Abstract
The increasing use of biobased fuels and fuel additives can potentially change the typical fuel-related contamination in soil and groundwater. Anaerobic biotransformation of the biofuel additive ethyl tert-butyl ether (EtBE), as well as of methyl tert-butyl ether (MtBE), benzene, and tert-butyl alcohol (TBA, a possible oxygenate metabolite), was studied at an industrially contaminated site and in the laboratory. Analysis of groundwater samples indicated that in the field MtBE was degraded, yielding TBA as major product. In batch microcosms, MtBE was degraded under different conditions: unamended control, with medium without added electron acceptors, or with ferrihydrite or sulfate (with or without medium) as electron acceptor, respectively. Degradation of EtBE was not observed under any of these conditions tested. TBA was partially depleted in parallel with MtBE. Results of microcosm experiments with MtBE substrate analogues, i.e., syringate, vanillate, or ferulate, were in line with the hypothesis that the observed TBA degradation is a cometabolic process. Microcosms with ferulate, syringate, isopropanol, or diethyl ether showed EtBE depletion up to 86.5% of the initial concentration after 83 days. Benzene was degraded in the unamended controls, with medium without added electron acceptors and with ferrihydrite, sulfate, or chlorate as electron acceptor, respectively. In the presence of nitrate, benzene was only degraded after addition of an anaerobic benzene-degrading community. Nitrate and chlorate hindered MtBE, EtBE, and TBA degradation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of renewable resources, such as bioethanol, as basis for automotive fuels is stimulated under the European Biofuels Directive (EC 2018) to reduce greenhouse gas emissions. Both fossil-based and biobased fuels may enter the groundwater through incidents and spillages. An overview compiled by Concawe (division of the European Petroleum Refiners Association) showed that oxygenates are present in groundwater, drinking water, surface water, runoff water, precipitation, and air in the European environment (Stupp et al. 2012). The oxygenate methyl tert-butyl ether (MtBE) is a synthetic volatile organic compound added to gasoline to increase its octane number, i.e., performance. Due to its high water solubility, low odor threshold, and health concerns, MtBE contamination is a widespread problem that requires remediation (Deeb et al. 2003; van Wezel et al. 2009). Although the tertiary carbon structure and stable ether bond suggest that the MtBE molecule will not be readily degraded by microorganisms under anaerobic conditions, degradation has been observed under a variety of redox conditions, including methanogenic, nitrate-reducing, manganese-reducing, iron-reducing, and sulfate-reducing conditions (Bradley et al. 2001a; Finneran and Lovley 2001; Fischer et al. 2005; Häggblom et al. 2007; Hyman 2013; Liu et al. 2016; Mormile et al. 1994; Pruden et al. 2005; Somsamak et al. 2005; Somsamak et al. 2006; Suflita and Mormile 1993; Waul et al. 2009; Wilson et al. 2005). Key metabolites of aerobic MtBE degradation are tert-butyl alcohol (TBA), tert-butyl formate (TBF) and 2-hydroxy isobutyric acid (HIBA) (Fiorenza and Rifai 2003). The detection of TBA indicates that ether bond cleavage is the initial step in anaerobic MtBE degradation. It has been found that under anoxic conditions TBA is often a recalcitrant product of MtBE degradation (Schmidt et al. 2004). However, mineralization of MtBE without accumulation of TBA has been shown under denitrifying and oxic conditions (Bradley et al. 2001b).
In the past years, MtBE has been partially replaced by ethyl tert-butyl ether (EtBE), a biofuel oxygenate synthesized from (bio)ethanol and isobutylene. Due to its high solubility and persistence, EtBE disperses rapidly in the environment (Le Digabel et al. 2013). Little is known about anaerobic EtBE degradation. Several studies have reported lack of any anaerobic degradation of EtBE (Hernandez-Perez et al. 2001; Mormile et al. 1994; Somsamak et al. 2001). In contrast, Yeh and Novak demonstrated EtBE degradation in soils under denitrifying and methanogenic conditions (Yeh and Novak 1994). Compound-specific stable isotope analysis (CSIA) revealed insignificant carbon isotope fractionation, and low hydrogen isotope fractionation up to + 14‰ along an anoxic EtBE plume at a contaminated field site in the Central West of Spain suggesting anaerobic biodegradation of EtBE (Bombach et al. 2015).
In addition to TBA being produced as metabolite during MtBE and EtBE degradation, it is also frequently used as a polar organic solvent in flavors, perfumes, or paint removers. TBA is highly soluble in water and is considered as a widespread contamination problem (Deeb et al. 2003). Several studies in the absence or presence of MtBE have concluded that TBA is either degraded with rates ranging between <0.14 and 2 μM/day/g dry soil or persists in anoxic groundwater (Bradley et al. 1999; Mormile et al. 1994; Somsamak et al. 2001; Yeh and Novak 1994). Under methanogenic conditions no TBA degradation has been observed (Bradley et al. 2002; Mormile et al. 1994). TBA consumption in anoxic microcosms with iron(III) as electron acceptor has been demonstrated in microcosms (Finneran and Lovley 2001). A direct link between TBA oxidation and electron acceptor reduction has, however, not been demonstrated. Aerobically, TBA can be degraded via 2-methyl-2-hydroxy-1-propanol, HIBA, 2-propanol, acetone, and hydroxyacetone to carbon dioxide and water.
Benzene is a natural constituent of crude oil, has a high octane number, and is commonly present in (bio)fuel blends (Chin and Batterman 2012). Massive production and use of benzene combined with its high mobility, relatively low anaerobic biodegradation rates, and carcinogenicity make benzene one of the most widespread groundwater contaminants of concern. Therefore, benzene is typically considered as risk determining compound in fuels. Anaerobic benzene biodegradation has been described under denitrifying, iron-reducing, sulfate-reducing, and methanogenic conditions (Coates et al. 2002).
Several studies have investigated the effects of mixtures of fossil-based and biobased fuels on degradation of the single components. Both antagonistic and synergistic effects have been reported during degradation of mixtures of benzene, toluene, ethylbenzene, xylene (BTEX), and MtBE. The presence of BTEX was shown to enhance aerobic MtBE degradation by a mixed microbial culture under continuous flow conditions (Sedran et al. 2002). On the contrary, biodegradation of MtBE in the subsurface was inhibited largely by the presence of ethylbenzenes and xylenes and partially inhibited by benzene and toluene until MtBE had migrated beyond the BTEX plume (Deeb et al. 2001). The preferential utilization of ethanol under aerobic, denitrifying, iron-reducing, sulfate-reducing, and methanogenic conditions resulted in a negative effect on BTEX degradation (Corseuil et al. 1998; Lovanh et al. 2002; Ruiz-Aguilar et al. 2002). Bioaugmentation with a benzene-enriched methanogenic consortium enhanced anaerobic benzene degradation in a mixture of BTEX and ethanol (Da Silva and Alvarez 2004).
The concurrence of MtBE, EtBE, TBA, and benzene may affect the microbial degradation potential for both fossil-based and biobased components. Nevertheless, to our knowledge, studies on the anaerobic degradation of a mixture of MtBE, EtBE, TBA, and benzene have not been reported. In this study, anoxic groundwater from a contaminated location was used to determine the microbial degradation potential for a mixture of benzene, MtBE, EtBE, and TBA, the latter being a degradation product of MtBE and EtBE.
The aims of the present study were therefore to (i) determine the potential of an indigenous microbial community from a contaminated site for the anaerobic degradation of MtBE, EtBE, TBA, and benzene in a mixture; (ii) determine the effect of electron acceptors nitrate, sulfate, amorphous 2-line ferrihydrite, and chlorate on the anaerobic degradation; and (iii) determine anaerobic TBA degradation and stimulate this process using MtBE and EtBE substrate analogues and potential metabolites as substrates.
Materials and methods
Field site, modeling, and sampling procedures
Experiments were focused on MtBE, EtBE, TBA, and benzene contaminated groundwater at an industrial site. At this site, the redox conditions varied from iron reducing to methanogenic, and the pH was around 7 (Table S1). At the impacted area, the contamination was located between 9 and 20 m depth below the surface in an anoxic aquifer. Two source zones of MtBE were present with a maximum MtBE concentration of about 4000 μM. The highest concentrations of EtBE, TBA, and benzene detected in 2009 were 1 μM (88 μg/l), 130 μM (9636 μg/l), and 260 μM (20,309 μg/l), respectively. The average MtBE, TBA, and benzene concentrations per layer in 2009, 2013, and 2015 are given in Table S2. The data for MtBE, TBA, and benzene depletion at the field site were compared with an existing hydrological model and contaminant fate and transport model that was calibrated using field data on measured heads, porosity, organic matter content, and long-term diver data on groundwater fluctuations (Harbaugh 2005; Harbaugh et al. 2017).
Groundwater was collected from a monitoring well located in one of the MtBE source zones, in gas tight bottles of 1 litre. The bottles were filled completely with groundwater to prevent the introduction of oxygen, were transported in a cool box with ice the same day to the laboratory, and were stored at 4 °C for preparation of microcosms within 14 days after sampling.
Microcosm incubations
Microcosms were prepared under a 80/20% N2/CO2 (v/v) flow in 250-ml serum bottles (Glasgerätebau Ochs GmbH, Bovenden, Germany) and crimp sealed with viton rubber stoppers (Rubber BV, Hilversum, The Netherlands) and aluminum crimp caps (Grace, MD, USA). Traces of O2 were stripped from the N2/CO2 gas as described previously (van der Waals et al. 2017). The microcosms contained 90 ml of groundwater contaminated with MtBE, TBA, and benzene and amended with 50 μM EtBE added from a 20 mM anoxic, autoclaved stock solution. One series of microcosms with different electron acceptors was amended with 10 ml of 10-fold concentrated anoxic, sterile mineral medium with vitamins that was prepared as described previously, but without the addition of nitrate (van der Waals et al. 2017). No medium was added to a second series of microcosms with the addition of only electron acceptors. Electron acceptors, i.e., nitrate (NaNO3), chlorate (NaClO3), iron (amorphous 2-line ferrihydrite) (Schwertmann and Cornell 2007), or sulfate (Na2SO4), were added from separately autoclaved stock solutions of 70.5 mM to final concentrations of 2.4 mM. The pH of the prepared medium was 7.0. The microcosms were incubated upside down and continuously shaken at 100 rpm (Certomat, B|Braun, Melsungen, Germany) in the dark at 20 °C. Abiotic control microcosms were autoclaved and contained 100 mg/l HgCl2 and 2 mg/l NaN3. Unamended control microcosms, not receiving any medium and/or electron acceptors, were used as representatives for the natural condition. When MtBE or benzene was depleted, the compound was added to approximately 100 μM using diluted anoxic, autoclaved 20 mM stock solutions.
TBA cometabolism experiment
Serum vials (10 ml, Grace) with an 80/20% N2/CO2 (v/v) headspace, crimp sealed with a viton rubber stopper and aluminum crimp cap were prepared with medium and 5 mM methanol (Merck, Darmstadt, Germany), isopropanol (Sigma-Aldrich, MO, USA), diethyl ether (Sigma-Aldrich), syringic acid (Fluka analytical, St. Louis, USA), ferulic acid (Sigma-Aldrich), or vanillic acid (Boom, Meppel, the Netherlands) neutralized with 1 M NaOH stock solution, respectively. These substrates were selected, since they are potential metabolites of MtBE and TBA degradation (methanol and isopropanol, respectively), or because they may be EtBE and MtBE analogues containing ethoxy (diethyl ether) or methoxy groups (syringic acid, ferulic acid, and vanillic acid), respectively. Serum vials were filled with 2.4 ml liquid from an unamended control or medium microcosm, respectively. The TBA concentration in the vials was regularly measured using solid phase microextraction (SPME) on a gas chromatograph with flame ionization detector (GC-FID) as described below. Concentrations of methanol, ethanol, isopropanol, and diethyl ether in the serum vials were also regularly measured using a GC-FID equipped with an SPME fiber.
TBA (Sigma-Aldrich), MtBE (Rathburn Chemicals, Walkerburn, Scotland), benzene (Janssen Chimica, Beerse, Belgium), and EtBE (Sigma-Aldrich) were of a purity of > 99%.
Analytical procedures
Headspace samples of 0.5 ml were taken from the microcosms to measure methane using a 1-ml sterile syringe (B|Braun) and 0.5 × 25 mm needle (Henke Sass Wolf, Tuttlingen, Germany) and measured on a Varian 3800 GC-FID as previously described (van der Waals et al. 2017). Liquid samples of 0.3 ml for measuring MtBE, EtBE, TBA, and benzene were taken from the microcosms using a 1-ml sterile syringe (B|Braun) and 0.5 × 16 mm needle (Henke Sass Wolf) and injected in a 10-ml headspace vial (Grace) containing 7 ml of 20 mg/l mercury chloride and 2 ml of 0.93 μg/ml 1-propanol as internal standard (Acros, Geel, Belgium). SPME was performed on these aqueous solutions using a 75-μm CAR/PDMS 24ga autosampler fiber, which was conditioned according to the manufacturer’s protocol (Sigma-Aldrich). Six standards from 10 to 200 μM in serum bottles crimp sealed with viton stoppers and aluminum caps were used for calibration. An extraction time of 30 min and a desorption time of 3 min were found as optimum (Fig. S1). A Combi-PAL autosampler (CTC analytics, Zwingen, Switzerland) with SPME fiber holder and agitator was used for this GC method. Each measurement sequence contained a calibration line and quality controls (Milli-Q and calibration sample) within the sequence because of degrading fiber adsorption potential due to high benzene concentrations (Black and Fine 2001). The coefficient of variation of this method was 5%. The potential metabolites detected with this method include methanol, TBF, ethanol, TBA, acetone, isopropanol, and N-propanol.
Ion analyses were done using 0.1 ml supernatant of shortly (5 s) centrifuged samples (5000×g) (Eppendorf centrifuge 5424, Hamburg, Germany) diluted up to 1 ml with Milli-Q water and measured on a Dionex ICS-1500 equipped with an Ionpac AS19 anion-exchange column and an AERS 500 suppressor (Dionex Corp., Sunnyvale, CA). The potassium hydroxide gradient to separate anions was as follows: 10 mM from 0 to 10 min and 10 to 45 mM from 10 to 25 min with a flow rate of 1 ml/min. The sample injection volume was 25 μl.
Compound stable isotope analysis (CSIA) was performed in 2009 (Supplementary information) and 2013 by Hydroisotop (www.hydroisotop.de) using isotope ratio mass spectrometry (GC-IRMS) related to the Vienna PDB standard. Groundwater samples were fixed in the field with a pellet sodium hydroxide in 100-ml serum bottles.
DNA extraction and molecular analyses
Biomass was concentrated from 10 ml microcosm samples by vacuum filtration on 0.2 μm pore-size filters (Merck Millipore). Filters were crushed with a sterilized wooden tooth pick and total DNA was extracted using the MoBio Powerlyzer DNA isolation kit (MoBio, CA, USA). DNA was stored at − 80 °C until further molecular analyses. The total bacterial 16S rRNA gene and the abcA gene encoding benzene carboxylase were quantified as described previously (van der Waals et al. 2017). For this study, a quantitative real-time PCR assay was designed using the Primer3 software (Koressaar and Remm 2007; Untergasser et al. 2012) to detect genes coding for isobutyryl-CoA mutase (icmA) that has been shown to be a key enzyme involved in MtBE degradation in Aquincola tertiaricarbonis (Rohwerder et al. 2006). The designed primer pair (F695) 5′-ACATCTCGGGCTACCACATC-3′ (R868) 5′-CCTCGAAGAAGTCACCTTGC-3′ with TaqMan probe 5′-CTGGCCAACCTGATCACCTACGT-3′ (757) was evaluated in silico using the publicly accessible NCBI BLAST search tool (Ye et al. 2012). The icmA gene assay was performed in 25 μl volumes as described elsewhere (van der Waals et al. 2017). The temperature program on an IQ5 Icycler (Bio-rad, Veenendaal, the Netherlands) was 5 min at 95 °C followed by 45 cycles of 20 s at 95 °C, 60 s at 58 °C and a final elongation step of 3 min at 72 °C. Normalized abcA and icmA gene counts were calculated as a concentration relative to the bacterial 16S rRNA gene count.
First-order degradation rate constant calculation
First-order degradation rate constants (k) were calculated according to Eq. 1 (Suarez and Rifai 1999).
where t2 − t1 is the time interval in days; x1 is the concentration at time t1 and x2 is the concentration at time t2.
Results
Field data
In 2015, MtBE concentrations had decreased strongly compared to measurements in 2009 and 2013. Based on the average concentration in all measured monitoring wells, only 2% of the MtBE was left in 2015 (Table S2). Benzene concentrations had decreased in the shallow layers of the aquifer up to 25 m below surface in 2015 as compared with 2013. MtBE, TBA, and benzene concentrations did not change in the deeper layer of 25–30 m below surface from 2009 till 2015. In 2009, the δ13C for MtBE ranged from − 24 to − 31‰. In 2013, MtBE δ13C had increased up to + 50‰. The TBA δ13C was constant ranging from − 27 to − 31‰ over 2009–2013. Overall, the fate and transport model achieved a fairly good correlation with long-term benzene and MtBE field monitoring data at a normalized root mean square deviation (NRMSD) of about 14%. Although the limited dataset of TBA demonstrated a NRMSD of about 14%, the overall observation was that correlation of TBA field data was unsatisfactory. In order to fit the TBA plume, complete lack of degradation was assumed. First-order degradation rate constants for benzene and MtBE of 0.0007 day−1 in the model were representative for the field plume situation.
In field samples from 2010, an average 16S rRNA total bacterial gene count of 5.0 ± 8.5 × 105 gene copies/ml sample was detected. The functional gene, icmA, was detected in 3 out of 14 groundwater monitoring wells. Normalized gene counts were relatively low, ranging from 1.5 × 10−5 to 2.7 × 10−4. The functional gene, abcA, was detected in 2009, 2013, and 2015 at stable concentrations with an average concentration of 1.2 ± 2.4 × 10−4 relative to the total bacterial 16S rRNA gene copies.
Biodegradation of MtBE, EtBE, TBA, and benzene in microcosms
In microcosms with groundwater, MtBE was degraded in the unamended control and with the addition of medium and ferrihydrite, at first-order rate constants of 0.02 ± 0.002 day−1, 0.07 day−1, and 0.04 day−1, respectively (Table 1). Under sulfate-reducing conditions with the addition of medium, the MtBE first-order degradation rate constant of 0.06 day−1 was higher than without the addition of medium (0.03 day−1) (Table 1). The concentration data for benzene, MtBE, and electron acceptors were used to calculate electron balances. Under ferrihydrite-reducing conditions, more electrons were released from benzene and MtBE (assuming complete oxidation to CO2) than the electrons used to reduce iron(III) to iron(II) with no detectable net production of metabolites (Table S3). Under sulfate-reducing conditions, fewer electrons were released from benzene and MtBE than the electrons used to reduce sulfate to sulfide (Table S3). In sterile control microcosms no degradation was observed.
EtBE did not show any degradation after 1140 days in the microcosms under different redox conditions, even after depletion of MtBE and benzene.
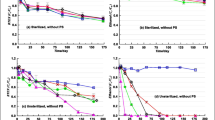
MtBE was re-added to the different microcosms after depletion, and an increase in TBA concentration was observed in medium without added electron acceptors, ferrihydrite-reducing or unamended microcosms corresponding to a percentage of 8, 30, and 19 ± 5%, respectively, compared to the concentration of MtBE degraded. The MtBE and TBA concentration profile under natural conditions is shown in Fig. 1. This TBA increase leveled out to the initial TBA concentration after about 200 days. Complete TBA mineralization has not been proven. After a second MtBE dosage, the TBA concentration increase corresponded to 1%, 34%, and 24 ± 7% of the MtBE degraded in medium, ferrihydrite-reducing and unamended microcosms, respectively. The TBA increase was leveled out for this second dosage after 80 days.
Benzene was degraded in unamended controls and with the addition of medium without added electron acceptors, chlorate, ferrihydrite, or sulfate, with first-order degradation rate constants between 0.01 and 0.02 day−1 (Table 1). In the presence of medium, the benzene first-order degradation rate constants were significantly higher than in the absence of medium under chlorate (0.39 day−1), ferrihydrite (0.08 day−1), or sulfate-reducing conditions (0.07 day−1) (Table 1). No degradation with nitrate was observed. The electron balance indicated that more electrons were used to reduce chlorate to chloride than were released by the oxidation of benzene (Table S3). Initially, no benzene degradation was observed in the nitrate-reducing microcosms. Supply of a 5% liquid inoculum from a benzene-degrading, denitrifying biofilm culture grown on benzene and nitrate with medium resulted in an average degradation rate constant of 0.08 ± 0.04 day−1 (van der Waals et al. 2017). Over two dosages of 100 μM benzene, on average 5.6 ± 3.1 mol nitrate was reduced in parallel with degradation of 1 mol benzene. No increased methane concentration was measured.
Total bacterial 16S rRNA genes in the microcosms were on average 2.0 ± 1.3 × 106 gene copies/ml sample. The icmA gene was only detected in the microcosms under nitrate-reducing conditions at normalized counts of 6.7 × 10−5 and 1.6 × 10−5 in the absence or presence of medium, respectively. The abcA gene was detected in 14 out of 20 microcosms at a normalized count of 5.0 ± 5.2 × 10−3. In the microcosms with addition of medium and the microcosm with groundwater plus nitrate, abcA genes were below the detection limit (< 1.1 copies/μl sample or < 6.0 × 10−4 relative to the total bacterial 16S rRNA gene copies). The abcA gene copy number in the microcosm with medium plus nitrate and reactor inoculum was 5.8 times higher than the bacterial 16S rRNA gene number.
Anaerobic TBA and EtBE degradation during growth on MtBE and EtBE analogues and potential metabolites
Microcosms with vanillate, ferulate, syringate, isopropanol, methanol, or diethyl ether as growth substrate analogues were prepared to determine whether TBA was cometabolically degraded. TBA was depleted with all substrates tested (Table 2). After two re-additions of 5 mM syringate, ferulate, or vanillate, to microcosms with medium, the cumulative amount of TBA depleted increased to 31.6, 33.6, and 42.9 μM, respectively in 136 days (Fig. 2). The amount of vanillate, ferulate, or syringate added was, respectively, 350, 447, or 475 mol/mol of TBA depleted. Also EtBE depletion was observed at significant amounts in microcosms with ferulate (both unamended control and medium), syringate (unamended control and medium), isopropanol (unamended control), or diethyl ether (unamended control and medium), respectively (Table 2).
The cumulative TBA depletion with vanillate (triangles), ferulate (diamonds), or syringate (circles) in MtBE-degrading groundwater microcosms with medium. The arrows indicate a 5-mM dosage of the substrate vanillate, ferulate, or syringate, respectively. An initial TBA concentration of 50 μM was present in the microcosms
Discussion
The aims of this study were to (i) determine the biodegradation potential of the microbial community for MtBE, EtBE, TBA, and benzene in a mixture in groundwater from a contaminated location; (ii) determine the effects of electron acceptors; and (iii) determine TBA and EtBE degradation using MtBE and EtBE substrate analogues and potential metabolites. A better understanding of the degradation rates under different redox conditions and cometabolic TBA depletion would enable the design of more effective bioremediation through biostimulation at contaminated sites.
Anaerobic MtBE degradation
MtBE concentrations decreased strongly in all monitoring wells from 2009 to 2015. The detection of TBA in almost all monitoring wells in 2013 indicates that demethylation is the first step in the MtBE biodegradation in the aquifer (Youngster et al. 2010). Fractionation of stable carbon isotopes of MtBE and TBA in groundwater in 2009 and 2013 confirmed degradation of MtBE. TBA degradation could not be demonstrated by CSIA.
MtBE was anaerobically degraded in microcosms with groundwater in unamended controls and after the addition of medium, ferrihydrite, or sulfate. This indicates that conditions prevailing at the field site favor MtBE degradation in batch cultures. The MtBE degradation rate constant implemented in the model and representative for the field situation of 0.0007 day−1 was 25 times lower compared with the rate observed in the enrichments (0.02 ± 0.002 day−1). Although data obtained in batch are only indicative for the field situation, these outcomes suggest the potential of biostimulation and/or augmentation of laboratory enriched microbial communities to increase the MtBE degradation rate in the field. The first-order MtBE degradation rate constant of 0.02 ± 0.002 day−1 in the unamended control was similar compared with previous rate constants found of 0.01–0.09 day−1 in methanogenic microcosms (Liu et al. 2016; Somsamak et al. 2006; Wilson et al. 2005). The first-order MtBE degradation rate constants obtained in this study under sulfate-reducing conditions of 0.03 and 0.06 day−1 in the absence and presence of medium, respectively, are comparable with a previous microcosm rate constant of 0.031 ± 0.007 day−1 (Wilson et al. 2005). The TBA concentration did not stochiometrically increase with the amount of MtBE degraded in the microcosms with sulfate. This suggests that MtBE may be completely oxidized to CO2 or other metabolites were formed, which were not detected with the GC-FID. The electrons released from MtBE and benzene degradation were in the range of 60 to 83% compared with the electrons used to reduce sulfate to sulfide. Somsamak et al. (2006) and Youngster et al. (2008) indicated that in their experiments anaerobic MtBE degradation was not directly coupled to sulfidogenesis (Somsamak et al. 2006; Youngster et al. 2008). They hypothesize that MtBE-degrading organisms might have produced acetate which was used as a carbon source for sulfate-reducing organisms. Up to our knowledge, this study found the highest first-order MtBE degradation rate constant under ferrihydrite-reducing conditions of 0.04 day−1. Landmeyer et al. (1998) reported a first-order rate constant of 0.0002 day−1 under iron-reducing conditions in microcosms (Landmeyer et al. 1998). In the ferrihydrite microcosms, much more benzene and MtBE was consumed in comparison to the amount of ferrihydrite that was reduced. This indicates that ferrihydrite reduction may not be the predominant electron accepting reaction. The electron imbalance could also be attributed to a lack of complete oxidation of benzene and MtBE to CO2. The fact that there was no iron(II) production in the sterile controls suggests that abiotic ferrihydrite reduction did not compromise/influence the measurements.
We hypothesized that icmA gene products might also be relevant in anaerobic MtBE degradation since (i) cobalamin-dependent enzymes are important in the metabolism of many anaerobes and (ii) no oxygenases are needed for the transformation of 2-HIBA to 3-hydroxybutyryl-CoA and the further metabolism of this intermediate (Janssen and Schink 1993). In the field, low concentrations of icmA genes were detected in three out of 14 monitoring wells. In microcosms, icmA was only detected under nitrate-reducing conditions at low normalized counts of 6.7 × 10−5 and 1.6 × 10−5. This low icmA concentration suggests that 2-hydroxyisobutyrate is not, or, slowly degraded. It may also be that under anaerobic conditions other isobutyryl-CoA mutase enzymes were active, which were not detected by the applied icmA assay (Rohwerder et al. 2006), or that MtBE and TBA were degraded via alternative metabolic pathways, not involving isobutyryl-CoA mutase.
Anaerobic EtBE degradation
No significant anaerobic degradation of EtBE was detected in the microcosms under different redox conditions, even after depletion of the (apparently) more easily degradable substrates MtBE and benzene. This result is in line with recent studies indicating the lack of anaerobic EtBE degradation (Hernandez-Perez et al. 2001; Somsamak et al. 2001). However, microcosms with ferulate, syringate, isopropanol, and diethyl ether demonstrated EtBE depletion of up to 86.5% of the initial concentration after 83 days. This suggests that bacteria capable of cometabolic EtBE degradation might be present at this location. We recommend additional research to confirm the possible cometabolic EtBE degradation during growth on methoxylated and ethoxylated substrates.
Anaerobic cometabolic TBA depletion
Interestingly, TBA concentrations did not stoichiometrically increase in parallel with MtBE depletion in the field nor in the microcosm experiment. The initial TBA increase after the re-addition of MtBE and the subsequent TBA decrease during MtBE degradation to its initial concentration in the microcosms suggests cometabolic TBA degradation. It is feasible that TBA was not depleted further after the primary growth substrate was consumed. This is in line with previous studies observing simultaneous anaerobic MtBE and TBA degradation (Table 3). A strong TBA decrease in microcosms with vanillate, ferulate, or syringate indicates cometabolic degradation with MtBE analogue substrates. Possibly, TBA degradation was catalyzed by enzymes induced for demethoxylation, as was shown previously under aerobic conditions (Lopes Ferreira et al. 2006). It is also feasible that TBA degradation is mediated by enzymes responsible for metabolism of tert-alcohol intermediates, which are formed during degradation of methoxylated aromatics. Re-addition of substrate analogues to the medium microcosms doubled the amount of TBA depleted, indicating that these compounds indeed induced cometabolic TBA depletion. Without the addition of these substrates, TBA was not depleted below its initial concentration of 50 μM, and EtBE was not degraded at all. The amount of vanillate (350 mol), ferulate (447 mol), or syringate (475 mol) was added in excess to degrade 1 mol of TBA. Therefore, the substrate concentration used in this study is rather high compared with a previously reported MtBE cometabolic degradation study using 1.6 mol vanillate or 1.7 mol syringate to degrade 1 mol of MtBE (Youngster et al. 2008). Further laboratory experiments will be important to test whether lower substrate concentrations can be used to cometabolically degrade TBA. Addition of the analogue growth substrates to the batches may have enriched microorganisms with enzymes catalyzing MtBE, EtBE, and TBA transformation. A previous study has demonstrated cometabolic MtBE degradation using syringate, vanillate, and guaiacol (Youngster et al. 2008). It was suggested that acetogenic bacteria capable of degrading methoxylated aromatics may be responsible for this enhanced MtBE degradation (Youngster et al. 2008). Anaerobic TBA degradation has also been observed in anaerobic microcosms without the addition of MtBE (Finneran and Lovley 2001). However, these microcosms contained aquifer sediment heavily contaminated with MtBE, suggesting residual MtBE adsorbed to the sediment might have caused possible cometabolic TBA degradation.
Anaerobic benzene degradation
The benzene degradation rate constant implemented in the model and representative for the field situation of 0.0007 day−1 was 14 to 557 times lower than the first-order rate constants measured in microcosms. The benzene first-order degradation rate constants in microcosms under chlorate, ferrihydrite, and sulfate-reducing conditions were higher in the presence of medium. This implies that organisms responsible for benzene degradation were stimulated by nutrients or vitamins supplied with the medium to increase the benzene degradation rate. Initially, benzene was not degraded in microcosms under nitrate-reducing conditions. After a 5% (v/v) inoculation of culture liquid from a benzene-degrading, denitrifying biofilm community a first-order benzene degradation rate constant of 0.08 ± 0.04 day−1 was obtained. This rate constant is lower compared with a rate constant of 0.52 ± 0.03 day−1 found in a previous microcosm study with this biofilm community containing a dense inoculation of biofilm material from the glass walls of the continuous culture (van der Zaan et al. 2012). The stoichiometry of anaerobic benzene degradation, 1 mol benzene consumed per 5.6 ± 3.1 mol nitrate reduced, was similar to that found previously (van der Zaan et al. 2012). The incomplete electron recovery of benzene degradation with chlorate or sulfate indicates that electrons released from the relatively high concentration of DOC of 32 mg/l were used to reduce these acceptors in our microcosms. No increased methane concentration was measured in these microcosms, indicating that methanogenesis was not involved in benzene degradation.
The abcA gene associated with benzene carboxylation was detected in all monitoring wells and microcosms, albeit in low numbers. The normalized abcA gene numbers in the microcosms under different redox conditions (5.0 ± 5.2 × 10−3) were higher than the gene numbers measured in the field (1.2 ± 2.4 × 10−4). This suggests that the organisms responsible for benzene degradation through carboxylation were enriched in the microcosms by the addition of nutrients and/or electron acceptors. Groundwater from a leachate-contaminated aquifer polluted with BTEX contained on average 2.4 × 10−4 normalized counts of the bssA gene that codes for benzylsuccinate synthase, an enzyme that is important in toluene degradation (Staats et al. 2011). This relative number of bssA genes is similar to the abcA gene count in this study. Our results suggest that bioaugmentation and biostimulation can be used to enhance the anaerobic benzene degradation rate at this location. These results are in line with a previous study indicating that benzene is degraded through an initial carboxylation, and the responsible organisms may be stimulated by the addition of nutrients and nitrate (van der Waals et al. 2017). Interestingly, the microcosm containing nitrate plus medium and an inoculum from a benzene-degrading, denitrifying biofilm culture showed an abcA gene count of 5.8 times higher than the bacterial 16S rRNA gene number. This high abcA gene count could be due to (i) multiple abcA gene copies in the DNA of the biofilm culture or (ii) the possibility of the abcA gene being mobile due to flanking transposon sequences that were observed on the same contig in a metagenome dataset derived from the chemostat biofilm (unpublished data).
Mixture experiment
Addition of nitrate plus a benzene-degrading community and chlorate (with and without medium) stimulated benzene degradation, but hindered MtBE, EtBE, and TBA degradation. TBA degradation may be significantly decreased by the presence of benzene in aerobic microcosms (Sedran et al. 2002; Sedran et al. 2004), while other studies indicated no effect of benzene on MtBE and TBA degradation in microcosms (Pruden and Suidan 2004). No EtBE degradation was observed in the mixture without substrate analogues. This result is in line with previous studies indicating (i) that under both aerobic and anaerobic conditions, structurally related compounds can have a negative effect on the degradation rates (Deshpande et al. 1987) or (ii) that MtBE might be preferentially degraded (Kharoune et al. 2001). Gunasekaran and colleagues reported inhibition of EtBE degradation in the presence of benzene, toluene, and xylene (Gunasekaran et al. 2013). Somsamak and colleagues also reported a lack of EtBE degradation in a mixture of MtBE, EtBE, and tert-amyl ether (TAME) (Somsamak et al. 2001). In this study, a stoichiometric increase of TBA with the degradation of MtBE was observed under sulfate-reducing conditions.
Addition of nitrate with a 5% (v/v) benzene-degrading culture inoculation and chlorate to the microcosms stimulated benzene degradation. For MtBE, EtBE, and TBA, electron acceptors such as nitrate appeared to hinder the degradation. Growth substrate analogues stimulated degradation. Cometabolic TBA depletion with MtBE and different methoxylated substrate analogues was indicated in this study. The addition of methoxylated compounds such as syringate, vanillate, and ferulate may be a useful method to enhance anaerobic MtBE, EtBE, and TBA degradation in situ. Further field studies and dedicated pilot testing could help to elucidate the potential of cometabolic TBA, MtBE, and EtBE degradation. The increasing likelihood of encountering mixtures of fossil-based and biobased fuels as cocontaminants in groundwater may require novel or sequential bioremediation approaches. This study describes that biostimulation with substrate analogues, medium and/or nitrate, chlorate, ferrihydrite, and sulfate and bioaugmentation approaches can potentially accelerate the degradation of MtBE, EtBE, TBA, and benzene.
References
Black L, Fine D (2001) High levels of monoaromatic compounds limit the use of solid-phase microextraction of methyl tert-butyl ether and tert-butyl alcohol. Environ Sci Technol 35:3190–3192. https://doi.org/10.1021/es010539c
Bombach P, Nägele N, Rosell M, Richnow HH, Fischer A (2015) Evaluation of ethyl tert-butyl ether biodegradation in a contaminated aquifer by compound-specific isotope analysis and in situ microcosms. J Hazard Mater 286:100–106. https://doi.org/10.1016/j.jhazmat.2014.12.028
Bradley PM, Landmeyer JE, Chapelle FH (1999) Aerobic mineralization of MTBE and tert-butyl alcohol by stream-bed sediment microorganisms. Environ Sci Technol 33:1877–1879. https://doi.org/10.1021/es990062t
Bradley PM, Chapelle FH, Landmeyer JE (2001a) Effect of redox conditions on MtBE biodegradation in surface water sediments. Environ Sci Technol 35:4643–4647. https://doi.org/10.1021/es010794x
Bradley PM, Chapelle FH, Landmeyer JE (2001b) Methyl t-butyl ether mineralization in surface-water sediment microcosms under denitrifying conditions. Appl Environ Microbiol 67:1975–1978. https://doi.org/10.1128/aem.67.4.1975-1978.2001
Bradley PM, Landmeyer JE, Chapelle FH (2002) TBA biodegradation in surface-water sediments under aerobic and anaerobic conditions. Environ Sci Technol 36:4087–4090. https://doi.org/10.1021/es011480c
Chin J-Y, Batterman SA (2012) VOC composition of current motor vehicle fuels and vapors, and collinearity analyses for receptor modeling. Chemosphere 86:951–958. https://doi.org/10.1016/j.chemosphere.2011.11.017
Coates JD, Chakraborty R, McInerney MJ (2002) Anaerobic benzene biodegradation--a new era. Res Microbiol 153:621–628
Corseuil HX, Hunt CS, Ferreira dos Santos RC, Alvarez PJJ (1998) The influence of the gasoline oxygenate ethanol on aerobic and anaerobic BTX biodegradation. Water Res 32:2065–2072. https://doi.org/10.1016/S0043-1354(97)00438-7
Da Silva MLB, Alvarez PJJ (2004) Enhanced anaerobic biodegradation of benzene-toluene-ethylbenzene-xylene-ethanol mixtures in bioaugmented aquifer columns. Appl Environ Microbiol 70:4720–4726. https://doi.org/10.1128/aem.70.8.4720-4726.2004
Deeb RA, Hu H-Y, Hanson JR, Scow KM, Alvarez-Cohen L (2001) Substrate interactions in BTEX and MTBE mixtures by an MTBE-degrading isolate. Environ Sci Technol 35:312–317. https://doi.org/10.1021/es001249j
Deeb RA, Chu K-H, Shih T, Linder S, Suffet I, Kavanaugh MC, Alvarez-Cohen L (2003) MTBE and other oxygenates: environmental sources, analysis, occurrence, and treatment. Environ Eng Sci 20:433–447. https://doi.org/10.1089/109287503768335922
Deshpande SD, Chakrabarti T, Subrahmanyam PVR (1987) Mixed-substrate utilization by acclimated activated sludge in batch and continuous-flow stirred tank reactors. Environ Sci Technol 21:1003–1008. https://doi.org/10.1021/es50001a015
EC (2018) Biofuels. Available from: https://ec.europa.eu/energy/en/topics/renewable-energy/biofuels. Accessed 20 Feb 2018
Finneran KT, Lovley DR (2001) Anaerobic degradation of methyl tert-butyl ether (MTBE) and tert-butyl alcohol (TBA). Environ Sci Technol 35:1785–1790. https://doi.org/10.1021/es001596t
Fiorenza S, Rifai HS (2003) Review of MTBE biodegradation and bioremediation. Bioremediat J 7:1–35. https://doi.org/10.1080/713914240-243
Fischer A, Oehm C, Selle M, Werner P (2005) Biotic and abiotic transformations of methyl tertiary butyl ether (MTBE). Environ Sci Pollut R 12:381–386. https://doi.org/10.1065/espr2005.08.277
Gunasekaran V, Stam L, Constantí M (2013) The effect of BTX compounds on the biodegradation of ETBE by an ETBE degrading bacterial consortium. Biotechnol Bioprocess Eng 18:1216–1223. https://doi.org/10.1007/s12257-013-0132-8
Häggblom MM, Youngster LKG, Somsamak P, Richnow HH (2007) Anaerobic biodegradation of methyl tert-butyl ether (MTBE) and related fuel oxygenates. In: Allen I. Laskin SS, Geoffrey MG (eds) Adv Appl Microbiol, vol Volume 62. Academic Press, pp 1–20. doi:https://doi.org/10.1016/S0065-2164(07)62001-2
Harbaugh AW (2005) MODFLOW-2005, the U.S. Geological Survey modular ground-water model—the gound-water flow process: U.S. Geological Survey Techniques and Methods 6-A16
Harbaugh AW, Langevin CD, Hughes JD, Niswonger RN, Konikow LF (2017) MODFLOW-2005 version 1.12.00, the U.S. Geological Survey modular groundwater model: U.S. Geological Survey Software Release, 03 February
Hernandez-Perez G, Fayolle F, Vandecasteele JP (2001) Biodegradation of ethyl t-butyl ether (ETBE), methyl t-butyl ether (MTBE) and t-amyl methyl ether (TAME) by Gordonia terrae. Appl Microbiol Biotechnol 55:117–121
Hyman M (2013) Biodegradation of gasoline ether oxygenates. Curr Opin Biotechnol 24:443–450. https://doi.org/10.1016/j.copbio.2012.10.005
Janssen PH, Schink B (1993) Pathway of anaerobic poly-β-hydroxybutyrate degradation by Ilyobacter delafieldii. Biodegradation 4:179–185. https://doi.org/10.1007/BF00695120
Kharoune M, Pauss A, Lebeault JM (2001) Aerobic biodegradation of an oxygenates mixture: ETBE, MTBE and TAME in an upflow fixed-bed reactor. Water Res 35:1665–1674. https://doi.org/10.1016/S0043-1354(00)00448-6
Koressaar T, Remm M (2007) Enhancements and modifications of primer design program Primer3. Bioinformatics 23:1289–1291. https://doi.org/10.1093/bioinformatics/btm091
Landmeyer JE, Chapelle FH, Bradley PM, Pankow JF, Church CD, Tratnyek PG (1998) Fate of MTBE relative to benzene in a gasoline-contaminated aquifer (1993–98). Ground Water Monit Remediat 18:93–102. https://doi.org/10.1111/j.1745-6592.1998.tb00168.x
Le Digabel Y, Demaneche S, Benoit Y, Vogel TM, Fayolle-Guichard F (2013) Ethyl tert-butyl ether (ETBE) biodegradation by a syntrophic association of Rhodococcus sp. IFP 2042 and Bradyrhizobium sp. IFP 2049 isolated from a polluted aquifer. Appl Microbiol Biotechnol 97:10531–10539. https://doi.org/10.1007/s00253-013-4803-3
Liu T, Ahn H, Sun W, McGuinness LR, Kerkhof LJ, Häggblom MM (2016) Identification of a Ruminococcaceae species as the methyl tert-butyl ether (MTBE) degrading bacterium in a methanogenic consortium. Environ Sci Technol 50:1455–1464. https://doi.org/10.1021/acs.est.5b04731
Lopes Ferreira N, Malandain C, Fayolle-Guichard F (2006) Enzymes and genes involved in the aerobic biodegradation of methyl tert-butyl ether (MTBE). Appl Microbiol Biotechnol 72:252–262. https://doi.org/10.1007/s00253-006-0494-3
Lovanh N, Hunt CS, Alvarez PJJ (2002) Effect of ethanol on BTEX biodegradation kinetics: aerobic continuous culture experiments. Water Res 36:3739–3746. https://doi.org/10.1016/S0043-1354(02)00090-8
Mormile MR, Liu S, Suflita JM (1994) Anaerobic biodegradation of gasoline oxygenates: extrapolation of information to multiple sites and redox conditions. Environ Sci Technol 28:1727–1732. https://doi.org/10.1021/es00058a026
Pruden A, Suidan M (2004) Effect of benzene, toluene, ethylbenzene, and p-xylene (BTEX) mixture on biodegradation of methyl tert-butyl ether (MTBE) and tert-butyl alcohol (TBA) by pure culture UC1. Biodegradation 15:213–227
Pruden A, Sedran MA, Suidan MT, Venosa AD (2005) Anaerobic biodegradation of methyl tert-butyl ether under iron-reducing conditions in batch and continuous-flow cultures. Water Environ Res 77:297–303
Rohwerder T, Breuer U, Benndorf D, Lechner U, Muller RH (2006) The alkyl tert-butyl ether intermediate 2-hydroxyisobutyrate is degraded via a novel cobalamin-dependent mutase pathway. Appl Environ Microbiol 72:4128–4135. https://doi.org/10.1128/aem.00080-06
Ruiz-Aguilar GML, Fernandez-Sanchez JM, Kane SR, Kim D, Alvarez PJJ (2002) Effect of ethanol and methyl-tert-butyl ether on monoaromatic hydrocarbon biodegradation: response variability for different aquifer materials under various electron-accepting conditions. Environ Toxicol Chem 21:2631–2639. https://doi.org/10.1002/etc.5620211215
Schmidt TC, Schirmer M, Weiß H, Haderlein SB (2004) Microbial degradation of methyl tert-butyl ether and tert-butyl alcohol in the subsurface. J Contam Hydrol 70:173–203. https://doi.org/10.1016/j.jconhyd.2003.09.001
Schwertmann U, Cornell RM (2007) Ferrihydrite. In: Iron oxides in the laboratory. Wiley-VCH Verlag GmbH, pp 103–112. doi:https://doi.org/10.1002/9783527613229.ch08
Sedran MA, Pruden A, Wilson GJ, Suidan MT, Venosa AD (2002) Effect of BTEX on degradation of MTBE and TBA by mixed bacterial consortium. J Environ Eng 128:830–835
Sedran MA, Pruden A, Wilson GJ, Suidan MT, Venosa AD (2004) Biodegradation of methyl tert-butyl ether and BTEX at varying hydraulic retention times. Water Environ Res 76:47–55
Somsamak P, Cowan RM, Häggblom MM (2001) Anaerobic biotransformation of fuel oxygenates under sulfate-reducing conditions. FEMS Microbiol Ecol 37:259–264. https://doi.org/10.1016/S0168-6496(01)00159-3
Somsamak P, Richnow HH, Häggblom MM (2005) Carbon isotopic fractionation during anaerobic biotransformation of methyl tert-butyl ether and tert-amyl methyl ether. Environ Sci Technol 39:103–109. https://doi.org/10.1021/es049368c
Somsamak P, Richnow HH, Häggblom MM (2006) Carbon isotope fractionation during anaerobic degradation of methyl tert-butyl ether under sulfate-reducing and methanogenic conditions. Appl Environ Microbiol 72:1157–1163. https://doi.org/10.1128/aem.72.2.1157-1163.2006
Staats M, Braster M, Röling WFM (2011) Molecular diversity and distribution of aromatic hydrocarbon-degrading anaerobes across a landfill leachate plume. Environ Microbiol 13:1216–1227. https://doi.org/10.1111/j.1462-2920.2010.02421.x
Stupp D, Gass M, Leiteritz H, Pijls C, Thornton S, Smith J, Dunk M, Grosjean T, den Haan K (2012) Gasoline ether oxygenate occurrence in Europe, and a review of their fate and transport characteristics in the environment
Suarez MP, Rifai HS (1999) Biodegradation rates for fuel hydrocarbons and chlorinated solvents in groundwater. Bioremediat J 3:337–362. https://doi.org/10.1080/10889869991219433
Suflita JM, Mormile MR (1993) Anaerobic biodegradation of known and potential gasoline oxygenates in the terrestrial subsurface. Environ Sci Technol 27:976–978. https://doi.org/10.1021/es00042a022
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG (2012) Primer3—new capabilities and interfaces. Nucl Acids Res 40:e115. https://doi.org/10.1093/nar/gks596
van der Waals MJ, Atashgahi S, da Rocha UN, van der Zaan BM, Smidt H, Gerritse J (2017) Benzene degradation in a denitrifying biofilm reactor: activity and microbial community composition. Appl Microbiol Biotechnol 101:1–14. https://doi.org/10.1007/s00253-017-8214-8
van der Zaan BM, Saia FT, Stams AJ, Plugge CM, de Vos WM, Smidt H, Langenhoff AA, Gerritse J (2012) Anaerobic benzene degradation under denitrifying conditions: Peptococcaceae as dominant benzene degraders and evidence for a syntrophic process. Environ Microbiol 14:1171–1181. https://doi.org/10.1111/j.1462-2920.2012.02697.x
van Wezel A, Puijker L, Vink C, Versteegh A, de Voogt P (2009) Odour and flavour thresholds of gasoline additives (MTBE, ETBE and TAME) and their occurrence in Dutch drinking water collection areas. Chemosphere 76:672–676. https://doi.org/10.1016/j.chemosphere.2009.03.073
Waul C, Arvin E, Schmidt JE (2009) Long term studies on the anaerobic biodegradability of MTBE and other gasoline ethers. J Hazard Mater 163:427–432. https://doi.org/10.1016/j.jhazmat.2008.06.113
Wilson JT, Adair C, Kaiser PM, Kolhatkar R (2005) Anaerobic biodegradation of MTBE at a gasoline spill site. Ground Water Monit Remediat 25:103–115. https://doi.org/10.1111/j.1745-6592.2005.00032.x
Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL (2012) Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 13:134. https://doi.org/10.1186/1471-2105-13-134
Yeh CK, Novak JT (1994) Anaerobic biodegradation of gasoline oxygenates in soils. Water Environ Res 66:744–752
Youngster LKG, Somsamak P, Häggblom MM (2008) Effects of co-substrates and inhibitors on the anaerobic O-demethylation of methyl tert-butyl ether (MTBE). Appl Microbiol Biotechnol 80:1113–1120. https://doi.org/10.1007/s00253-008-1697-6
Youngster LKG, Kerkhof LJ, Häggblom MM (2010) Community characterization of anaerobic methyl tert-butyl ether (MTBE)-degrading enrichment cultures. FEMS Microbiol Ecol 72:279–288. https://doi.org/10.1111/j.1574-6941.2010.00841.x
Acknowledgements
We like to thank Fredericke Hannes (Deltares, Utrecht) for expert advice and technical assistance. This work was carried out within the BE-Basic R&D Program, which was supported through a FES subsidy from the Dutch Ministry of Economic Affairs, Agriculture and Innovation (EL&I). This project was also supported by the Dutch governmental top sector policy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 123 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
van der Waals, M.J., Pijls, C., Sinke, A.J.C. et al. Anaerobic degradation of a mixture of MtBE, EtBE, TBA, and benzene under different redox conditions. Appl Microbiol Biotechnol 102, 3387–3397 (2018). https://doi.org/10.1007/s00253-018-8853-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-8853-4