Abstract
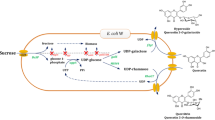
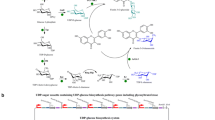
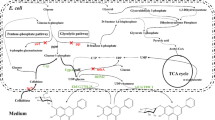
Quercetin, a flavonol aglycone, is one of the most abundant flavonoids with high medicinal value. The bioavailability and pharmacokinetic properties of quercetin are influenced by the type of sugars attached to the molecule. To efficiently diversify the therapeutic uses of quercetin, Escherichia coli was harnessed as a production factory by the installation of various plant and bacterial UDP-xylose sugar biosynthetic genes. The genes encoding for the UDP-xylose pathway enzymes phosphoglucomutase (nfa44530), glucose-1-phosphate uridylyltransferase (galU), UDP-glucose dehydrogenase (calS8), and UDP-glucuronic acid decarboxylase (calS9) were overexpressed in E. coli BL21 (DE3) along with a glycosyltransferase (arGt-3) from Arabidopsis thaliana. Furthermore, E. coli BL21(DE3)/∆pgi, E. coli BL21(DE3)/∆zwf, E. coli BL21(DE3)/∆pgi∆zwf, and E. coli BL21(DE3)/∆pgi∆zwf∆ushA mutants carrying the aforementioned UDP-xylose sugar biosynthetic genes and glycosyltransferase and the galU-integrated E. coli BL21(DE3)/∆pgi host harboring only calS8, calS9, and arGt-3 were constructed to enhance whole-cell bioconversion of exogeneously supplied quercetin into 3-O-xylosyl quercetin. Here, we report the highest production of 3-O-xylosyl quercetin with E. coli BL21 (DE3)/∆pgi∆zwf∆ushA carrying UDP-xylose sugar biosynthetic genes and glycosyltransferase. The maximum concentration of 3-O-xylosyl quercetin achieved was 23.78 mg/L (54.75 μM), representing 54.75 % bioconversion, which was an ~4.8-fold higher bioconversion than that shown by E. coli BL21 (DE3) with the same set of genes when the reaction was carried out in 5-mL culture tubes with 100 μM quercetin under optimized conditions. Bioconversion was further improved by 98 % when the reaction was scaled up in a 3-L fermentor at 36 h.




Similar content being viewed by others
References
Aquino R, Morelli S, Lauro MR, Abdo S, Saija A, Tomaino A (2001) Phenolic constituents and antioxidant activity of an extract of Anthurium versicolor leaves. J Nat Prod 64:1019–1023
Buer CS, Imin N, Djordjevic MA (2010) Flavonoids: new roles for old molecules. J Integr Plant Biol 52:98–111
Chen X (2011) Fermenting next generation glycosylated therapeutics. ACS Chem Biol 6:14–17
Choi JA, Kim JY, Lee JY, Kang CM, Kwon HJ, Yoo YD, Kim TW, Lee YS, Lee SJ (2001) Induction of cell cycle arrest and apoptosis in human breast cancer cells by quercetin. J Int Oncol 19:837–844
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in E. coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645
Du J, Shao Z, Zhao H (2011) Engineering microbial factories for synthesis of value-added products. J Ind Microbiol Biotechnol 38:873–890
Dudek-Makuch M, Matlawska I (2011) Flavonoids from the flowers of Aesculus hippocastanum. Acta Pol Pharm 68:403–408
Griffith BR, Langenhan JM, Thorson JS (2005) ‘Sweetening’ natural products via glycorandomization. Curr Opin Biotechnol 16:622–630
Hwang EI, Kaneko M, Ohnishi Y, Horinouchi S (2003) Production of plant-specific flavanones by Escherichia coli containing an artificial gene cluster. Appl Environ Microbiol 69:2699–2706
Joseph AC, Yajun Y, Koffas MA (2006) Biosynthesis of isoprenoids, polyunsaturated fatty acids and flavonoids in Saccharomyces cerevisiae. Microb Cell Fact 5:20
Kajjout M, Rolando C (2011) Regiospecific synthesis of quercetin-O-β-d-glucosylated and O-β-d-glucuronidated isomers. Tetrahedron 67:4731–4741
Katsuyama Y, Funa N, Miyahisa I, Horinouchi S (2007) Synthesis of unnatural flavonoids and stilbenes by exploiting the plant biosynthetic pathway in Escherichia coli. Chem Biol 14:613–621
Kelly GS (2011) Quercetin. Altern Med Rev 16:172–194
Kim BG, Jung NR, Joe EJ, Hur HG, Lim Y, Chong Y, Ahn JH (2010) Bacterial synthesis of a flavonoid deoxyaminosugar conjugate in Escherichia coli expressing a glycosyltransferase of Arabidopsis thaliana. ChemBioChem 11:2389–2392
Kim BG, Sung SH, Ahn JH (2012) Biological synthesis of quercetin 3-O-N-acetylglucosamine conjugate using engineered Escherichia coli expressing UGT78D2. Appl Microbiol Biotechnol 93:2447–2453
Lim EK, Doucet CJ, Hou B, Jackson RG, Abrams SR, Bowles DJ (2005) Resolution of (±) abscisic acid using an Arabidopsis glycosyltransferase. Tetrahedron Asymmetry 16:143–147
Lim EK, Ashford DA, Hou B, Jackson RG, Bowles DJ (2004) Arabidopsis glycosyltransferases as biocatalysts in fermentation for regioselective synthesis of diverse quercetin glucosides. Biotechnol Bioeng 87:623–631
Lim H, Jin JH, Park H, Kim HP (2011) New synthetic anti-inflammatory chrysin analog 5,7-dihydroxy-8-(pyridine-4yl) flavones. Eur J Pharmacol 670:617–622
Lu Y, Foo LY (1997) Identification and quantification of major polyphenols in apple pomace. Food Chem 59:187–194
Malla S, Koffas MA, Kazlauskas RJK, Kim BG (2011) Production of 7-O-methyl aromadendrin, a medicinally valuable flavonoid, in Escherichia coli. Appl Environ Microbiol 78:684–694
Markham KR, Ternai B, Stanley R, Geiger H, Mrbry TJ (1978) Carbon-13 NMR studies of flavonoids—III: Naturally occurring flavonoid glycosides and their acylated derivatives. Tetrahedron 34:1389–1397
Méndez C, Salas JA (2001) Altering the glycosylation pattern of bioactive compounds. Trends Biotechnol 19:449–456
Minami H, Kim JS, Ikezawa N, Takemura T, Katayama T, Kumagai H, Sato F (2008) Microbial production of plant benzyl isoquinoline alkaloids. Proc Natl Acad Sci U S A 105:7393–7398
Nguyen TT, Tran E, Nguyen TH, Do PT, Huynh TH, Huynh HH (2004) The role of activated MEK-ERK pathway in quercetin-induced growth inhibition and apoptosis in A549 lung cancer cells. Carcinogenesis 25:647–659
Ong CS, Tran E, Nguyen TT, Ong CK, Lee SK, Lee JJ, Ng CP, Leong C, Huynh H (2004) Quercetin-induced growth inhibition and cell death in nasopharyngeal carcinoma cells are associated with increase in bad and hypophosphorylated retinoblastoma expressions. Oncol Rep 11:727–733
Park SR, Ahn MS, Han AR, Park JW, Yoon YJ (2011) Enhanced flavonoid production in Streptomyces venezuelae via metabolic engineering. J Microbiol Biotechnol 21:1143–1146
Plazoni A, Bucar F, Maleš Ž, Mornar A, Nigovi B, Kujundži N (2009) Identification and quantification of flavonoids and phenolic acids in burr parsley (Caucalis platycarpos), using high performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. Molecules 14:2466–2490
Pourcel L, Routaboul JM, Cheynier V, Lepiniec L, Debeaujon I (2007) Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trends Plant Sci 12:29–36
Ramos S (2007) Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J Nutr Biochem 18:427–442
Ruffing A, Chen RR (2006) Metabolic engineering of microbes for oligosaccharide and polysaccharide synthesis. Microb Cell Fact 5:25
Salas JA, Méndez C (2007) Engineering the glycosylation of natural products in actinomycetes. Trends Microbiol 15:219–232
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Sánchez-del-Campo L, Sáez-Ayala M, Chazarra S, Cabezas-Herrera J, Rodríguez-López JN (2009) Binding of natural and synthetic polyphenols to human dihydrofolate reductase. Int J Mol Sci 10:5398–5410
Simkhada D, Kim EM, Lee HC, Sohng JK (2009) Metabolic engineering of Escherichia coli for the biological synthesis of 7-O-xylosyl naringenin. Mol Cells 28:397–401
Simkhada D, Lee HC, Sohng JK (2010) Genetic engineering approach for the production of rhamnosyl and allosyl flavonoids from Escherichia coli. Biotechnol Bioeng 107:154–162
Sugamoto K, Matsusita Y, Matsui K, Kurogi C, Matsui T (2011) Synthesis and antibacterial activity of chalcones bearing prenyl or geranyl groups from Angelica keiskei. Tetrahedron 67:5346–5359
Tang LJ, Chen X, Sun YN, Jia Y, Lu J, Han Y, Jiang X, Cheng CC, He CC, Qiu PH, Li XK (2011) Synthesis and biological studies of 4′,7,8-trihydroxy-isoflavone metal complexes. J Inorg Biochem 105:1623–1629
Thorson JS, Barton WA, Hoffmeister D, Albermann C, Nikolov DB (2004) Structure-based enzyme engineering and its impact on in vitro glycorandomization. ChemBioChem 5:16–25
Trantas E, Panopoulos N, Ververidis F (2009) Metabolic engineering of the complete pathway leading to heterologous biosynthesis of various flavonoids and stilbenoids in Saccharomyces cerevisiae. Metab Eng 11:355–366
Vogt T, Jones P (2000) Glycosyltransferases in plant natural product synthesis: characterization of a supergene family. Trends Plant Sci 5:380–6
Williams GJ, Yang J, Zhang C, Thorson JS (2010) Recombinant E. coli prototype strains for in vivo glycorandomization. ACS Chem Biol 6:95–100
Willits MG, Giovanni M, Prata RTN, Kramer CM, De Luca V, Steffens JC, Graser G (2004) Biofermentation of modified flavonoids: an example of in vivo diversification of secondary metabolites. Phytochemistry 65:31–41
Winkel-Shirley B (2001) Flavonoid biosynthesis, a colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126:485–493
Yan Y, Chemler J, Huang L, Martens S, Koffas MA (2005) Metabolic engineering of anthocyanin biosynthesis in Escherichia coli. Appl Environ Microbiol 71:3617–3623
Yoon JA, Kim BG, Lee WJ, Lim Y, Chong Y, Ahn JH (2012) Production of a novel quercetin glycoside through metabolic engineering of Escherichia coli. Appl Environ Microbiol 78:4256–4262
Acknowledgment
This work was supported a grant from the Next-Generation BioGreen 21 Program (SSAC, grant no. PJ008013), Rural Development Administration and the Converging Research Center Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (20090082333), Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pandey, R.P., Malla, S., Simkhada, D. et al. Production of 3-O-xylosyl quercetin in Escherichia coli . Appl Microbiol Biotechnol 97, 1889–1901 (2013). https://doi.org/10.1007/s00253-012-4438-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4438-9