inhibitory cross-talk upon introduction of a new metabolic pathway into an existing metabolic network. Proc Natl Acad Sci U S A 109:E2856–E2864
Kim J, Kershner JP, Novikov Y, Shoemaker RK, Copley SD (2010) Three serendipitous pathways in E. coli can bypass a block in pyridoxal-5’-phosphate synthesis. Mol Syst Biol 6:436
Kim J, Flood JJ, Kristofich MR, Gidfar C, Morgenthaler AB, Fuhrer T, Sauer U, Snyder D, Cooper VS, Ebmeier CC, Old WM, Copley SD (2019) Hidden resources in the Escherichia coli genome restore PLP synthesis and robust growth after deletion of the essential gene pdxB. Proc Natl Acad Sci U S A 116:24164–24173
Kraemer K, Semba RD, Eggersdorfer M, Schaumberg DA (2012) Introduction: the diverse and essential biological functions of vitamins. Ann Nutr Metab 61:185–191
Kuznetsova E, Proudfoot M, Gonzalez CF, Brown G, Omelchenko MV, Borozan I, Carmel L, Wolf YI, Mori H, Savchenko AV, Arrowsmith CH, Koonin EV, Edwards AM, Yakunin AF (2006) Genome-wide analysis of substrate specificities of the Escherichia coli haloacid dehalogenase-like phosphatase family. J Biol Chem 281:36149–36161
Lam HM, Winkler ME (1990) Metabolic relationship between pyridoxine (vitamin B6) and serine biosynthesis in Escherichia coli. J Bacteriol 172:6518–6528
Mittenhuber G (2001) Phylogenetic analyses and comparative genomics of vitamin B6 (pyridoxine) and pyridoxal phosphate biosynthesis pathways. J Mol Microbiol Biotechnol 3:1–20
Mukherjee T, Hanes J, Tews I, Ealick SE, Begley TP (2011) Pyridoxal phosphate: biosynthesis and catabolism. Biochim Biophys Acta 1814:1585–1596
Newton GL, Rawat M, La Clair JJ, Jothivasan VK, Budiarto T, Hamilton CJ, Claiborne A, Helmann JD, Fahey RC (2009) Bacillithiol is an antioxidant thiol produced in Bacilli. Nat Chem Biol 5:625–627
Notebaart RA, Szappanos B, Kintses B, Pál F, Györkei A, Bogos B, Lázár V, Spohn R, Csörgö B, Wagner A, Ruppin E, Pál C, Papp B (2014) Network-level architecture and the evolutionary potential of underground metabolism. Proc Natl Acad Sci U S A 111:11762–11767
Notebaart RA, Kintses B, Feist AM, Papp B (2018) Underground metabolism: network-level perspective and biotechnological applications. Curr Opin Biotechnol 49:108–114
Oberhardt MA, Zarecki R, Reshef L, Xia F, Duran-Frigola M, Schreiber R, Henry CS, Ben-Tal N, Dwyer DJ, Grophna U, Ruppin E (2016) Systems-wide prediction of enzyme promiscuity reveals a new underground route for pyridoxal 5’-phosphate production in E. coli. PLoS Comput Biol 12:e1004705
Oka T, Sugitatsu H, Nordin H, Thakur MK, Aoyama M, Sasagawa T, Suzuki I, Tsuji H (2001) Pyridoxal 5’-phosphate inhibits DNA binding of HNF1. Biochim Biohys Acta 1568:189–196
Parra M, Stahl S, Hellmann H (2018) Vitamin B6 and its role in cell metabolism and physiology. Cells 7:84
Percudani R, Peracchi A (2003) A genomic overview of pyridoxal-phosphate-dependent enzymes. EMBO Rep 4:850–854
Percudani R, Peracchi A (2009) The B6 database: a tool for the description and classification of vitamin B6-dependent enzymatic activities and of the corresponding protein families. BMC Bioinformatics 10:273
Prunetti L, El Ycoubi B, Schiavon CR, Kirkpatrick E, Huang L, Bailly M, El Badawi-Sidhu M, Harrison K, Gregory JF, Fiehn O, Hanson AD, de Crécy-Lagard V (2016) Evidence that COG0325 proteins are involved in PLP homeostasis. Microbiology 162:694–706
Raschle T, Amrhein N, Fitzpatrick TB (2005) On the two components of pyridoxal 5’-phosphate synthase in Bacillus subtilis. J Biol Chem 280:32291–32300
Richts B, Rosenberg J, Commichau FM (2019) A survey of pyridoxal 5’-phosphate-dependent proteins in the Gram-positive model bacterium Bacillus subtilis. Front Mol Biosci 6:32
Rosenberg IH (2012) A history of the isolation and identification of vitamin B6. Ann Nutr Metab 61:236–238
Rosenberg J, Commichau FM (2019) Harnessing underground metabolism for pathway development. Trends Biotechnol 37:29–37
Rosenberg J, Müller P, Lentes S, Thiele MJ, Zeigler DR, Tödter D, Paulus H, Brantl S, Stülke J, Commichau FM (2016) ThrR, a DNA-binding transcription factor involved in controlling threonine biosynthesis in Bacillus subtilis. Mol Microbiol 101:879–893
Rosenberg J, Ischebeck T, Commichau FM (2017) Vitamin B6 metabolism in microbes and approaches for fermentative production. Biotechnol Adv 35:31–40
Rosenberg J, Yeak KC, Commichau FM (2018) A two-step evolutionary process establishes a non-native vitamin B6 pathway in Bacillus subtilis. Environ Microbiol 20:156–168
Rudolph J, Kim J, Copley SD (2010) Multiple turnovers of the nicotino-enzyme PdxB require α-keto acids as cosubstrates. Biochemistry 49:9249–9255
Sachla A, Helmann JD (2019) A bacterial checkpoint protein for ribosome assembly moonlights as an essential metabolite-proofreading enzyme. Nat Commun 10:1526
Sakai A, Kita M, Katsuragi T, Ogasawara N, Tani Y (2002a) yaaD and yaaE are involved in vitamin B6 biosynthesis in Bacillus subtilis. J Biosci Bioeng 93:309–312
Sakai A, Kita M, Katsuragi T, Tani Y (2002b) serC is involved in vitamin B6 biosynthesis in Escherichia coli but not in Bacillus subtilis. J Biosci Bioeng 93:334–337
Seack J, Perovic S, Gamulin V, Schroder HC, Beutelmann P, Muller IM, Muller WE (2001) Identification of highly conserved genes: SNZ and SNO in the marine sponge Suberites domuncula: their gene structure and promoter activity in mammalian cells. Biochim Biophys Acta 1520:21–34
Smirnov SV, Sokolov PM, Kodera T, Sugiyama M, Hibi M, Shimizu S, Yokozeki K, Ogawa J (2012) A novel family of bacterial dioxygenases that catalyse the hydroxylation of free L-amino acids. FEMS Microbial Lett 331:97–104
Smith AM, Brown WC, Harms E, Smith JL (2015) Crystal structures capture three states in the catalytic cycle of a pyridoxal phosphate (PLP) synthase. J Biol Chem 290:5226–5239
Strohmeier M, Raschle T, Mazurkiewicz J, Rippe K, Sinning I, Fitzpatrick TB, Tews I (2006) Structure of a bacterial pyridoxal 5 ′-phosphate synthase complex. Proc Natl Acad Sci U S A 103:19284–19289
Sugimoto R, Saito N, Shimada T, Tanaka K (2018) Identification of YbhA as the pyridoxal 5’-phosphate (PLP) phosphatase in Escherichia coli: importance pf PLP homeostasis on the bacterial growth. J Gen Appl Microbiol 63:362–368
Suvorova IA, Rodionov DA (2016) Comparative genomics of pyridoxal 5’-phosphate-dependent transcription factor regulons in bacteria. Microb Genom 2:200047
Tanaka T, Tateno Y, Gojobori T (2005) Evolution of vitamin B6 (pyridoxine) metabolism by gain and loss of genes. Mol Biol Evol 22:243–250
Tazoe M, Ichikawa K, Hoshino Z (2006) Flavin adenine dinucleotide-dependent 4-phospho-D-erythronate dehydrogenase is responsible for the 4-phosphohydroxy-L-threonine pathway in vitamin B6 biosynthesis in Sinorhizobium meliloti. J Bacteriol 188:4635–4645
Thiaville JJ, Flood J, Yurgel S, Prunetti L, Elbadawi-Sidhu M, Hutinet G, Forouhar F, Zhang X, Ganesan V, Reddy P, Fiehn O, Gerlt JA, Hunt JF, Copley SD, De Crécy-Lagard V (2016) Members of a novel kinase family (DUF1537) can recycle toxic inter- mediates into an essential metabolite. ACS Chem Biol 19:2304–2311
Tramonti A, Fiascarelli A, Milano T, di Salvo ML, Nogués I, Pascarella S, Contestabile R (2015) Molecular mechanism of PdxR – a transcriptional activator involved in the regulation of vitamin B6 biosynthesis in the probiotic bacterium Bacillus clausii. FEBS J 282:2966–2984
Tremino L, Forcada-Nadal A, Contreras A, Rubio V (2017) Studies on cyanobacterial protein PipY shed light on structure, potential functions, and vitamin B6-dependent epilepsy. FEBS Lett 591:3431–3442
Ullah N, Andaleeb H, Mudogo CN, Falke S, Betzel C, Wrenger C (2020) Solution structures and dynamic assembly of the 24-meric plasmodial Pdx1-Pdx2 complex. Int J Mol Sci 21:5971
Vander Horn PB, Backstrom AD, Stewart V, Begley TP (1993) Structural genes for thiamine biosynthetic enzymes (thiCEFGH) in Escherichia coli K-12. J Bacteriol 175:982–992
Verbeek J (1975) Vitamin behavior in premixes. Feedstuffs 47:4
Vu HN, Ito T, Downs DM (2020) The role of YggS in vitamin B6 homeostasis in Salmonella enterica is informed by heterologous expression of yeast SNZ3. J Bacteriol 202:e00383–e00320
Yang Y, Zhao G, Winkler ME (1996) Identification of the pdxK gene that encodes pyridoxine (vitamin B6) kinase in Escherichia coli K-12. FEMS Microbiol Lett 141:89–95
Yang Y, Tsui HC, Man TK, Winkler ME (1998) Identification and function of the pdxY gene, which encodes a novel pyridoxal kinase involved in the salvage pathway of pyridoxal 5 ′ -phosphate biosynthesis in Escherichia coli K-12. J Bacteriol 180:1814–1821
Yang G, Anderson DW, Baier F, Dohmen E, Hong N, Carr PD, Caroline S, Kamerlin L, Jackson CJ, Bornberg-Bauer E, Tokuriki N (2019) Higher-order epistasis shapes the fitness landscape of a xenobiotic-degrading enzyme. Nat Chem Biol 15:1120–1128
Zhang X, Carter MS, Vetting MW, Francisco BS, Zhao S, Al-Obaidi NF, Solbiati JO, Thiaville JJ, de Crécy-Lagard V, Jacobson MP, Almo SC, Gerlt JA (2016) Assigment of function to a domain of unknown function: DUF1537 is a new kinase family in catabolic pathways for acid sugars. Proc Natl Acad Sci U S A 113:E4161–E4169
Zhao G, Winkler ME (1995) Kinetic limitation and cellular amount of pyridoxine (pyridoxamine) 5’-phosphate oxidase of Escherichia coli K-12. J Bacteriol 177:883–891
Zhao G, Pease AJ, Bharani N, Winkler ME (1995) Biochemical characterization of gapB-encoded erythrose 4-phosphate dehydrogenase of Escherichia coli K-12 and its possible role in pyridoxal 5’-phosphate biosynthesis. J Bacteriol 177:2804–2812
Zhu J, Burgner JW, Hamrs W, Belitsky BR, Smith JL (2005) A new arrangement of (beta/alpha)8 barrels in the synthase subunit of PLP synthase. J Biol Chem 280:27914–27923
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the grant Co1139/3-1 from the Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Contributions
B.R. and F.M.C. wrote and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Richts, B., Commichau, F.M. Underground metabolism facilitates the evolution of novel pathways for vitamin B6 biosynthesis. Appl Microbiol Biotechnol 105, 2297–2305 (2021). https://doi.org/10.1007/s00253-021-11199-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11199-w