Abstract
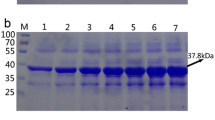
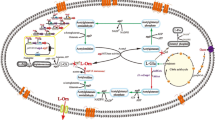
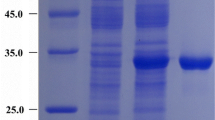
This study aims to use neutral pH optimum arginase as the catalyst for high-efficiency l-ornithine production. Sulfobacillus acidophilus arginase was firstly cloned and overexpressed in Escherichia coli. The purified enzyme was obtained, and the molecular mass determination showed that this arginase was a hexamer. S. acidophilus arginase possessed similarities with the other arginases such as the conserved sequences, purification behavior, and the necessity for Mn2+ as a cofactor. The maximum enzyme activity was obtained at pH 7.5 and 70 °C. Thermostability and pH stability analysis showed that the arginase was stable at 30–60 °C and pH 7.0–8.5, respectively. The kinetic parameters suggested that S. acidophilus arginase could efficiently hydrolyze l-arginine. Bioconversion with this neutral pH optimum arginase had the advantages of avoiding producing by-product, high molar yield, and high-level production of l-ornithine. When the bioconversion was performed with a fed-batch strategy and a coupled-enzyme system involving S. acidophilus arginase and Jack bean urease, the final production of 2.87 mol/L was obtained with only 1.72 mmol/L l-arginine residue, and the molar yield was 99.9%. The highest production record suggests that S. acidophilus arginase has a great prospect in industrial l-ornithine production.






Similar content being viewed by others
References
Abhishek S, Shiv Kumar M, Mashkoor A, Nayeem SM, Shashank D, Apurba Kumar S (2013) Structural and functional insights into the regulation of Helicobacter pylori arginase activity by an evolutionary nonconserved motif. Biochemistry 52:508–519. https://doi.org/10.1021/bi301421v
Bewley MC, Jeffrey PD, Patchett ML, Kanyo ZF, Baker EN (1999) Crystal structures of Bacillus caldovelox arginase in complex with substrate and inhibitors reveal new insights into activation, inhibition and catalysis in the arginase superfamily. Structure 7(4):435–448. https://doi.org/10.1016/s0969-2126(99)80056-2
Caldwell RB, Toque HA, Narayanan SP, Caldwell RW (2015) Arginase: an old enzyme with new tricks. Trends Pharmacol Sci 36:395–405. https://doi.org/10.1016/j.tips.2015.03.006
Cheng L, Mu W, Zhang T, Jiang B (2010) An L-arabinose isomerase from Acidothermus cellulolytics ATCC 43068: cloning, expression, purification, and characterization. Appl Microbiol Biot 86:1089–1097. https://doi.org/10.1007/s00253-009-2322-z
Dala EE, Szajáni B (1994) Immobilization, characterization, and laboratory-scale application of bovine liver arginase. Appl Biochem Biotech 49:203–215. https://doi.org/10.1007/BF02783058
D’Antonio EL, Ullman B, Roberts SC, Dixit UG, Wilson ME, Hai Y, Christianson DW (2013) Crystal structure of arginase from Leishmania mexicana and implications for the inhibition of polyamine biosynthesis in parasitic infections. Arch Biochem Biophys 535:163–176. https://doi.org/10.1016/j.abb.2013.03.015
Huang K, Zhang T, Jiang B, Mu MW, Miao M (2016a) Characterization of a thermostable arginase from Rummeliibacillus pycnus SK31.001. J Mol Catal B-Enzym 133:S68–S75. https://doi.org/10.1016/j.molcatb.2016.11.020
Huang K, Zhang T, Jiang B, Mu WM, Miao M (2016b) A coupled system involving arginase and urease for L-ornithine production. J Mol Catal B-Enzym 133:S303–S310. https://doi.org/10.1016/j.molcatb.2017.01.018
Huang K, Zhang T, Jiang B, Mu WM, Miao M (2016c) Cloning, expression, and characterization of a thermostable L-arginase from Geobacillus thermodenitrificans NG80-2 for L-ornithine production. Biotechnol Appl Biochem 63:391–397. https://doi.org/10.1002/bab.1385
Huang K, Guan X, Jiang B, Li S (2018) Construction of a food-grade arginase expression system and its applicsation in L-ornithine production with whole cell biocatalyst. Process Biochem 73:94–101. https://doi.org/10.1016/j.procbio.2018.07.020
Jenkinson CP, Grody WW, Cederbaum SD (1996) Comparative properties of arginase. Comp Biochem Physiol 114:107–132. https://doi.org/10.1016/0305-0491(95)02138-8
Kanyo ZF, Scolnick LR, Ash DE, Christianson DW (1996) Structure of a unique binuclear manganese cluster in arginase. Nature 383:554–557. https://doi.org/10.1038/383554a0
Maharem TM, Zahran WE, Hassan RE, Fattah MMA (2017) Unique properties of arginase purified from camel liver cytosol. Int J Biol Macromol 108:88–97. https://doi.org/. https://doi.org/10.1016/j.ijbiomac.2017.11.141
Polacco JC, Mazzafera P, Tezotto T (2013) Opinion-nickel and urease in plants: still many knowledge gaps. Plant Sci 199-200:79–90. https://doi.org/10.1016/j.plantsci.2012.10.010
Rafnsson A, Matic LP, Lengquist M, Mahdi A, Shemyakin A, Paulsson-Berne G, Hansson GK, Gabrielsen A, Hedin U, Yang J, Pernow J (2020) Endothelin-1 increases expression and activity of arginase 2 via ETB receptors and is co-expressed with arginase 2 in human atherosclerotic plaques. Atherosclerosis 292:215–223. https://doi.org/10.1016/j.atherosclerosis.2019.09.020
Reddy SRR, Campbell JW (1968) A low molecular weight arginase in the earthworm. Biochim Biophys Acta 159:557–560. https://doi.org/10.1016/0005-2744(68)90145-9
Siddappa S, Basrur V, Vittal RR, Marathe GK (2018) Biochemical and functional characterization of an atypical plant L-arginase from Cilantro (Coriandrum sativam L.). Int J Biol Macromol 118:844–856. https://doi.org/10.1016/j.ijbiomac.2018.06.096
Song W, Niu P, Chen XL, Liu LM (2014) Enzymatic production of L-ornithine from L-arginine with recombinant thermophilic arginase. J Mol Catal B-Enzym 110:1–7. https://doi.org/10.1016/j.molcatb.2014.09.005
Stasyuk N, Smutok O, Gayda G, Vus B, Koval'Chuk Y, Gonchar M (2012) Bi-enzyme L-arginine-selective amperometric biosensor based on ammonium-sensing polyaniline-modified electrode. Biosens Bioelectron 37:46–52. https://doi.org/10.1016/j.bios.2012.04.031
Stone EM, Glazer ES, Chantranupong L, Cherukuri P, Breece RM, Tierney DL, Curley SA, Iverson BL, Georgiou G (2010) Replacing Mn2+ with Co2+ in human arginase I enhances cytotoxicity toward L-arginine auxotrophic cancer cell lines. ACS Chem Biol 5:333–342. https://doi.org/10.1021/cb900267j
Viator RJ, Rest RF, Hildebrandt E, Mcgee DJ (2008) Characterization of Bacillus anthracis arginase: effects of pH, temperature, and cell viability on metal preference. BMC Biochem 9:15. https://doi.org/10.1186/1471-2091-9-15
Wang M, Xu M, Rao Z, Yang T, Zhang X (2015) Construction of a highly efficient Bacillus subtilis 168 whole-cell biocatalyst and its application in the production of L-ornithine. J Ind Microbiol Biotechnol 42:1427–1437. https://doi.org/10.1007/s10295-015-1672-z
Yu JJ, Park KB, Kim SG, Oh SH (2013) Expression, purification, and biochemical properties of arginase from Bacillus subtilis 168. J Microbiol 51:222–228. https://doi.org/10.1007/s12275-013-2669-9
Zhan Y, Liu J, Mao P, Zhang H, Liu Q, Jiao Q (2013) Biotransformation of L-ornithine from L-arginine using whole-cell recombinant arginase. World J Microb Biot 29:2167–2172. https://doi.org/10.1007/s11274-013-1382-5
Zhang J, Zhang X, Wu C, Lu D, Guo G, Mao X, Zhang Y, Wang DC, Li D, Zou Q (2011) Expression, purification and characterization of arginase from Helicobacter pylori in its apo form. PLoS One 6:e26205. https://doi.org/10.1371/journal.pone.0026205
Zhang T, Guo YJ, Wang H, Mu WM, Miao M (2013) Arginase from Bacillus thuringiensis SK 20.001: purification, characteristics, and implications for L-ornithine biosynthesis. Process Biochem 48:663–668. https://doi.org/10.1016/j.procbio.2013.02.023
Zhang X, Liu J, Yu X, Wang F, Yi L, Li Z, Liu Y, Ma L (2015) High-level expression of human arginase I in Pichia pastoris and its immobilization on chitosan to produce L-ornithine. BMC Biotechnol 15:1–10. https://doi.org/10.1186/s12896-015-0184-2
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31701515) and Youth Scientific Talent Program of the National Grain Industry (LQ2018204).
Author information
Authors and Affiliations
Contributions
KH and XG conceived and designed research. KH and SRZ conducted experiments. SL contributed new reagents or analytical tools. KH, SRZ, HDS, and JL analyzed data. KH wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. Arginase from S. acidophilus was cloned and overexpressed in E. coli
2. This thermostable arginase had a neutral pH optimum
3. A urease-coupled system and the fed-batch strategy was used for l-ornithine production
4. This arginase and the conversion process have important industrial application potential
Rights and permissions
About this article
Cite this article
Huang, K., Zhang, S., Guan, X. et al. Thermostable arginase from Sulfobacillus acidophilus with neutral pH optimum applied for high-efficiency l-ornithine production. Appl Microbiol Biotechnol 104, 6635–6646 (2020). https://doi.org/10.1007/s00253-020-10721-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10721-w