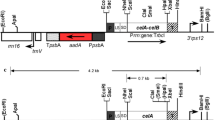
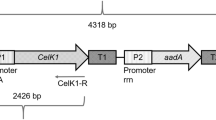
Abstract
The bulk production of recombinant enzymes by either prokaryotic or eukaryotic organisms might contribute to replace environmentally non-friendly chemistry-based industrial processes with enzyme-based biocatalysis, provided the cost of enzyme production is low. In this context, it is worth noting that the production of recombinant proteins by photosynthetic organisms offer both eukaryotic (nuclear) and prokaryotic (chloroplast) alternatives, along with the advantage of an autotrophic nutrition. Compared to nuclear transformation, chloroplast transformation generally allows a higher level of accumulation of the recombinant protein of interest. Furthermore, among the photosynthetic organisms, there is a choice of using either multicellular or unicellular ones. Tobacco, being a non-food and non-feed plant, has been considered as a good choice for producing enzymes with applications in technical industry, using a transplastomic approach. Also, unicellular green algae, in particular Chlamydomonas reinhardtii, have been proposed as candidate organisms for the production of recombinant proteins. In the light of the different features of these two transplastomic systems, we decided to make a direct comparison of the efficiency of production of a bacterial endoglucanase. With respect to the amount obtained, 14 mg g−1 of biomass fresh weight equivalent to 8–10% of the total protein content and estimated production cost, 1.5–2€ kg−1, tobacco proved to be far more favorable for bulk enzyme production when compared to C. reinhardtii which accumulated this endoglucanase at 0.003% of the total protein.





Similar content being viewed by others
References
Battistel P (2014) Controllo dei costi energetici. Colt Protette 43:16–24
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Daniell H, Singh ND, Mason H, Streatfield SJ (2009) Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci 14:669–679. doi:10.1016/j.tplants.2009.09.009
Demain AL, Vaishnav P (2009) Production of recombinant proteins by microbes and higher organisms. Biotechnol Adv 27:297–306. doi:10.1016/j.biotechadv.2009.01.008
Doria E, Longoni P, Scibilia L, Iazzi N, Cella R, Nielsen E (2011) Isolation and characterization of a Scenedesmus acutus strain to be used for bioremediation of urban wastewater. J Appl Phycol 24:375–383. doi:10.1007/s10811-011-9759-z
Fernández-Robledo JA, Vasta GR (2010) Production of recombinant proteins from protozoan parasites. Trends Parasitol 26:244–254. doi:10.1016/j.pt.2010.02.004
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem. doi:10.1351/pac198759020257
Giovanardi M, Baldisserotto C, Ferroni L, Longoni P, Cella R, Pancaldi S (2014) Growth and lipid synthesis promotion in mixotrophic Neochloris oleoabundans (Chlorophyta) cultivated with glucose. Protoplasma 251:115–125. doi:10.1007/s00709-013-0531-x
Hacker DL, De Jesus M, Wurm FM (2009) 25 years of recombinant proteins from reactor-grown cells - where do we go from here? Biotechnol Adv 27:1023–1027. doi:10.1016/j.biotechadv.2009.05.008
Harun R, Danquah MK, Forde GM (2010) Microalgal biomass as a fermentation feedstock for bioethanol production. J Chem Technol Biotechnol 85:199–203. doi:10.1002/jctb.2287
Harun R, Yip JWS, Thiruvenkadam S, Ghani WAWAK, Cherrington T, Danquah MK (2014) Algal biomass conversion to bioethanol—a step-by-step assessment. Biotechnol J 9:73–86. doi:10.1002/biot.201200353
Houdebine L-M (2009) Production of pharmaceutical proteins by transgenic animals. Comp Immunol Microbiol Infect Dis 32:107–121. doi:10.1016/j.cimid.2007.11.005
Jin S, Daniell H (2015) The engineered chloroplast genome just got smarter. Trends Plant Sci 20:622–640. doi:10.1016/j.tplants.2015.07.004
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Leelavathi S, Gupta N, Maiti S, Ghosh A, Reddy VS (2003) Overproduction of an alkali- and thermo-stable xylanase in tobacco chloroplasts and efficient recovery of the enzyme. Mol Breed 11:59–67. doi:10.1023/A:1022168321380
Liu G, Zhang J, Bao J (2016) Cost evaluation of cellulase enzyme for industrial-scale cellulosic ethanol production based on rigorous aspen plus modeling. Bioprocess Biosyst Eng 39:133–140. doi:10.1007/s00449-015-1497-1
Longoni P, Leelavathi S, Doria E, Reddy VS, Cella R (2015) Production by tobacco transplastomic plants of recombinant fungal and bacterial Cell-Wall degrading enzymes to Be used for cellulosic biomass saccharification. Biomed Res Int 2015:289759. doi:10.1155/2015/289759
Lunin VV, Sergeeva YE, Galanina LA, Mysyakina IS, Ivashechkin AA, Bogdan VI, Feofilova EP (2012) Biodiesel fuel production from lipids of filamentous fungi. Appl Biochem Microbiol 49:46–52. doi:10.1134/S0003683813010122
Mayfield SP, Franklin SE (2005) Expression of human antibodies in eukaryotic micro-algae. Vaccine 23:1828–1832. doi:10.1016/j.vaccine.2004.11.013
Michelet L, Lefebvre-Legendre L, Burr SE, Rochaix J-D, Goldschmidt-Clermont M (2011) Enhanced chloroplast transgene expression in a nuclear mutant of Chlamydomonas. Plant Biotechnol J 9:565–574. doi:10.1111/j.1467-7652.2010.00564.x
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Pantaleoni L, Longoni P, Ferroni L, Baldisserotto C, Leelavathi S, Reddy VS, Pancaldi S, Cella R (2014) Chloroplast molecular farming: efficient production of a thermostable xylanase by Nicotiana tabacum plants and long-term conservation of the recombinant enzyme. Protoplasma 251:639–648. doi:10.1007/s00709-013-0564-1
Potvin G, Zhang Z (2010) Strategies for high-level recombinant protein expression in transgenic microalgae: a review. Biotechnol Adv 28:910–918. doi:10.1016/j.biotechadv.2010.08.006
Rasala BA, Muto M, Lee PA, Jager M, Cardoso RMF, Behnke CA, Kirk P, Hokanson CA, Crea R, Mendez M, Mayfield SP (2010) Production of therapeutic proteins in algae, analysis of expression of seven human proteins in the chloroplast of Chlamydomonas reinhardtii. Plant Biotechnol J 8:719–733. doi:10.1111/j.1467-7652.2010.00503.x
Reddy VS, Leelavathi S, Selvapandiyan A, Raman R, Giovanni F, Shukla V, Bhatnagar RK (2002) Analysis of chloroplast transformed tobacco plants with cry1Ia5 under rice psbA transcriptional elements reveal high level expression of Bt toxin without imposing yield penalty and stable inheritance of transplastome. Mol Breed 9:259–269. doi:10.1023/A:1020357729437
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. doi:10.1038/nmeth.2019
Scotti N, Rigano MM, Cardi T (2012) Production of foreign proteins using plastid transformation. Biotechnol Adv 30:387–397. doi:10.1016/j.biotechadv.2011.07.019
Slade R, Bauen A (2013) Micro-algae cultivation for biofuels: cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 53:29–38. doi:10.1016/j.biombioe.2012.12.019
Specht EA, Mayfield SP (2013) Synthetic oligonucleotide libraries reveal novel regulatory elements in Chlamydomonas chloroplast mRNAs. ACS Synth Biol 2:34–46. doi:10.1021/sb300069k
Studier FW (2005) Protein production by auto-induction in high density shaking cultures. Protein Expr Purif 41:207–234
Surzycki R, Greenham K, Kitayama K, Dibal F, Wagner R, Rochaix J-D, Ajam T, Surzycki S (2009) Factors effecting expression of vaccines in microalgae. Biol J Int Assoc Biol Stand 37:133–138. doi:10.1016/j.biologicals.2009.02.005
Tschofen M, Knopp D, Hood E, Stöger E (2016) Plant molecular farming: much more than medicines. Annu Rev Anal Chem 9:271–294. doi:10.1146/annurev-anchem-071015-041706
Tusé D, Tu T, McDonald KA (2014) Manufacturing economics of plant-made biologics: case studies in therapeutic and industrial enzymes. Biomed Res Int 2014:e256135. doi:10.1155/2014/256135
Verma D, Kanagaraj A, Jin S, Singh ND, Kolattukudy PE, Daniell H (2010) Chloroplast-derived enzyme cocktails hydrolyse lignocellulosic biomass and release fermentable sugars. Plant Biotechnol J 8:332–350. doi:10.1111/j.1467-7652.2009.00486.x
Verma D, Jin S, Kanagaraj A, Singh ND, Daniel J, Kolattukudy PE, Miller M, Daniell H (2013) Expression of fungal Cutinase and Swollenin in tobacco chloroplasts reveals novel enzyme functions and/or substrates. PLoS One 8:e57187. doi:10.1371/journal.pone.0057187
Watson J, Koya V, Leppla SH, Daniell H (2004) Expression of Bacillus anthracis protective antigen in transgenic chloroplasts of tobacco, a non-food/feed crop. Vaccine 22:4374–4384. doi:10.1016/j.vaccine.2004.01.069
Wooley R, Ruth M, Glassner D, Sheehan J (1999) Process design and costing of bioethanol technology: a tool for determining the status and direction of Research and Development. Biotechnol Prog 15:794–803. doi:10.1021/bp990107u
Zedler JAZ, Mullineaux CW, Robinson C (2016) Efficient targeting of recombinant proteins to the thylakoid lumen in Chlamydomonas reinhardtii using a bacterial Tat signal peptide. Algal Res 19:57–62. doi:10.1016/j.algal.2016.07.007
Zittelli GC, Rodolfi L, Bassi N, Biondi N, Tredici MR (2013) Photobioreactors for microalgal biofuel production. In: Borowitzka MA, Moheimani NR (eds) Algae for biofuels and energy. Springer Netherlands, Dordrecht, pp 115–131
Acknowledgements
We thank Prof. Paolo Iadarola and Dr. Marco Fumagalli for their technical help. We gratefully acknowledge Fondazione Bussolera-Branca (FBB) for the generous financial support. The authors are indebted to Dr. Fabio Cei (President of FBB) and Prof. Roberto Schmid for their continuous support and interest in our work. Also, we are thankful to ICGEB and DBT, Govt. of India, for the financial support. This work was supported in part (P.L. and M.G.-C.) by the University of Geneva and the Swiss National Science Foundation (31003A_146300).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 2468 kb).
Rights and permissions
About this article
Cite this article
Faè, M., Accossato, S., Cella, R. et al. Comparison of transplastomic Chlamydomonas reinhardtii and Nicotiana tabacum expression system for the production of a bacterial endoglucanase. Appl Microbiol Biotechnol 101, 4085–4092 (2017). https://doi.org/10.1007/s00253-017-8164-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8164-1