Abstract
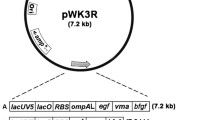
Inteins, also known as “protein introns,” have been found to be present in many microbial species and widely employed for the expression and purification of recombinant proteins in Escherichia coli. However, interestingly, until now there has not been much information on the identification and application of inteins to protein expression in Bacillus subtilis. In this article, for the first time, despite the likelihood of absence of inteins in B. subtilis, this bacterium was shown to be able to facilitate auto-catalytic cleavages of fusions formed between inteins and recombinant proteins. Employing a construct expressing the intein, Ssp DnaB, (DnaB), which was fused at its N-terminus with the cellulose-binding domain (CellBD) of an endoglucanase encoded by the cenA gene of Cellulomonas fimi, the construct was demonstrated to be capable of mediating intracellular expression of basic fibroblast growth factor (bFGF), followed by auto-processing of the CellBD-DnaB-bFGF fusion to result in bFGF possessing the 146-residue authentic structure. The mentioned fusion was shown to result in a high yield of 84 mg l−1 of biologically active bFGF. Future work in improving the growth of B. subtilis may enable the use of this bacterium, working in cooperation with inteins, to result in a new platform for efficient expression of valuable proteins.





Similar content being viewed by others
References
Akita S, Akino K, Imaizumi T, Hirano A (2008) Basic fibroblast growth factor accelerates and improves second-degree burn wound healing. Wound Repair Regen 16:635–641
Akita S, Akino K, Hirano A (2013) Basic fibroblast growth factor in scarless wound healing. Adv Wound Care 2:44–49
Amitai G, Callahan BP, Stanger MJ, Belfort G, Belfort M (2009) Modulation of intein activity by its neighboring extein substrates. Proc Natl Acad Sci U S A 106:11005–11010
Baneyx F, Mujacic M (2004) Recombinant protein folding and misfolding in Escherichia coli. Nat Biotech 22:1399–1408
Bentley WE, Mirjalili N, Andersen DC, Davis RH, Kompala DS, Bentley WE (2009) Plasmid-encoded protein: the principal factor in the “metabolic burden” associated with recombinant bacteria. Biotechnology Bioengineering, 1990. Biotechnol Bioeng 102:1284–1297 discussion 83
Chen JQ, Fu G, Gai YM, Zheng P, Dw Z, Wen JP (2015) Combinatorial Sec pathway analysis for improved heterologous protein secretion in Bacillus subtilis: identification of bottlenecks by systematic gene overexpression. Microb Cell Factories 14:92
Cottingham IR, Millar A, Emslie E, Colman A, Schnieke AE, McKee C (2001) A method for the amidation of recombinant peptides expressed as intein fusion proteins in Escherichia coli. Nat Biotechnol 19:974–977
Din N, Damude HG, Gilkes NR, Miller RC, Warren RA, Kilburn DG (1994) C1-Cx revisited: intramolecular synergism in a cellulase. Proc Natl Acad Sci U S A 91:11383–11387
Elleuche S, Pöggeler S (2010) Inteins, valuable genetic elements in molecular biology and biotechnology. Appl Microbiol Biotechnol 87:479–489
Esipov RS, Makarov DA, Stepanenko VN, Kostromina MA, Muravyova TI, Andreev YA, Dyachenko IA, Kozlov SA, Grishin EV (2017) Pilot production of the recombinant peptide toxin of Heteractis crispa as a potential analgesic by intein-mediated technology. Protein Expr Purif 145:71–76
Fu ZB, Ng KL, Lam TL, Wong WKR (2005) Cell death caused by hyper-expression of a secretory exoglucanase in Escherichia coli. Protein Expr Purif 42:67–77
Fu ZB, Ng KL, Lam CC, Leung KC, Yip WH, Wong WKR (2006) A two-stage refinement approach for the enhancement of excretory production of an exoglucanase from Escherichia coli. Protein Expr Purif 48:205–214
Gemini (2018) Human basic fibroblast growth factor-147 (bFGF-147). https://www.gembio.com/product/human-basic-fibroblast-growth-factor-147-bfgf-147. Accessed 07 Feb 2018
Greenwood JM, Gilkes NR, Miller RC, Kilburn DG, Warren RAJ (1994) Purification and processing of cellulose-binding domain-alkaline phosphatase fusion proteins. Biotechnol Bioeng 44:1295–1305
He Q, Fu AY, Li TJ (2015) Expression and one-step purification of the antimicrobial peptide cathelicidin-BF using the intein system in Bacillus subtilis. J Ind Microbiol Biotechnol 42:647–653
He WW, Mu WM, Jiang B, Yan X, Zhang T (2016) Construction of a food grade recombinant Bacillus subtilis based on replicative plasmids with an auxotrophic marker for biotransformation of d-fructose to d-allulose. J Agric Food Chem 64:3243–3250
Hirata R, Ohsumk Y, Nakano A, Kawasaki H, Suzuki K, Anraku Y (1990) Molecular structure of a gene, VMA1, encoding the catalytic subunit of H(+)-translocating adenosine triphosphatase from vacuolar membranes of Saccharomyces cerevisiae. J Biol Chem 265:6726–6733
Hoppenreijs VP, Pels E, Vrensen GF, Treffers WF (1994) Basic fibroblast growth factor stimulates corneal endothelial cell growth and endothelial wound healing of human corneas. Invest Ophthalmol Vis Sci 35:931–944
Ikawa K, Araki H, Tsujino Y, Hayashi Y, Igarashi K, Hatada Y, Hagihara H, Ozawa T, Ozaki K, Kobayashi T, Ito S (1998) Hyperexpression of the gene for a Bacillus α-amylase in Bacillus subtilis cells: enzymatic properties and crystallization of the recombinant enzyme. Biosci Biotechnol Biochem 62:1720–1725
Ingham AB, Sproat KW, Tizard MLV, Moore RJ (2005) A versatile system for the expression of nonmodified bacteriocins in Escherichia coli. J Appl Microbiol 98:676–683
Jacobs MF, Andersen JB, Borchert TV, Kontinen VP (1995) Identification of a Bacillus subtilis secretion mutant using a beta-galactosidase screening procedure. Microbiology 141(Pt 7):1771–1779
Kang Z, Yang S, Du GC, Chen J (2014) Molecular engineering of secretory machinery components for high-level secretion of proteins in Bacillus species. J Ind Microbiol Biotechnol 41:1599–1607
Krejci E, Pesevski Z, Nanka O, Sedmera D (2016) Physiological role of FGF signaling in growth and remodeling of developing cardiovascular system. Physiol Res 65:425
Kurland CG, Dong HJ (1996) Bacterial growth inhibition by overproduction of protein. Mol Microbiol 21:1–4
Kwon EY, Kim KM, Kim MK, Lee IY, Kim BS (2011) Production of nattokinase by high cell density fed-batch culture of Bacillus subtilis. Bioprocess Biosyst Eng 34:789–793
Kwong KWY, Wong WKR (2013) A revolutionary approach facilitating co-expression of authentic human epidermal growth factor and basic fibroblast growth factor in both cytoplasm and culture medium of Escherichia coli. Appl Microbiol Biotechnol 97:9071–9080
Kwong KWY, Ng KL, Lam CC, Wang YY, Wong WKR (2013) Authentic human basic fibroblast growth factor produced by secretion in Bacillus subtilis. Appl Microbiol Biotechnol 97:6803–6811
Kwong KWY, Ng KL, Wong WKR (2016a) Engineering versatile protein expression systems mediated by inteins in Escherichia coli. Appl Microbiol Biotechnol 100:255–262
Kwong KWY, Sivakumar T, Wong WKR (2016b) Intein mediated hyper-production of authentic human basic fibroblast growth factor in Escherichia coli. Sci Rep 6:33948
Lam TL, Wong RSC, Wong WKR (1997) Enhancement of extracellular production of a Cellulomonas fimi exoglucanase in Escherichia coli by the reduction of promoter strength. Enzym Microb Technol 20:482–488
Lam KHE, Chow KC, Wong WKR (1998) Construction of an efficient Bacillus subtilis system for extracellular production of heterologous proteins. J Biotechnol 63:167–177
Li WF, Zhou XX, Lu P (2004) Bottlenecks in the expression and secretion of heterologous proteins in Bacillus subtilis. Res Microbiol 155:605–610
Ng KL, Lam CC, Kwong WY, Dik WN, Lai CY, Au Yong LW, Qian PY, Wong WKR (2016) Enhancement of fish growth employing feed supplemented with recombinant fish growth hormone expressed in Bacillus subtilis. Res J Biotechnol 11:1
Nijland R, Kuipers OP (2008) Optimization of protein secretion by Bacillus subtilis. Recent Pat Biotechnol 2:79–87
Okabe K, Hayashi R, Aramaki-Hattori N, Sakamoto Y, Kishi K (2013) Wound treatment using growth factors. Mod Plast Surg 3:108–112
Palva I (1982) Molecular cloning of α-amylase gene from Bacillus amyloliquefaciens and its expression in B. subtilis. Gene 19:81–87
Peprotech (2018) Recombinant human FGF-basic (154 a.a.) https://www.peprotech.com/peprogmp-recombinant-human-fgf-basic. Accessed 07 Feb 2018
Phan TTP, Nguyen HD, Schumann W (2012) Development of a strong intracellular expression system for Bacillus subtilis by optimizing promoter elements. J Biotechnol 157:167–172
Prospec (2018) FGF 2 human. https://www.prospecbio.com/fgf-2_human/. Accessed 07 Feb 2018
Saida F, Uzan M, Odaert B, Bontems F (2006) Expression of highly toxic genes in E. coli: special strategies and genetic tools. Curr Protein Pept Sci 7:47–56
Setrerrahmane S, Zhang Y, Dai GZ, Lv J, Tan SH (2014) Efficient production of native lunasin with correct N-terminal processing by using the pH-induced self-cleavable Ssp DnaB mini-intein system in Escherichia coli. Appl Biochem Biotechnol 174:612–622
Shah NH, Muir TW (2014) Inteins: nature’s gift to protein chemists. Chem Sci 5:446–461
Shenandoah (2018) Human FGF-basic 154 aa (FGF2), https://www.shenandoah-bt.com/Human_FGF-basic_154.html. Accessed 07 Feb 2018
Sivakesava S, Xu ZN, Chen YH, Hackett J, Huang RC, Lam E, Lam TL, Siu KL, Wong WKR (1999) Production of excreted human epidermal growth factor (hEGF) by an efficient recombinant Escherichia coli system. Process Biochem 34:893–900
Sivakumar T, Lai CYN, Lam CC, Hu XH, Wang H, Ng KL, Wong WKR (2018) Purification of authentic human basic fibroblast growth factor expressed in both the cytoplasm and culture medium of Escherichia coli. Int J Chromatogr Sep Tech. https://doi.org/10.29011/2577-218X.00015
Starokadomskyy PL, Okunev OV, Irodov DM, Kordium VA (2008) Utilization of protein splicing for purification of the human growth hormone. Mol Biol 42:966–972
Taguchi S, Ooi T, Mizuno K, Matsusaki H (2015) Advances and needs for endotoxin-free production strains. Appl Microbiol Biotechnol 99:9349–9360
Terpe K (2006) Overview of bacterial expression systems for heterologous protein production: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol 72:211–222
Unger EF, Goncalves L, Epstein SE, Chew EY, Trapnell CB, Cannon RO, Quyyumi AA, Loscalzo F, Stiber JA (2000) Effects of a single intracoronary injection of basic fibroblast growth factor in stable angina pectoris. Am J Cardiol 85:1414–1419
Vicario-Abejon C, Johe KK, Hazel TG, Collazo D, McKay RD (1995) Functions of basic fibroblast growth factor and neurotrophins in the differentiation of hippocampal neurons. Neuron 15:105–114
Wan W, Wang DM, Gao XL, Hong J (2011) Expression of family 3 cellulose-binding module (CBM3) as an affinity tag for recombinant proteins in yeast. Appl Microbiol Biotechnol 91:789–798
Wang XY, Quinn PJ (2010) Endotoxins: lipopolysaccharides of gram-negative bacteria. Endotoxins: structure, function and recognition. Springer
Wang YY, Fu ZB, Ng KL, Lam CC, Chan AK, Sze KF, Wong WKR (2011) Enhancement of excretory production of an exoglucanase from Escherichia coli with phage shock protein A (PspA) overexpression. J Microbiol Biotechnol 21:637–645
Westers L, Westers H, Quax WJ (2004) Bacillus subtilis as cell factory for pharmaceutical proteins: a biotechnological approach to optimize the host organism. Biochim Biophys Acta 1694:299–310
Wong WK, Gerhard B, Guo ZM, Kilburn DG, Anthony R, Warren J, Miller RC (1986) Characterization and structure of an endoglucanase gene cenA of Cellulomonas fimi. Gene 44:315–324
Wu WY, Miller KD, Coolbaugh M, Wood DW (2011) Intein-mediated one-step purification of Escherichia coli secreted human antibody fragments. Protein Expr Purif 76:221–228
Xu ZN, LIU g CPL, Wong WKR (2000) Factors influencing excretive production of human epidermal growth factor (hEGF) with recombinant Escherichia coli K12 system. Bioprocess Biosyst Eng 23:669–674
Yang MM, Zhang WW, Ji SY, Cao PH, Chen YL, Zhao X (2013) Generation of an artificial double promoter for protein expression in Bacillus subtilis through a promoter trap system. PLoS One 8:e56321
Ye RQ, Kim JH, Kim BG, Szarka S, Sihota E, Wong SL (1999) High-level secretory production of intact, biologically active staphylokinase from Bacillus subtilis. Biotechnol Bioeng 62:87–96
Zhang YJ, Zhang K, Wan Y, Zi J, Wang Y, Wang J, Wang LL, Xue XC (2015) A pH-induced, intein-mediated expression and purification of recombinant human epidermal growth factor in Escherichia coli. Biotechnol Prog 31:758–764
Acknowledgements
We thank Biosciences Central Research Facility at HKUST for their mass spectrometry services.
Funding
This study was funded by RGC project: GRF16101515 and Research Contracts: 13142580CLIL07W011 and 1617219-0.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Hu, X., Lai, C.Y.N., Sivakumar, T. et al. Novel strategy for expression of authentic and bioactive human basic fibroblast growth factor in Bacillus subtilis. Appl Microbiol Biotechnol 102, 7061–7069 (2018). https://doi.org/10.1007/s00253-018-9176-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9176-1