Abstract
Comparative analyses determined the relationship between the structure of bisphenol A (BPA) as well as of seven bisphenol analogues (bisphenol B (BPB), bisphenol C (BPC), bisphenol E (BPE), bisphenol F (BPF), bisphenol Z (BPZ), bisphenol AP (BPAP), bisphenol PH (BPPH)) and their biotransformability by the biphenyl-degrading bacterium Cupriavidus basilensis SBUG 290. All bisphenols were substrates for bacterial transformation with conversion rates ranging from 6 to 98% within 216 h and 36 different metabolites were characterized. Transformation by biphenyl-grown cells comprised four different pathways: (a) formation of ortho-hydroxylated bisphenols, hydroxylating either one or both phenols of the compounds; (b) ring fission; (c) transamination followed by acetylation or dimerization; and (d) oxidation of ring substituents, such as methyl groups and aromatic ring systems, present on the 3-position. However, the microbial attack of bisphenols by C. basilensis was limited to the phenol rings and its substituents, while substituents on the carbon bridge connecting the rings were not oxidized. All bisphenol analogues with modifications at the carbon bridge could be oxidized up to ring cleavage, while substituents at the 3-position of the phenol ring other than hydroxyl groups did not allow this reaction. Replacing one methyl group at the carbon bridge of BPA by a hydrophobic aromatic or alicyclic ring system inhibited both dimerization and transamination followed by acetylation. While most of the bisphenol analogues exhibited estrogenic activity, four biotransformation products tested were not estrogenically active.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For more than six decades, bisphenol A (BPA) has been used in the production of polycarbonate plastics and epoxy resins (EPA 2014; Hoekstra and Simoneau 2013; Usman and Ahmad 2016) with an annual production of more than 3.8 million tons (Michałowicz 2014). It is one of the most extensively used bisphenols with applications in thermal paper production (EPA 2014; Pivnenko et al. 2015), food contact material, electronic devices, water pipes, or health care (Michałowicz 2014; Vandenberg et al. 2007). There are many studies pointing to environmental contamination resulting in unavoidable human exposure, with risks particularly associated with its potential to act as endocrine agent (Usman and Ahmad 2016). In reaction to public concern, some countries, especially in North America and the European Union, regulated the production and restricted the usage of BPA (Barroso 2011; Goldinger et al. 2015). As a result, chemicals with similar structure, referred to as bisphenol analogues, were introduced for industrial applications to replace BPA (Chen et al. 2016). For example, in thermal paper production, 19 different bisphenol analogues have been documented (EPA 2014). Bisphenol analogues include, among others, bisphenol AP (BPAP), bisphenol B (BPB), bisphenol C (BPC), bisphenol E (BPE), bisphenol F (BPF), bisphenol PH (BPPH), and bisphenol Z (BPZ). All of these have in the meantime found widespread applications. BPAP, for example, is used in polymer materials and in the fine chemical industry (Zhang et al. 2013a). BPF is applied in the manufacture of fire-resistant polymers (Delfosse et al. 2012) and used in lacquers, varnishes, liners, adhesives plastics, and water pipes (Fromme et al. 2002). The increasing usage of bisphenol analogues raised questions about their environmental fate, degradability, and endocrine activity. Many of them have been documented in various environmental compartments like indoor dust, sediment, sludge, and surface waters, in foods and food containers, personal care products, as well as in human samples as comprehensively summarized by Chen et al. (2016). Many of these substances constitute serious public health risks. Bisphenol analogues, such as BPE or BPF, show estrogenic activity similar to that of BPA while BPB or BPZ are even more active (Kojima et al. 2019). BPF is known to exhibit genotoxicity (Cabaton et al. 2009) and oxidative toxicity (Michałowicz et al. 2015). It also enhances reactive oxygen species formation, increases lipid peroxidation, and changes the activities of superoxide dismutase, catalase, and glutathione peroxidase in human erythrocytes (Macczak et al. 2017). Even though there is an increasing number of studies regarding the estimation of human exposure, uptake and health risks, toxicity, estrogenicity, and environmental fate of these bisphenol analogues, the knowledge currently available is not sufficient to assess the impact of these compounds on human health and the environment. Chen et al. (2016) pointed out the serious lack of information regarding environmental persistence, toxicity, and elucidation of metabolic pathways and products of bisphenol analogues. With the present study, we narrow some of these gaps by investigating the biotransformation of seven bisphenol analogues by the biphenyl-degrading bacterium Cupriavidus basilensis SBUG 290 that was isolated from environmental samples and is able to transform BPA efficiently (Zühlke et al. 2017). Our objectives were (i) to determine the biotransformation pathway of the various compounds by structure elucidation of the transformation products formed, (ii) to establish principles of the structure-biotransformation-relationship, and (iii) to determine the estrogenicity of the transformation products compared to that of the bisphenol analogues.
Materials and methods
Strain
The bacterial strain was isolated from compost soil by enrichment cultures with 4-chlorobiphenyl (Becher et al. 2000) and later identified by 16S rRNA analyses as C. basilensis (Zühlke et al. 2017). The bacterial strain is deposited in the strain collection of the Department of Biology of the University of Greifswald (SBUG) as C. basilensis SBUG 290.
Biotransformation experiments
2,2-Bis-(4-hydroxy-3-methylphenyl)-propane (bisphenol C, BPC), 1,1-bis-(4-hydroxyphenyl)ethane (bisphenol E, BPE), 1,1-bis-(4-hydroxyphenyl)methane (bisphenol F, BPF), and 4,4′-cyclohexylidenebisphenol (bisphenol Z, BPZ) were purchased from Sigma-Aldrich (Steinheim, Germany), and 1,1-bis-(4-hydroxyphenyl)-1-phenylethane (bisphenol AP, BPAP), 2,2-bis-(4-hydroxyphenyl)butane (bisphenol B, BPB), and 2,2-bis(2-hydroxy-5-biphenylyl)propane (bisphenol PH, BPPH) were purchased from Tokyo Chemical Industry GmbH Co (Tokyo, Japan), at highest purity available. Cells of C. basilensis were cultivated for 8 h in nutrient broth (NB) and afterwards for an additional 72 h with biphenyl as substrate as described earlier (Zühlke et al. 2017). For biotransformation experiments, 500-ml-flasks containing 100 ml mineral salts medium for bacteria (MMb; pH 6.3; Hundt et al. 1998; Stope et al. 2002) were used. The medium was supplemented with 0.002% (20 mg L−1) or 0.006% (60 mg L−1) of the bisphenols, each applied from 5% stock solutions in dimethylformamide. After 72 h of cultivation with biphenyl, cells were harvested by centrifugation (15,890×g, 25 min and 4 °C), washed twice in MMb and finally resuspended in a small amount of MMb. This cell suspension was transferred to the medium supplemented with the bisphenols until an optical density (OD500 nm) of 3.0 was reached. This corresponds to approximate 5.2 × 109 cells mL−1. Cells were incubated on a rotary shaker at 30 °C and 180 rpm for 216 h (HT FORS). Flasks with (i) MMb and the respective bisphenol in the absence of cells and (ii) with MMb without bisphenols but with cells (OD500 nm = 3.0) served as controls. Biotransformation experiments were carried out in independent duplicate experiments.
Analytical methods for detection of bisphenols and transformation products
For monitoring the transformation of the bisphenol analogues and the formation of transformation products, samples of 1 mL of cell suspension were removed periodically: the first time directly after cell transfer to MMb with bisphenols (this corresponds to 0 h) and then each or every second day until 216 h of incubation. Cells were pelleted by centrifugation (3587×g, 5 min, room temperature), and the cell-free supernatant was analyzed by high-performance liquid chromatography (HPLC) using an Agilent Technologies 1200 Series system (Santa Clara, USA). Components were separated on a LiChroCART® 125-4 RP-18 endcapped 5 μm (Merck, Darmstadt, Germany) column applying a solvent system of methanol and phosphoric acid (0.1%, v/v) with a linear gradient from 30 to 100% methanol over a period of 14 min at a flow rate of 1 mL min−1. A diode array detector was used for signal recording. Extraction and product isolation were carried out according to Zühlke et al. (2017). On this basis, the supernatant of 10 to 20 centrifuged cultures (corresponding to 1 to 2 L volume) was extracted and the products were isolated as described for the products I-IV of BPA transformation (Zühlke et al. 2017). Usually, a bisphenol concentration of 0.006% was used to yield the highest product concentrations. This did not apply for BPZ where cells were exposed to 0.002% of substrate and for BPF where product IVBPF was only detected after applying 0.002% BPF.
Analytical methods for structure elucidation of transformation products
High-performance liquid chromatography-mass spectrometry (HPLC-MS) and gas chromatography-mass spectrometry (GC-MS) were performed on the equipment and with the separation conditions described earlier (Zühlke et al. 2017). Nuclear magnetic resonance (NMR) spectra of all products were recorded on a Bruker Avance-II instrument (Bruker Biospin GmbH, Rheinstetten, Germany) at 600.27 MHz (1H-NMR) and 150.1 MHz (13C-NMR) in MeOH-d4.
Determination of estrogenicity of bisphenols and transformation products
To determine estrogenic properties of certain transformation products and the bisphenol analogues from which they were formed, the Arxula adeninivorans estrogen screen kit (A-YES assay) from new_diagnostics GmbH (Freising, Germany) was used as described (Zühlke et al. 2016). Estrogenicity was determined at least in duplicate.
Results
In the present study, we investigated the biotransformation of seven BPA analogues, which differ from BPA by the addition or by the lack of substituents at the ring linking carbon bridge (as with BPAP, BPB, BPE, BPF, and BPZ) or at the phenol rings (as with BPC and BPPH). For the chemical structures, see middle column of Fig. 1.
Overview of detected and characterized products, with corresponding abbreviations used in the text, formed during the incubation of Cupriavidus basilensis SBUG 290 with eight different bisphenols. Products of BPA transformation refer to Zühlke et al. (2017). aDimer consisting of modified bisphenol-monomers with at least one ortho-quinonimine, an ortho-quinoid and an unmodified ring
Transformation rates of bisphenol A analogues
Biphenyl-grown cells of C. basilensis were able to transform all of these compounds but could not use them as substrates for growth. Using a concentration of 0.006%, transformation rates ranged between 6 and 98% within 216 h of incubation: BPC (98%) > BPB (62%) > BPE (31%) > BPF (6%), see Supplementary Figs. S1-S5. Rates for transformation of BPAP, BPZ, and BPPH were not determined due to their low solubility in the medium (Supplementary Figs. S3 and S5). By comparison, 85% of BPA was transformed using the same incubation conditions.
Transformation of bisphenol analogues to products—principle and overview
During transformation of seven bisphenol A analogues, 36 different metabolites were characterized biochemically and the structures for 24 of these products were identified (Supplementary Figs. S1-S5). HPLC analysis provided initial information on the structure of the products formed, which were then named according to the already characterized transformation products of BPA transformation (Zühlke et al. 2017). Additional abbreviations were used to indicate the structure of products, e.g., BP-OH for the ortho-hydroxylated product I (Fig. 1) or BPB-OH if the respective bisphenol is specified. Structure elucidation was based on HPLC, HPLC-MS, GS-MS, and/or NMR analysis, as well as comparison with already identified products.
Identification of transformation products of bisphenol analogues with unsubstituted phenol rings and different substituents at the carbon bridge
C. basilensis converted bisphenols with unsubstituted phenol rings and different substituents at the carbon bridge to three (BPAP, BPE, BPF) or up to four (BPB, BPZ) transformation products (Fig. 1). Analytical data of the transformation products were very similar to data on the characterized products of BPA transformation (Zühlke et al. 2017).
Identification of one-ring ortho-hydroxylated products (designated as products I; BP-OH)
During the incubation of C. basilensis with BPAP, BPB, BPE, BPF, and BPZ for 216 h, one product each was detected by HPLC analysis. These products have a similar UV-Vis spectrum compared to that of product IBPA (Zühlke et al. 2017), with two absorption maxima at around 220 and 280 nm (Table 1). By analogy, the products were named product I with subscript bisphenol analogue abbreviations. These products were extracted at pH 7 and they eluted 1.0 to 1.4 min earlier from the RP-18-column than did their parent compounds, suggesting a more hydrophilic character. Using mass spectrometry analyses, the mass differences of m/z 16 between the products and the parent compounds (Supplementary Table S1) indicated the introduction of a hydroxyl group. GC-MS analyses did not detect products I in each case, most probably due to low product amounts, or to insufficient methylation. However, for product I of BPA, BPAP, BPE, and BPZ transformation, the detection of mono-, di-, and/or trimethylated derivatives of the products confirmed the presence of an additional hydroxyl group, resulting in a trihydroxylated molecule. A second hydroxyl group in ortho-position to the phenolic hydroxyl group was verified by NMR analyses for product IBPE (Supplementary Table S2). Thus, it is postulated that C. basilensis ortho-hydroxylated one phenol ring of bisphenols (Fig. 1).
Identification of products ortho-hydroxylated at both phenol rings (designated as products II; BP-2xOH)
HPLC analyses of the culture supernatant of C. basilensis incubated with BPAP and BPZ revealed one product each with UV-Vis spectra having two maxima, one at around 220 nm and the other at around 280 nm (Table 1). Both the UV-Vis spectra and the shift in the retention time at the RP-18-column compared to the substrates (about 2 min earlier) showed strong similarity to the data of product IIBPA (Zühlke et al. 2017). Both products were extracted at pH 7. The GC-MS data showed a mass difference of m/z 32 between the products and the respective bisphenol analogues and pointed to another hydroxyl group on the aromatic ring system in addition to products I (Supplementary Table S3). Furthermore, both products II were detected in the HPLC-MS negative ion mode only (Supplementary Table S3) as was product IIBPA. Because of the similarity of the HPLC, GC-MS, and HPLC-MS data of products II with corresponding data of product IIBPA, whose structure was confirmed by NMR analyses (Zühlke et al. 2017), a structure of products ortho-hydroxylated on both phenol rings was postulated for product IIBPAP and product IIBPZ (Fig. 1).
Identification of ring fission products with lactone structure (designated as products III; BP-lactone)
During incubation of C. basilensis with BPAP, BPB, BPE, BPF, and BPZ, one product each eluted about 2–3 min earlier than the substrates from the RP-18-column. The UV-Vis spectra of the products with two absorption maxima at around 220 and 290–300 nm (Table 1) corresponded to that of product IIIBPA (Zühlke et al. 2017), and these products are therefore designated as products III with subscript bisphenol analogue abbreviations. HPLC-MS analyses of the products III, all present in the extract at pH 2, revealed a higher mass peak for the underivatized products III in comparison to the respective bisphenol analogues with a mass difference of m/z 46 (Supplementary Table S4). GC-MS analyses did not detect products III in each case, most probably due to low yield or insufficient methylation. However, for product III of BPB, BPE, and BPZ transformation, the detection of a mono- and dimethylated derivative of the respective product confirmed the presence of two groups, which can be methylated. Based on NMR analyses of product IIIBPA (Zühlke et al. 2017) and product IIIBPE (Supplementary Table S5), all products III could be identified as ring fission products with lactone structure (Fig. 1).
Identification of ortho-hydroxylated products with an acetamide substituent (designated as products IV; BP-acetamide)
After incubation of C. basilensis with BPB, BPE, and BPF, products IV were detected by HPLC analyses, which showed absorption maxima at around 230–240 and 280 nm and thus were similar to the ones of product IVBPA (Zühlke et al. 2017). These products also had reduced retention times (∆ 0.9–1.3 min) compared to the bisphenol analogues (Table 1). The difference of m/z 57 between the products IV, extracted at pH 7, and the bisphenol analogues (Supplementary Table S6) strongly hinted at an acetamide substituent as shown for product IVBPA (Zühlke et al. 2017). NMR analyses of product IVBPE (Supplementary Table S7) confirmed this structure. Thus, all products IV were postulated to be ortho-hydroxylated products with an acetamide substituent (Fig. 1).
Identification of dimers of modified bisphenol monomers (designated as products V; BP-dimer)
HPLC analysis of the culture supernatant during the incubation of C. basilensis with BPB revealed one product, named product VBPB, with a retention time shift similar to product VBPA (Zühlke et al. 2017). The similarity of the UV-Vis spectrum with absorption maxima at 226, 298, and 486 nm (Table 1) with that of product VBPA, which was postulated to be a dimer consisting of an ortho-quinonimine, an ortho-quinoid and an unmodified ring and further structures, suggests a similar overall structure for product VBPB. However, final structure elucidation was not possible by the methods available.
Identification of ring fission products with lactone structure hydroxylated on the remaining phenol ring (designated as products VI; BP-lactone-OH)
One product was only detected by HPLC analysis during the incubation of C. basilensis with BPZ and named product VIBPZ. The UV-Vis spectrum was similar to that of the ring fission product IIIBPZ, but product VIBPZ eluted 1 min earlier from the RP-18-column (Table 1). HPLC-MS analysis revealed a base ion peak at m/z 331 (positive ion mode) and m/z 329 (negative ion mode). The molecular mass of m/z 330 and the difference of m/z 16 between product VIBPZ and product IIIBPZ (Supplementary Table S8) pointed to a hydroxylated ring fission product with lactone structure. GC-MS analysis also detected one trimethylated derivative of product VIBPZ confirming the presence of an additional hydroxyl group. These structural data led to the identification of product VIBPZ as 4-[1-(3,4-dihydroxyphenyl)-cyclohexyl]-6-oxo-6H-pyran-2-carboxylic acid, a ring fission product with lactone structure, ortho-hydroxylated on the uncleaved phenol ring (Fig. 1).
Postulated structures and chemical names of all products identified are summarized in Supplementary Table S9. Products detected by HPLC but without any further information as to their structure are listed in Supplementary Table S10.
Identification of transformation products of bisphenol analogues with substituted phenol rings
C. basilensis converted BPC (ortho-substituted with a methyl group at each phenol ring) to five major products (Fig. 1; Supplementary Fig. S4). BPPH (ortho-substituted with an aromatic ring system at each phenol ring) was transformed to two major products (Supplementary Fig. S5), and another seven minor products were formed in very low amounts (Supplementary Table S10). Because of the low yields, these were not further characterized.
Identification of product IBPC (BPC-OH)
The UV-Vis spectrum of product IBPC was similar to that of product IBPA (Zühlke et al. 2017) and all other products I, with absorption maxima at 224 and 282 nm, and a retention time reduced by about 1 min compared to the parent compound (Table 2). These data pointed to a trihydroxylated derivative. HPLC, GC-MS (Supplementary Table S11), and NMR analyses (Supplementary Table S12) led to the proposed structure 5-[1-(4-hydroxy-3-methyl-phenyl)-1-methyl-ethyl]-3-methyl-benzene-1,2-diol for product IBPC, a product ortho-hydroxylated at one phenol ring (Fig. 1).
Identification of product 1BPC (BPC-CH2OH)
In contrast to product IBPC, where one phenol ring was hydroxylated, GC-MS and NMR analyses of product 1BPC pointed to an additional substituent at the methyl substituent of one phenol ring (Supplementary Tables S11-S13). The presence of no additional proton signals to those of BPC in the aromatic range and an additional methylene signal at 4.6 ppm in the 1H NMR spectrum and at 61.4 ppm in the 13C NMR spectrum led to the identification of product 1BPC as 2-(hydroxymethyl)-4-[1-(4-hydroxy-3-methyl-phenyl)-1-methyl-ethyl]-phenol (Fig. 1).
Identification of product 2BPC (BPC-COOH)
Product 2BPC was analyzed by HPLC-UV-Vis, HPLC-MS, GC-MS, and NMR. The UV-Vis spectrum has three maxima at 226, 286, and 314 nm (Table 2) and is readily distinguishable from the spectra of the substrate and all other products, but it is very similar to the spectrum of product 4BPC (see data below). The NMR analyses of product 2BPC showed the presence of only one aromatic methyl substituent (1H 2.12 ppm, 13C 16.5 ppm), and one aromatic carboxyl group (13C 174.3 ppm) formed from the second aromatic methyl substituent (Supplementary Tables S11, S14). All other NMR signals are comparable with those of the substrate. These data led to the description of product 2BPC as 2-hydroxy-5-[1-(4-hydroxy-3-methyl-phenyl)-1-methyl-ethyl]benzoic acid (Fig. 1).
Identification of product 3BPC (BPC-CH2OH-OH)
HPLC analysis and the UV-Vis spectrum of product 3BPC indicated an additional ortho-hydroxylation of the aromatic ring of product 1BPC (Table 2). Experiments determined a retention time shift of Δ1.9 min between BPC and product 1BPC. A similar retention time shift was seen between product IBPC and product 3BPC. Whereas a Δ1.9 min was thus indicative for the oxidation of the methyl group to CH2OH, a retention time shift of Δ0.9 min was characteristic for ring-ortho-hydroxylation between BPC and product IBPC, as well as between product 1BPC and product 3BPC (Table 2). HPLC and HPLC-MS (Supplementary Table S11) analyses led to the proposed structure of 3-(hydroxymethyl)-5-[1-(4-hydroxy-3-methyl-phenyl)-1-methyl-ethyl]benzene-1,2-diol for product 3BPC, a one-ring ortho-hydroxylated product with an additional hydroxylated methyl substituent at the same ring (Fig. 1).
Identification of product 4BPC (BPC-COOH-OH)
Because of low yield, product 4BPC was only characterized by HPLC-UV-Vis and by comparison of these data with those of product 2BPC. The UV-Vis spectrum of product 4BPC has three maxima at 226, 286, and 316 nm (Table 2) and is very similar to that of product 2BPC (BPC-COOH, see data above). The HPLC retention time shift of product 2BPC (BPC-COOH) compared to that of product 4BPC (BPC-COOH-OH) is the same as the HPLC retention time shift of product 1BPC (BPC-CH2OH) compared to that of product 3BPC (BPC-CH2OH-OH). Both are Δ0.9 min, indicating an additional hydroxyl group (see data above). Furthermore, the time shift of product 2BPC (BPC-COOH) compared to that of product 1BPC (BPC-CH2OH) is the same as the time shift of product 4BPC (BPC-COOH-OH) compared to that of product 3BPC (BPC-CH2OH-OH). Both are Δ 2.2 min, pointing to an oxidation of the hydroxymethyl group to a carboxyl group. This indicated that one methyl group of BPC was oxidized to a carboxyl group and one hydroxy group was introduced into the same aromatic ring. All data indicate comparable structure patterns for product 4BPC and product 2BPC so that product 4BPC can be described as 2,3-dihydroxy-5-[1-(4-hydroxy-3-methyl-phenyl)-1-methyl-ethyl]benzoic acid (Fig. 1).
Identification of product 2BPPH (BPPH-COOH)
The structure of product 2BPPH was analyzed by comparing the NMR and MS data with those of product 5BPPH (see below), and product 2BPC of BPC transformation, and the parent compound (Supplementary Tables S14-S18). The very complex NMR data of product 2BPPH showed the presence of one carboxyl group at the aromatic ring similar to that of product 5BPPH and product 2BPC. Furthermore, the signals of two additional aromatic rings similar to those in the substrate BPPH were detected. The HPLC-MS data (Supplementary Table S18) also support the structure of product 2BPPH as 2-hydroxy-5-[1-(4-hydroxy-3-phenyl-phenyl)-1-methyl-ethyl]benzoic acid (Fig. 1).
Identification of product 5BPPH (BPPH-2xCOOH)
Before considering product 2BPPH, we first determined the structure of product 5BPPH, because of its more easily identifiable NMR signals. The NMR data of product 5BPPH are very similar to those for the aromatic ring with the carboxyl group of product 2BPC (Supplementary Tables S14, S17), indicating the cleavage of both substituted phenyl rings and further degradation to carboxyl groups. The HPLC-MS data (Supplementary Table S18) fit this proposed structure, and therefore, product 5BPPH can be described as 5-[1-(3-carboxy-4-hydroxy-phenyl)-1-methyl-ethyl]-2-hydroxy-benzoic acid (Fig. 1).
Kinetics of product formation
Measured amounts of one-ring ortho-hydroxylated products I (BP-OH) reached their respective maximum within 24–48 h of incubation with BPB, BPC, BPE, and BPF. This was considerably postponed for products I of BPAP and BPZ. In all cases, concentrations of products I then decreased again. Similar kinetics were determined for product 1BPC (BPC-CH2OH) of BPC transformation, where a methyl substituent of one phenol ring was oxidized, and for product IIBPZ (BPZ-2xOH) of BPZ transformation, where both phenol rings are ortho-hydroxylated. The other products, e.g., products III (BP-lactone) or products IV (BP-acetamide) accumulated in the supernatant (Supplementary Figs. S1-S5).
Estrogenicity of some products and bisphenols
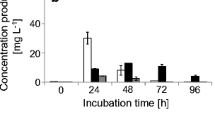
The A-YES-assay was used to determine the estrogenic activity of selected parent compounds and transformation products. In this assay, the human estrogen receptor expressed in yeast drives a phytase gene whose activity can be followed spectrophotometrically. While BPE induced phytase activity at a concentration of 1 mg L−1 (Fig. 2a) and BPC at 0.25 mg L−1 and are thus estrogenically active, phytase activity was not induced by BPPH (Fig. 2c). Those bisphenol-derived products that were formed by C. basilensis in sufficient amounts for purification were isolated and analyzed for their estrogenic properties. Neither product IBPE (BPE-OH) of BPE, product 1BPC (BPC-CH2OH) and product 3BPC (BPC-CH2OH-OH) of BPC nor product 2BPPH (BPPH-COOH) of BPPH exhibited estrogenic activities (Fig. 2a–c).
Reporter gene activity (phytase) of the transgenic yeast Arxula adeninivorans at different concentrations of a product IBPE (BPE-OH; open bars) compared to BPE (dark gray–filled bars), b product 1BPC (BPC-CH2OH; light gray–filled bars) and product 3BPC (BPC-CH2OH-OH; open bars) compared to BPC (dark gray–filled bars), and c product 2BPPH (BPPH-COOH; open bars) compared to BPPH (dark gray–filled bars)
Discussion
In this study, we extended previous investigations on the transformation of BPA by C. basilensis SBUG 290 (Zühlke et al. 2017) to five bisphenols with unsubstituted phenol rings (BPB, BPE, BPF, BPAP, and BPZ) and two with ring substituents (BPC and BPPH). These bisphenols all now have widespread industrial applications.
For relatively water-soluble bisphenols, biotransformation rates within 9 days (216 h) were determined by HPLC (Supplementary Figs. S1-S5). The extent of transformation by C. basilensis ranged from 98 to 6% (BPC > BPA > BPB > BPE > BPF). Data comparing microbial removal of bisphenols are scarce, and they are in contrast to our findings. For example, in the Gram-positive bacterium Arthrobacter sp., the removal efficiency was BPF > BPA, although the final degradation extents of BPF and BPA were rather similar (Ren et al. 2016). A similar order (BPA, BPB, BPF, BPS > BPE > BPC) was determined for biodegradation of bisphenols by the 4-tert-butylphenol utilizing Sphingobium fuliginis OMI (Ogata et al. 2012). In wastewater treatment plants (with mixed culture conditions), the order was BPAP > BPP > BPF > BPZ > BPC > BPS > BPB > BPA > BPE > BPAF (Wang et al. 2019). In contrast, Sun et al. (2017) estimated BPS > BPA > BPF in a wastewater treatment plant, while BPAP and BPE were persistent. In this latter case, besides biodegradation, also bioadsorption was taken into account as a modulating factor of these values. Bisphenols in river sediments were ranked by their biodegradability under aerobic conditions BPF >> BPA > BPC > BPB >> BPS (Ike et al. 2006), confirmed by Chang et al. (2014) with BPF > BPA > BPB. In contrast, using anaerobic conditions, the BPS degradation rate was clearly enhanced: BPF > BPS, BPA > BPE > BPB (Ike et al. 2006). Thus, at least, the very low conversion rate of BPF by C. basilensis does not correspond to the values estimated for microbial populations in rivers and wastewater. On the other hand, BPC and BPB removal is relatively efficient in C. basilensis SBUG 290. Doubtless, the removal rate of bisphenols will vary with the type of microorganism involved, whether the cells are growing or non-growing, the type of degradation pathway (primary attack at the aromatic ring or at the carbon bridge), the conditions of incubation (single species, e.g., in labs, or mixed culture, e.g., in waste water treatment plants), and the substrate concentration available.
Whereas the extents of biotransformation of the different bisphenol analogues by C. basilensis diverge, we found that the transformation mechanisms of those bisphenols with unsubstituted phenol rings but with varying substituents at the ring linking carbon bridge (BPB, BPE, BPF, BPAP, BPZ) are similar to the mode of BPA transformation. C. basilensis initially hydroxylated one phenol ring of these bisphenols in the ortho-position. The corresponding one-ring ortho-hydroxylated intermediates were substrates for (a) ortho-hydroxylation of the other phenol ring, (b) ring cleavage, and/or (c) transamination followed by acetylation or dimer formation (Fig. 3). All bisphenols with unsubstituted phenol rings were hydroxylated at one phenol ring, while intermediates hydroxylated at both phenol rings were detected for BPAP and BPZ only. In contrast to C. basilensis, the 4-tert-butylphenol utilizing S. fuliginis OMI is able to ortho-hydroxylate both phenol rings in BPB, BPE, BPC, and BPS (Ogata et al. 2012). Corresponding products of BPA were also formed by an undefined soil microbial consortium (Choi and Lee 2017).
Proposed pathway for the biotransformation of BPAP, BPB, BPE, BPF, and BPZ by Cupriavidus basilensis SBUG 290 via product I by (a) a second ortho-hydroxylation, (b) ring fission and combination of a second ortho-hydroxylation and ring fission, as well as (c) transamination followed by acetylation or dimerization
Likewise, all five bisphenols with unsubstituted phenol rings were subjected to ring cleavage analogously to the transformation of BPA by C. basilensis, resulting in products with lactone structure. Kinetics of product formation (Supplementary Fig. S3) indicated that the compound hydroxylated at both phenol rings was an additional substrate for ring cleavage only in the case of BPZ, but not for BPAP. Hydroxylation and subsequent cleavage of one phenol ring of bisphenols by C. basilensis SBUG 290 serve for detoxification (Zühlke et al. 2017), which has also been reported for corresponding products of p-tert-amylphenol transformation by this strain (Schlueter et al. 2014). This is due to the fact that C. basilensis SBUG 290 is not able to grow with these compounds, just as S. fuliginis OMI (Ogata et al. 2012), a strain able to cleave the aromatic ring system of BPA resulting in formation of monoaromatic compounds, is unable to grow on BPA. Aerobic soil biodegradation of BPA, BPAF, and BPS via ring cleavage has also been postulated (Choi and Lee 2017).
The third pathway - transamination of a one-ring ortho-hydroxylated intermediate followed by acetylation - was shown only for BPB, BPE, and BPF. The formation of these products was inhibited when the carbon bridge was substituted with an aromatic (BPAP) or alicyclic (BPZ) ring system or when the phenol rings were substituted. The formation of dimers of modified bisphenol monomers, in analogy to BPA, was only detected in the case of BPB.
All bisphenols with unsubstituted phenol rings differ in the substituents at the carbon bridge. Irrespective of the nature of these substituents, C. basilensis SBUG 290 could neither oxidize them nor cleave the bridge and thus could not use the bisphenols as carbon and energy source. This is in contrast to the situation with C. basilensis JF1 (Fischer et al. 2010), which introduces oxygen into the molecule followed by cleavage into 4-(2-propanol)-phenol and p-hydroquinone. Other bacterial strains that target the carbon bridge are often able to degrade bisphenols. A rearrangement of the bridge enabled cleavage of bisphenols (BPA) into monocyclic aromatic hydrocarbons as reported for strain MV1 (Lobos et al. 1992; Spivack et al. 1994), Sphingomonas bisphenolicum AO1, Sphingomonas sp. TTNP3 (Kolvenbach et al. 2007; Zhang et al. 2013b) or Shewanella haliotis MH137742 (de Santana et al. 2019).
Bisphenols with substituted phenol rings were substrates for novel transformation reactions compared to the biotransformation of BPA by C. basilensis SBUG 290. BPC und BPPH are characterized by additional ortho-substituents at their phenol rings. These substituents prevented ring cleavage, transamination followed by acetylation and dimerization, but at the same time served as additional targets for bacterial transformation. When an aromatic ring system is connected to the phenol ring, resulting in a biphenyl-like structure (BPPH), biphenyl-grown cells of C. basilensis SBUG 290 can cleave this substituted ring and oxidize it up to a carboxyl group (Fig. 4), probably in the same manner as biphenyl (Wesche et al. 2005). When the phenol ring is substituted with a methyl group in the ortho-position (BPC), one phenol ring of BPC was not only ortho-hydroxylated, but the methyl group was also oxidized (Fig. 4), as reported here for the first time. A corresponding reaction was observed for the anaerobic bacterium Castellaniella defragrans, where a limonene dehydrogenase hydroxylated the methyl group of cyclic monoterpenes (Puentes-Cala et al. 2018). Fungi hydroxylate methyl groups at alicyclic ring systems, too (Schlüter et al. 2019). In S. bisphenolicum AO1, a cytochrome P450 monooxygenase catalyzed hydroxylation of methyl substituents at the BPA carbon bridge (Sasaki et al. 2005a, b, 2008). This was not detected for C. basilensis SBUG 290, where oxidation of the methyl groups is restricted to the substituents at the phenol rings. However, another P450 system that oxygenates thiocarbamate herbicides was characterized in a Cupriavidus species (C. metallidurans: De Mot and Parret 2002; Warman et al. 2012), but neither its occurrence in C. basilensis nor its substrate selectivity is hitherto known.
Another mechanism for bisphenol transformation is the formation of conjugates. The formation of BPA glucosides has been shown in Aspergillus fumigatus (Yim et al. 2003), in Cunninghamella elegans (Keum et al. 2009) and in plants (Morohoshi et al. 2003; Nakajima et al. 2007), and BPA glucuronides were detected in rats (Inoue et al. 2001). Bacteria can form conjugates as well. While Bacillus amyloliquefaciens (Zühlke et al. 2016) converts various bisphenols into phosphate conjugates as a detoxification mechanism, no conjugates were detected in C. basilensis SBUG 290.
When bisphenols enter the microbial cell, but cannot be used as carbon and energy source, the cells need to detoxify these hazardous compounds. As a result, certain structure-biotransformation relationships become apparent (Fig. 5). All bisphenols with unsubstituted phenol rings are substrate for (a) hydroxylation and (b) ring cleavage with lactone formation (Fig. 5). When the carbon bridge is substituted with small ligands like methyl and ethyl groups and/or hydrogen, (c) transamination followed by acetylation was carried out, too. Larger substituents, like aromatic or alicyclic rings, prevented this pathway. Microbial dimerization of transformation products seems to be possible only when methyl and/or ethyl groups are present at the carbon bridge. Bisphenols with ortho-substituted phenol rings are not substrates either for ring cleavage or for transamination followed by acetylation or dimerization. Thus, the basic structure of the bisphenol molecule itself is, with one exception (ring-hydroxylation of BPC), not modified, and only (d) the substituents at the phenolic ring were oxidized.
Structure-biotransformation-relationship of bisphenols used as substrates for bacterial transformation by Cupriavidus basilensis SBUG 290. Different bisphenols are shown as simplified structures. Dashed lines indicate substituents, which vary in case of bisphenols with unsubstituted phenols (Fig. 1). Green: transformation reaction detected; red: transformation reaction not detected
Depending upon the structure of the bisphenols, C. basilensis SBUG 290 formed 5 products with BPA and 36 products with 7 bisphenol analogues as parent compounds. This may reflect what happens in the environment where a consortium of microorganisms may catalyze various reactions. Because of this, quantification of pollutants should also include important metabolites or derivatives as well as their toxic and endocrine activities. In terms of estrogenic activity, BPC is more estrogenic active than BPE and the non-estrogenic BPPH, while the transformation products of BPC, BPE, and BPPH tested did not exhibit estrogenic activity. Estrogenicity of bisphenols themselves correlates with their hydrophobicity, making BPAP, BPZ, BPB, or BPC more estrogenic than BPA and BPE or BPF (Kitamura et al. 2005; Kojima et al. 2019; Zühlke et al. 2016). Despite being very hydrophobic, BPPH is a larger molecule and might thus mask important coactivator regions (Heldring et al. 2007). This may also apply to its products. In contrast, the reduced estrogenic activity of the hydroxylated products of BPC and BPE might be due to reduced hydrophobicity resulting in a weaker affinity towards the hydrophobic ligand binding side of the ERα receptor (Coleman et al. 2003; Gao et al. 1999), which corresponds to previous results (Kitamura et al. 2005; Skledar and Masic 2016). In addition, lactone formation as well as transamination followed by acetylation may also lead to reduced endocrine activity as confirmed for the corresponding products of BPA transformation by C. basilensis SBUG 290 (Zühlke et al. 2017). Thus, C. basilensis SBUG 290 has a broad repertoire of transformation mechanisms, accepting not only bisphenols but also biphenyl, 4-chlorobiphenyl, dibenzofuran, 9H-carbazol, and p-tert-amylphenol (Becher et al. 2000; Hundt et al. 1998; Schlueter et al. 2014; Waldau et al. 2009; Wesche et al. 2005). As shown above for bisphenols, C. basilensis not only performs single biotransformation steps but also allows versatile transformation cascades for the individual bisphenol analogues leading to more hydrophilic products with decreased estrogenicity. Compared to reversible conjugate formation (Gonzalez-Gil et al. 2019; Zühlke et al. 2016), these products might be stable or serve as substrates for further degradation by other microorganisms in the environment.
Change history
16 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00253-021-11421-9
References
Barroso J (2011) Commission Directive 2011/8/EU of 28 January 2011 amending Directive 2002/72/EC as regards the restriction of use of bisphenol A in plastic infant feeding bottles. Off J Eur Union 26:11–14
Becher D, Specht M, Hammer E, Francke W, Schauer F (2000) Cometabolic degradation of dibenzofuran by biphenyl-cultivated Ralstonia sp strain SBUG 290. Appl Environ Microbiol 66(10):4528–4531. https://doi.org/10.1128/aem.66.10.4528-4531.2000
Cabaton N, Dumont C, Severin I, Perdu E, Zalko D, Cherkaoui-Malki M, Chagnon MC (2009) Genotoxic and endocrine activities of bis(hydroxyphenyl)methane (bisphenol F) and its derivatives in the HepG2 cell line. Toxicology 255(1–2):15–24. https://doi.org/10.1016/j.tox.2008.09.024
Chang B-V, Liu J-H, Liao C-S (2014) Aerobic degradation of bisphenol-A and its derivatives in river sediment. Environ Technol 35(4):416–424
Chen D, Kannan K, Tan HL, Zheng ZG, Feng YL, Wu Y, Widelka M (2016) Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity - a review. Environ Sci Technol 50(11):5438–5453. https://doi.org/10.1021/acs.est.5b05387
Choi YJ, Lee LS (2017) Aerobic soil biodegradation of bisphenol (BPA) alternatives bisphenol S and bisphenol AF compared to BPA. Environ Sci Technol 51(23):13698–13704. https://doi.org/10.1021/acs.est.7b03889
Coleman KP, Toscano WA, Wiese TE (2003) QSAR models of the in vitro estrogen activity of bisphenol A analogs. QSAR Comb Sci 22(1):78–88. https://doi.org/10.1002/qsar.200390008
De Mot R, Parret AH (2002) A novel class of self-sufficient cytochrome P450 monooxygenases in prokaryotes. Trends Microbiol 10(11):502–508. https://doi.org/10.1016/S0966-842X(02)02458-7
de Santana FS, Gracioso LH, Karolski B, Baltazar MDG, Mendes MA, do Nascimento CAO, Perpetuo EA (2019) Isolation of bisphenol A-tolerating/degrading Shewanella haliotis strain MH137742 from an estuarine environment. Appl Biochem Biotechnol 189(1):103–115. https://doi.org/10.1007/s12010-019-02989-0
Delfosse V, Grimaldi M, Pons J-L, Boulahtouf A, le Maire A, Cavailles V, Labesse G, Bourguet W, Balaguer P (2012) Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes. Proc Natl Acad Sci U S A 109(37):14930–14935. https://doi.org/10.1073/pnas.1203574109
EPA (2014) Bisphenol A alternatives in thermal paper - final report. https://www.epa.gov/saferchoice/publications-bpa-alternatives-thermal-paper-partnership
Fischer J, Kappelmeyer U, Kastner M, Schauer F, Heipieper HJ (2010) The degradation of bisphenol A by the newly isolated bacterium Cupriavidus basilensis JF1 can be enhanced by biostimulation with phenol. Int Biodeterior Biodegrad 64(4):324–330. https://doi.org/10.1016/j.ibiod.2010.03.007
Fromme H, Küchler T, Otto T, Pilz K, Müller J, Wenzel A (2002) Occurrence of phthalates and bisphenol A and F in the environment. Water Res 36(6):1429–1438. https://doi.org/10.1016/s0043-1354(01)00367-0
Gao H, Katzenellenbogen JA, Garg R, Hansch C (1999) Comparative QSAR analysis of estrogen receptor ligands. Chem Rev 99(3):723–744. https://doi.org/10.1021/cr980018g
Goldinger DM, Demierre AL, Zoller O, Rupp H, Reinhard H, Magnin R, Becker TW, Bourqui-Pittet M (2015) Endocrine activity of alternatives to BPA found in thermal paper in Switzerland. Regul Toxicol Pharmacol 71(3):453–462. https://doi.org/10.1016/j.yrtph.2015.01.002
Gonzalez-Gil L, Carballa M, Corvini PFX, Lema JM (2019) Reversibility of enzymatic reactions might limit biotransformation of organic micropollutants. Sci Total Environ 665:574–578. https://doi.org/10.1016/j.scitotenv.2019.02.143
Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA (2007) Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87(3):905–931. https://doi.org/10.1152/physrev.00026.2006
Hoekstra EJ, Simoneau C (2013) Release of bisphenol A from polycarbonate -a review. Crit Rev Food Sci Nutr 53(4):386–402. https://doi.org/10.1080/10408398.2010.536919
Hundt K, Wagner M, Becher D, Hammer E, Schauer F (1998) Effect of selected environmental factors on degradation and mineralization of biaryl compounds by the bacterium Ralstonia pickettii in soil and compost. Chemosphere 36(10):2321–2335. https://doi.org/10.1016/S0045-6535(97)10201-6
Ike M, Chen M, Danzl E, Sei K, Fujita M (2006) Biodegradation of a variety of bisphenols under aerobic and anaerobic conditions. Water Sci Technol 53(6):153–159
Inoue H, Yokota H, Makino T, Yuasa A, Kato S (2001) Bisphenol A glucuronide, a major metabolite in rat bile after liver perfusion. Drug Metab Dispos 29(8):1084–1087
Keum YS, Lee YH, Kim J-H (2009) Metabolism of methoxychlor by Cunninghamella elegans ATCC36112. J Agric Food Chem 57(17):7931–7937. https://doi.org/10.1021/jf902132j
Kitamura S, Suzuki T, Sanoh S, Kohta R, Jinno N, Sugihara K, Yoshihara S, Fujimoto N, Watanabe H, Ohta S (2005) Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol Sci 84(2):249–259. https://doi.org/10.1093/toxsci/kfi074
Kojima H, Takeuchi S, Sanoh S, Okuda K, Kitamura S, Uramaru N, Sugihara K, Yoshinari K (2019) Profiling of bisphenol A and eight its analogues on transcriptional activity via human nuclear receptors. Toxicology 413:48–55. https://doi.org/10.1016/j.tox.2018.12.001
Kolvenbach B, Schlaich N, Raoui Z, Prell J, Zühlke S, Schäffer A, Guengerich F, Corvini P (2007) Degradation pathway of bisphenol A: does ipso substitution apply to phenols containing a quaternary α-carbon structure in the para position? Appl Environ Microbiol 73(15):4776–4784. https://doi.org/10.1128/aem.00329-07
Lobos JH, Leib T, Su T-M (1992) Biodegradation of bisphenol A and other bisphenols by a gram-negative aerobic bacterium. Appl Environ Microbiol 58(6):1823–1831
Macczak A, Cyrkler M, Bukowska B, Michałowicz J (2017) Bisphenol A, bisphenol S, bisphenol F and bisphenol AF induce different oxidative stress and damage in human red blood cells (in vitro study). Toxicol in Vitro 41:143–149. https://doi.org/10.1016/j.tiv.2017.02.018
Michałowicz J (2014) Bisphenol A - sources, toxicity and biotransformation. Environ Toxicol Pharmacol 37(2):738–758. https://doi.org/10.1016/j.etap.2014.02.003
Michałowicz J, Mokra K, Bak A (2015) Bisphenol A and its analogs induce morphological and biochemical alterations in human peripheral blood mononuclear cells (in vitro study). Toxicol in Vitro 29(7):1464–1472. https://doi.org/10.1016/j.tiv.2015.05.012
Morohoshi K, Shiraishi F, Oshima Y, Koda T, Nakajima N, Edmonds JS, Morita M (2003) Synthesis and estrogenic activity of bisphenol A mono-and di-β-D-glucopyranosides, plant metabolites of bisphenol A. Environ Toxicol Chem 22(10):2275–2279
Nakajima N, Teramoto T, Kasai F, Sano T, Tamaoki M, Aono M, Kubo A, Kamada H, Azumi Y, Saji H (2007) Glycosylation of bisphenol A by freshwater microalgae. Chemosphere 69(6):934–941. https://doi.org/10.1016/j.chemosphere.2007.05.088
Ogata Y, Goda S, Toyama T, Sei K, Ike M (2012) The 4-tert-butylphenol-utilizing bacterium Sphingobium fuliginis OMI can degrade bisphenols via phenolic ring hydroxylation and meta-cleavage pathway. Environ Sci Technol 47(2):1017–1023. https://doi.org/10.1021/es303726h
Pivnenko K, Pedersen GA, Eriksson E, Astrup TF (2015) Bisphenol A and its structural analogues in household waste paper. Waste Manag 44:39–47. https://doi.org/10.1016/j.wasman.2015.07.017
Puentes-Cala E, Liebeke M, Markert S, Harder J (2018) Limonene dehydrogenase hydroxylates the allylic methyl group of cyclic monoterpenes in the anaerobic terpene degradation by Castellaniella defragrans. J Biol Chem 293(24):9520–9529. https://doi.org/10.1074/jbc.RA117.001557
Ren L, Jia Y, Ruth N, Shi Y, Wang J, Qiao C, Yan Y (2016) Biotransformations of bisphenols mediated by a novel Arthrobacter sp. strain YC-RL1. Appl Microbiol Biotechnol 100(4):1967–1976. https://doi.org/10.1007/s00253-015-7076-1
Sasaki M, Akahira A, Oshiman K-I, Tsuchido T, Matsumura Y (2005a) Purification of cytochrome P450 and ferredoxin, involved in bisphenol A degradation, from Sphingomonas sp. strain AO1. Appl Environ Microbiol 71(12):8024–8030. https://doi.org/10.1128/AEM.71.12.8024-8030.2005
Sasaki M, Maki J-I, Oshiman K-I, Matsumura Y, Tsuchido T (2005b) Biodegradation of bisphenol A by cells and cell lysate from Sphingomonas sp. strain AO1. Biodegradation 16(5):449–459
Sasaki M, Tsuchido T, Matsumura Y (2008) Molecular cloning and characterization of cytochrome P450 and ferredoxin genes involved in bisphenol A degradation in Sphingomonas bisphenolicum strain AO1. J Appl Microbiol 105(4):1158–1169. https://doi.org/10.1111/j.1365-2672.2008.03843.x
Schlueter R, Röder A, Czekalski N, Gliesche D, Mikolasch A, Schauer F (2014) Novel mechanisms of biotransformation of p-tert-amylphenol by bacteria and fungi with special degradation abilities and simultaneous detoxification of the disinfectant. Appl Microbiol Biotechnol 98(1):373–384. https://doi.org/10.1007/s00253-013-5312-0
Schlüter R, Dallinger A, Kabisch J, Duldhardt I, Schauer F (2019) Fungal biotransformation of short-chain n-alkylcycloalkanes. Appl Microbiol Biotechnol 103(10):4137–4151. https://doi.org/10.1007/s00253-019-09749-4
Skledar DG, Masic LP (2016) Bisphenol A and its analogs: do their metabolites have endocrine activity? Environ Toxicol Pharmacol 47:182–199. https://doi.org/10.1016/j.etap.2016.09.014
Spivack J, Leib T, Lobos J (1994) Novel pathway for bacterial metabolism of bisphenol A. Rearrangements and stilbene cleavage in bisphenol A metabolism. J Biol Chem 269(10):7323–7329
Stope MB, Becher D, Hammer E, Schauer F (2002) Cometabolic ring fission of dibenzofuran by Gram-negative and Gram-positive biphenyl-utilizing bacteria. Appl Microbiol Biotechnol 59(1):62–67. https://doi.org/10.1007/s00253-0002-0979-7
Sun Q, Wang Y, Li Y, Ashfaq M, Dai L, Xie X, Yu C-P (2017) Fate and mass balance of bisphenol analogues in wastewater treatment plants in Xiamen City, China. Environ Pollut 225:542–549
Usman A, Ahmad M (2016) From BPA to its analogues: is it a safe journey? Chemosphere 158:131–142. https://doi.org/10.1016/j.chemosphere.2016.05.070
Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV (2007) Human exposure to bisphenol A (BPA). Reprod Toxicol 24(2):139–177
Waldau D, Methling K, Mikolasch A, Schauer F (2009) Characterization of new oxidation products of 9H-carbazole and structure related compounds by biphenyl-utilizing bacteria. Appl Microbiol Biotechnol 81(6):1023–1031. https://doi.org/10.1007/s00253-008-1723-8
Wang H, Liu Z-H, Zhang J, Huang R-P, Yin H, Dang Z, Wu P-X, Liu Y (2019) Insights into removal mechanisms of bisphenol A and its analogues in municipal wastewater treatment plants. Sci Total Environ 692:107–116. https://doi.org/10.1016/j.scitotenv.2019.07.134
Warman AJ, Robinson JW, Luciakova D, Lawrence AD, Marshall KR, Warren MJ, Cheesman MR, Rigby SE, Munro AW, McLean KJ (2012) Characterization of Cupriavidus metallidurans CYP116B1–A thiocarbamate herbicide oxygenating P450–phthalate dioxygenase reductase fusion protein. FEBS J 279(9):1675–1693. https://doi.org/10.1111/j.1742-4658.2012.08543.x
Wesche J, Hammer E, Becher D, Burchhardt G, Schauer F (2005) The bphC gene-encoded 2,3-dihydroxybiphenyl-1,2-dioxygenase is involved in complete degradation of dibenzofuran by the biphenyl-degrading bacterium Ralstonia sp SBUG 290. J Appl Microbiol 98(3):635–645. https://doi.org/10.1111/j.1365-2672.2004.02489.x
Yim S-H, Kim HJ, Lee I-S (2003) Microbial metabolism of the environmental estrogen bisphenol A. Arch Pharm Res 26(10):805–808
Zhang L, Fang P, Yang LJ, Zhang J, Wang X (2013a) Rapid method for the separation and recovery of endocrine-disrupting compound bisphenol AP from wastewater. Langmuir 29(12):3968–3975. https://doi.org/10.1021/la304792m
Zhang W, Yin K, Chen L (2013b) Bacteria-mediated bisphenol A degradation. Appl Microbiol Biotechnol 97(13):5681–5689. https://doi.org/10.1007/s00253-013-4949-z
Zühlke M-K, Schlüter R, Henning A-K, Lipka M, Mikolasch A, Schumann P, Giersberg M, Kunze G, Schauer F (2016) A novel mechanism of conjugate formation of bisphenol A and its analogues by Bacillus amyloliquefaciens: detoxification and reduction of estrogenicity of bisphenols. Int Biodeterior Biodegrad 109:165–173. https://doi.org/10.1016/j.ibiod.2016.01.019
Zühlke M-K, Schlüter R, Mikolasch A, Zühlke D, Giersberg M, Schindler H, Henning A-K, Frenzel H, Hammer E, Lalk M (2017) Biotransformation and reduction of estrogenicity of bisphenol A by the biphenyl-degrading Cupriavidus basilensis. Appl Microbiol Biotechnol 101(9):3743–3758. https://doi.org/10.1007/s00253-016-8061-z
Acknowledgments
The authors thank Robert Jack for reviewing the manuscript and Stephanie Markert for helpful discussions.
Funding
Open access funding enabled and organized by Projekt DEAL. Funding provided by Universität Greifswald. MKZ received financial support from the European Social Fund (Landesgraduiertenstipendium; Mecklenburg-Vorpommern, Germany) and the Institute of Marine Biotechnology e.V. (IMaB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This work is dedicated by all colleagues to the late Frieder Schauer.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective Open Access order.
Electronic supplementary material
ESM 1
(PDF 969 kb).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zühlke, MK., Schlüter, R., Mikolasch, A. et al. Biotransformation of bisphenol A analogues by the biphenyl-degrading bacterium Cupriavidus basilensis - a structure-biotransformation relationship. Appl Microbiol Biotechnol 104, 3569–3583 (2020). https://doi.org/10.1007/s00253-020-10406-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10406-4