Abstract
Isobutene (2-methylpropene) is one of those chemicals for which bio-based production might replace the petrochemical production in the future. Currently, more than 10 million metric tons of isobutene are produced on a yearly basis. Even though bio-based production might also be achieved through chemocatalytic or thermochemical methods, this review focuses on fermentative routes from sugars. Although biological isobutene formation is known since the 1970s, extensive metabolic engineering is required to achieve economically viable yields and productivities. Two recent metabolic engineering developments may enable anaerobic production close to the theoretical stoichiometry of 1isobutene + 2CO2 + 2H2O per mol of glucose. One relies on the conversion of 3-hydroxyisovalerate to isobutene as a side activity of mevalonate diphosphate decarboxylase and the other on isobutanol dehydration as a side activity of engineered oleate hydratase. The latter resembles the fermentative production of isobutanol followed by isobutanol recovery and chemocatalytic dehydration. The advantage of a completely biological route is that not isobutanol, but instead gaseous isobutene is recovered from the fermenter together with CO2. The low aqueous solubility of isobutene might also minimize product toxicity to the microorganisms. Although developments are at their infancy, the potential of a large scale fermentative isobutene production process is assessed. The production costs estimate is 0.9 € kg−1, which is reasonably competitive. About 70% of the production costs will be due to the costs of lignocellulose hydrolysate, which seems to be a preferred feedstock.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, the need for renewable resources is constantly brought under discussion, providing interesting alternatives for sustainable production of chemicals and fuels, compared to the use of fossil feedstocks. With finite fossil feedstocks, it might be interesting to switch to fermentative processes in order to produce chemicals. Isobutene (2-methylpropene, see Fig. 1), which is currently produced at large scale by petrochemically cracking crude oil, could be one of those chemicals.
Recently, the company Global Bioenergies has patented their research on the fermentative production of isobutene, showing that bio-based isobutene production is possible (Marlière 2010, 2011a, b). Since isobutene is a gaseous compound at fermentative conditions, it might easily be recovered from the bioreactor. Moreover, if this compound is produced cost efficiently, its conversion into biofuels, or any other possible product, could become attractive.
Even though bio-based isobutene production seems favorable, there is still little knowledge on how to realize this production in a fermentation process. It might resemble the production of isoprene. Isoprene contains two double bonds of carbon, making it suitable for addition polymerization (Lee et al. 2011). Genencor, a division of Danisco U.S. Inc., is already working on the production of BioIsoprene—isoprene produced through fermentation (Singh 2010; Whited et al. 2011). Escherichia coli is used as the producing organism, using glucose as the carbon source. The isoprene is recovered from the fermenter off-gas and polymerized to rubber. Although bioisoprene production is promising, the highest yield of the produced isoprene on sugars reported in the literature is currently only 0.11 g g−1 (Lee et al. 2011). In line with the inspiring developments in biotechnological isoprene production, this review aims to summarize the status and perspectives for economically competitive biotechnological isobutene production.
Isobutene market and current petrochemical production
Isobutene is a key precursor for numerous chemicals. Currently, isobutene is obtained from a number of petrochemical sources (Romanow-Garcia and Hoffman 2007; Obenaus et al. 2005): a C4 stream from a steam cracker with the butadiene removed, butene–butane fractions from a catalytic cracker, and n-butane (from LPG) which is isomerized to isobutane and then dehydrogenated to isobutene. LyondellBasell derives isobutene from the dehydration of tert-butanol which is coproduced in its propene oxide process. The worldwide demand for isobutene has been estimated to exceed 10 million metric tons per year (OECD 2003) and its market value at 18 billion euro (de Guzman 2011). Due to the presence of its reactive double bond, isobutene can take part in various kinds of chemical reactions, resulting in a great variety of products. Well-known examples of these reactions are hydrogenation, oxidation, hydration, and numerous other additions. A schematic overview of the main reactions with isobutene and resulting products is presented in Fig. 1. One of the most widely used reactions in the industry is the electrophilic addition of methanol leading to methyl tert-butyl ether (MTBE), an antiknocking agent in fuels, dedicated to the automotive industry. However, MTBE might be prohibited in the near future because of health and environmental concerns. Therefore, other fuel additives have to be considered, for instance production of the more environmentally friendly ethyl tert-butyl ether (ETBE) from isobutene and ethanol, or isooctane, where isobutene reacts with isobutane.
Besides these important applications, isobutene is also used in a variety of polymerization reactions. One of the resulting products is butyl rubber, a polymer of isobutene and isoprene, which is used for the production of tires, gas masks, baseballs, and even chewing gum.
A more futuristic application of isobutene will be the production of antioxidants. When high purity isobutene reacts with p-cresol or anisole, synthetic antioxidants are obtained, which can be used in the food industry and are expected to have an increasing demand. Oxidation of isobutene leads to methacrolein and subsequently to methacrylic acid, a building block for poly(methyl methacrylate) plastics. Finally, tert-butanol and tert-amines can be produced from isobutene with water and ammonia, respectively, for use in various chemical processes and products (Romanow-Garcia and Hoffman 2007; Obenaus et al. 2005).
Although overexposure should be prevented, isobutene shows little toxicity to humans (Obenaus et al. 2005). It is flammable in air between 1.8 and 8.8 vol.% at 20 °C and can lead to explosions (Obenaus et al. 2005).
Enzyme reactions for isobutene biosynthesis
Before treating complete metabolic pathways from sugars to isobutene, the known enzymatic reactions yielding isobutene as product are discussed. These reactions are candidates for the final step in a metabolic pathway.
Isovalerate decarboxylation by cytochrome P450
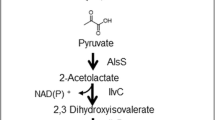
Biological isobutene formation was mentioned for the first time by Fukuda et al. (1984, 1987). Out of a total of 178 tested strains from 80 genera, strains of 33 fungi, 31 yeasts, and 6 bacteria produced traces of isobutene under aerobic growth conditions. The highest production rate was found for the yeast Rhodotorula minuta var. texensis IFO 1102. Optimized volume-specific and dry cell mass-specific rates were 0.45 mg L −1broth h−1 and 41 μg g−1 h−1, respectively, in the presence of l-phenylalanine as an inducer (Fujii et al. 1987). It was shown that isobutene was formed by the decarboxylation of isovalerate, which is produced in the catabolic pathway of l-leucine (Fujii et al. 1985; Fukuda et al. 1994) (see Fig. 2). The responsible enzyme was a microsomal cytochrome P450 (cytrochrome P450rm) with an activity of 0.011 μmol min−1 g −1protein in the presence of NADPH, O2, and a second cytochrome P450, which was an NADPH reductase (Fujii et al. 1988; Fukuda et al. 1994). The latter components are characteristic of monooxygenases.
In line with the monooxygenase characteristics, the physiological role of cytochrome P450rm seems to be the 4-hydroxylation of benzoate (Fig. 2), which is part of the l-phenylalanine dissimilation pathway of R. minuta (Shimaya and Fujii 2000). From the dissimilarity of benzoate 4-hydroxylation and isovalerate decarboxylation, one might anticipate that many other conversions would be catalyzed by the enzyme, but almost none have been described. The gene was successfully overexpressed in Yarrowia lipolytica (Shiningavamwe et al. 2006), but the resulting strain was only tested for benzoate hydroxylation.
Decarboxylation of 3-hydroxyisovalerate by mevalonate diphosphate decarboxylase
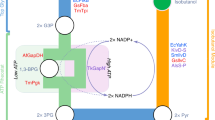
As shown in Fig. 3, isobutene can also be produced by decarboxylation with concomitant dehydration of 3-hydroxyisovalerate (3-hydroxy-3-methylbutyrate) (Gogerty and Bobik 2010; Marlière 2010). This is a side activity of mevalonate diphosphate decarboxylase (MDD, EC 4.1.1.33), an enzyme involved in ergosterol or lower isoprenoid biosynthesis. The MDD family is present in a large variety of microorganisms, though none of which are known to produce isobutene naturally.
Reactions catalyzed by mevalonate diphosphate decarboxylase. The natural reaction is shown at the bottom. ATP is required to phosphorylate the 3-hydroxy group, which is thought to leave as phosphate during decarboxylation (Gogerty and Bobik 2010)
The research groups of Marlière (Global Bioenergies) and Bobik (Iowa State University) both designed a synthetic approach implementing the MDD decarboxylation activity into an E. coli host to induce isobutene biosynthesis via 3-hydroxyisovalerate.
A patent (Marlière 2010) describes MDDs for production of isobutene and also for analogous formation of 1-pentene, 1-butene, propene, and ethene. It describes gene sequences of Saccharomyces cerevisiae and 10 other organisms coding for MDDs selected from a larger library in search for a kinetically favorable MDD. Upon expression in E. coli, the highest isobutene formation activity was found for MDD from Picrophilus torridus, an obligate aerobic archaeon. Protein engineering using random mutagenesis was mentioned to result in improved production of isobutene. Regrettably, no values for isobutene formation rates or yields are disclosed by Marlière (2010).
Besides the use of a different E. coli recombinant variant and expression vector, Gogerty and Bobik (2010) also used a MDD from S. cerevisiae (ScMDD) and showed that it was able to convert 3-hydroxyisovalerate into isobutene. In addition, error-prone PCR resulted in an increase of enzyme activity. Regarding whole cells, a 38-fold higher isobutene production was observed among the constructs containing mutated ScMDD, leading to an activity of 0.33 μg h−1 g −1cells . When purified, though, enzyme assays of mutated ScMDD did not result in such an increase in activity. This might be due to instability of the enzyme. The authors estimated that the level of cellular activity is about 106-fold below the activity needed for a commercial process.
Dehydration of isobutanol by oleate hydratase
Isobutanol can be converted to alkenes by a reaction at >100 °C using dehydration catalysts such as some acids, alumina and silica catalysts, and metal salts. The reaction can be carried out in both gas and liquid phases and leads to a mixture of 1-butene, cis-2-butene, trans-2-butene, and isobutene. The ratio is determined by the thermodynamics, reaction conditions, and catalysts used, but there is no known method for cleanly dehydrating isobutanol to >99% isobutene (Peters et al. 2010).
Until recently, enzymatic dehydration of isobutanol was considered to be virtually impossible. Hydrolyases, the class of enzymes catalyzing water addition to a C=C bond and also the reverse water elimination reaction, usually require a C=O group conjugated to the C=C bond (Jin and Hanefeld 2011). An exception is the well-known oleate hydratase (EC 4.2.1.53), which occurs in many microorganisms but was only recently purified, using Elizabethkingia meningoseptica (formerly Pseudomonas sp.). The gene was cloned and expressed in E. coli (Bevers et al. 2009). The enzyme is a monomer of 73 kDa and contains a nonessential calcium ion. The reaction mechanism still remains to be elucidated. This oleate hydratase converts oleic acid to (R)-10-hydroxystearate (Fig. 4). Its substrate specificity is narrow. Nevertheless, Marlière (2011b) traced 165 homologues to the E. meningoseptica sequence in order to find activity for dehydration of isobutanol and other alcohols. Upon cloning and expression in E. coli and partial purification of the enzymes, several showed isobutene formation from aqueous isobutanol (see Fig. 4) or aqueous tert-butanol. No information was given about the types of enzyme or amounts of isobutene.
Reactions catalyzed by oleate hydratase. The natural reaction is shown at the bottom. The top reaction is mentioned by Marlière (2011b)
Isobutene formation from xenobiotic precursors
Roberts et al. (1991) reported that isoforms of cytochrome P450 from rabbit liver microsomes deformylate trimethylacetaldehyde into isobutene. The activity was up to 0.86 mol min−1 per mol P450, when using 0.2 μmol L−1 of P450, 0.6 μmol L−1 of rabbit liver reductase, 1 mmol L−1 of aldehyde, and 1 mmol L−1 of NADPH. This xenobiotic reaction has not been studied for synthetic purposes.
Schäfer et al. (2011) detected isobutene in cultures of betaproteobacterial strains (Aquincola tertiaricarbonis L108, Methylibium petroleiphilum PM1, and Methylibium sp. strain R8) growing on MTBE or ETBE. Isobutene was formed from the intermediate tert-butanol. A putative tert-alkyl alcohol monooxygenase belonging to the Rieske nonheme mononuclear iron enzymes was assumed to have a side reaction leading to this unusual water elimination reaction. This reaction will not be useful in a pathway towards isobutene because MTBE, ETBE, and tert-butanol are to be produced from isobutene (see Fig. 1).
Metabolic pathways
Figure 5 shows metabolic pathways ending with one of the aforementioned isobutene-producing enzyme activities. All start with the conversion of glucose to pyruvate via glycolysis. The remaining part of these pathways will be treated subsequently.
Pathway via isovalerate
In Fig. 5, the pathway from glucose straight down to 2-oxoisocaproate (4-methyl-2-oxopentanoate) is generally used for leucine biosynthesis. In the subsequent three steps from 2-oxoisocaproate to isobutene, two CO2 equivalents are split off. Even though R. minuta produces isobutene via this pathway without metabolic engineering, there are several aspects to be considered regarding the industrial use of this isobutene production mechanism:
-
In addition to 2 mol pyruvate, 2 mol acetyl-CoA is required per mol isobutene. This will translate into a low maximum yield of isobutene on glucose.
-
O2 is required, whereas anaerobic fermentation is preferred to aerobic fermentation with respect to costs and with respect to explosion risks for isobutene/air mixtures in fermentation off-gas.
-
So far, the highest achieved isobutene production rate is 0.45 mg L−1 h−1 (Fujii et al. 1987), whereas >1 g L−1 h−1 should be achieved for commercial production of low value chemicals (Gogerty and Bobik 2010).
-
The production pathway should preferably be transferred into one of the common industrial microorganisms such as E. coli or S. cerevisiae, to create robustness of the producing organisms throughout the process.
-
The isobutene-producing cytochrome P450 requires the presence of heme, a cofactor that poorly lends itself for recombinant expression in bacteria and for improvement of enzyme parameters.
Pathways via 3-hydroxyisovalerate
The pathway on the right-hand side of Fig. 5 can be used for the production of isobutene via 3-hydroxyisovalerate (Marlière 2010; Gogerty and Bobik 2010). The requirement of acetyl-CoA for the production of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) from acetoacetyl-CoA implies that at least 3 mol pyruvate or acetyl-CoA per mol isobutene is required, translating into a net equation such as
This implies a maximum yield of 0.21 gisobutene g −1glucose . The formed NADH has to be regenerated into NAD+ through aeration. Gogerty and Bobik (2010) have suggested several options to improve the isobutene yield by producing 3 instead of 2 mol acetyl-CoA per mol glucose.
Lately, Marlière (2011a) patented a method for enzymatic production of 3-hydroxyisovalerate from acetyl-CoA and acetone. As shown in Fig. 5, such an enzymatic activity could shorten the pathway from acetoacetyl-CoA to 3-hydroxyisovalerate considerably. Still, 3 mol pyruvate or acetyl-CoA will be needed for the production of 1 mol 3-hydroxyisovalerate and, ultimately, isobutene. Therefore, this invention results in the same issues posed before: 1.5 mol glucose is needed for the production of 1 mol isobutene, and aeration is required.
Hypothetically, a pathway via 2-hydroxyisovalerate (Fig. 5, center) would use only 2 mol of acetyl-CoA per mol of isobutene and still use the aforementioned 3-hydroxyisovalerate decarboxylation into isobutene. This pathway would partly be identical to the pathway via isobutanol (see next section) but would require 2-oxoisovalerate reduction to l- or d-2-hydroxyisovalerate. Formation of the latter enantiomer can be achieved by a d-lactate dehydrogenase (Chambellon et al. 2008). An enzyme for subsequent isomerization of d-2-hydroxyisovalerate to 3-hydroxyisovalerate is not known but might be found upon screening. The conversion of isocitrate to citrate by aconitate hydratase (EC 4.2.1.3) is an example of a comparable isomerization reaction.
Pathway via isobutanol
Examples of naturally isobutanol-producing microorganisms are S. cerevisiae, when fed with valine as a carbon source (Dickinson et al. 1998), and Lactococcus lactis (Atsumi et al. 2009). However, commercially relevant isobutanol biosynthesis has been developed by the group of Liao using constructs in E. coli (Atsumi et al. 2008). The pathway runs via acetolactate and 2-oxoisovalerate, instead of acetoacetyl-CoA, (Fig. 5, left-hand side). Atsumi et al. (2008) achieved 82% of the theoretical yield of 1 mol isobutanol per mol glucose. Recently, an isobutanol production rate of 1.35 g L−1 h−1 was achieved during the first 22 h at 0.35 L scale and at a cell density of 7 g L−1 (Baez et al. 2011). The rate dropped to an average of 0.75 g L−1 h−1 between 22 and 72 h. Competitive catabolic pathways were removed successfully from the E. coli strain. An imbalance between NADH and NADPH might appear according to Fig. 5, where production of 1 mol isobutanol per mol glucose would produce 1 mol NADH and would consume 1 mol NADPH. However, in E. coli, such imbalance will be prevented by a transhydrogenase and by NADPH production via the pentose phosphate pathway (Sauer et al. 2004). An insufficient supply of NADPH might inhibit isobutanol production using a Corynebacterium glutamicum strain (Smith et al. 2010). Introducing a transhydrogenase and using a pathway via pyruvate carboxylase and/or PEP carboxylase, malate dehydrogenase, and malic enzyme solved this issue (Blombach et al. 2011). Using Clostridium cellulolyticum, it has been shown that direct production of isobutanol from cellulose is possible (Higashide et al. 2011). Production from CO2 is also an interesting alternative, using photosynthetic microorganisms such as algae and cyanobacteria (Atsumi et al. 2009).
The companies Gevo and Lanxess are developing fermentative isobutanol production and its chemical dehydration to isobutene. Commercial production has been announced to start in 2012 (de Guzman 2011). Other companies such as Butamax, a joint venture of Dupont and BP, are also pursuing fermentative isobutanol production, and the number of patents in this field is increasing rapidly. Although in this section we focus on fermentative conversion of sugars to isobutanol, bio-based production of isobutanol can also be done chemically. One possibility is to produce synthesis gas from biomass and subsequently convert it into isobutanol in the presence of a catalyst containing metals such as copper or magnesium (Subramani and Gangwal 2008).
Introducing the aforementioned oleate hydratase with isobutanol-dehydrating activity (Marlière 2011a) in an isobutanol-producing strain can theoretically result in the production of 1 mol isobutene per mol glucose according to the following stoichiometry (Fig. 5, left-hand pathway):
This corresponds to a maximum product yield of 0.31 gisobutene g −1glucose . E. coli is used for developing production via this pathway, but achieved yields or productivities of isobutene have not been disclosed so far (Marlière 2011b). Probably the isobutanol-dehydrating activity needs to be significantly improved to reach commercially viable productivities.
The intermediates isobutyraldehyde and isobutanol will be relatively toxic to microbial cells (Atsumi et al. 2009; Baez et al. 2011), so their concentration should be kept low by relatively fast consecutive reactions. That would also minimize their loss by evaporation.
Summary of metabolic options
In theory, several pathways should be able to produce 1 mol isobutene per mol glucose. Such pathways would be redox neutral and might be able to generate ATP. Some ATP generation is required to support cell growth and cellular maintenance. Cell growth will reduce the achievable yield somewhat below the maximum of 0.31 gisobutene g −1glucose . For subsequent calculations, 0.25 g g−1 will be used. Isobutene production according to the maximum stoichiometry will result in CO2 and H2O production, but does not involve O2. Therefore, anaerobic conditions will suffice.
Process design
The development of microbial hosts is still in its infancy stage with process details not published yet. However, considering process aspects at this stage will already be helpful to evaluate the direction of metabolic engineering and to evaluate the potential of fermentative isobutene production. Since isobutene is a large scale product, a fermentative isobutene plant capacity might be 50,000 metric tons per year.
Choice of substrate
To achieve an economically and ecologically sustainable process, future isobutene processes might be focused on using lignocellulosic hydrolysate as a substrate. This might be obtained by processes that are still in the development stage (Geddes et al. 2011). Analogous to ethanol-producing microorganisms, isobutene-producing microorganisms could be engineered for conversion of all C5- and C6-sugars in lignocellulose hydrolysate into product and for tolerance towards potential inhibitors such as furanics, phenolics, and acetic acid. In ethanol manufacturing, the achievable mass concentrations of ethanol will be at most 50% of the feed concentration of lignocellulose hydrolysate. Therefore, efficient ethanol distillation relies on fermentation of relatively concentrated hydrolysates. Isobutene production might use more dilute hydrolysate because the isobutene concentration in the off-gas will not depend on it. Besides, the risk that contaminants from the hydrolysate complicate the product recovery is much smaller for isobutene than for other fermentation products.
Fermentation
The aqueous solubility of isobutene is only 267 mg L−1 at 30 °C and 1 atm (Zhang et al. 2002). If the most favorable fermentation stoichiometry can be achieved, the mole fractions in the fermenter off-gas would be about 2/3CO2 and 1/3isobutene. Then, according to its partial vapor pressure, the dissolved isobutene concentration will be of the order of magnitude of 0.1 g L−1, assuming gas–liquid equilibrium. Comparison with the data for other hydrocarbons suggests that 0.1 g L−1 might be not yet toxic to the microorganism (Straathof 2003), but experimental verification will be necessary.
The fermentation can be done without aeration according to the desired stoichiometry. Chemostats would operate continuously at the aforementioned dissolved isobutene concentration and batch and fed-batch fermenters after a very short initial phase.
Choice of host microorganism
The availability of molecular biology tools and genetic accessibility is essential to facilitate rapid progress in strain development programs. For this reason, E. coli and S. cerevisiae are often used microbial platforms in industrial biotechnology. Perhaps, an even more important process aspect is choosing a microorganism that can perform under the conditions that are optimal for the overall process design. The chemical properties and low solubility of isobutene in water eliminates product tolerance as a consideration, and both microorganisms are able to grow under both aerobic and, the ultimately desirable, anaerobic conditions. However, robustness in lignocellulosic hydrolysates, absence of phage-related problems, and operation at low pH to avoid contaminations might still create an incentive for the use of S. cerevisiae. On the contrary, E. coli has the potential to operate at slightly elevated temperatures and production rates and additionally does not have the compartmentalized metabolism that creates metabolic engineering challenges in S. cerevisiae. With both platforms having benefits, the ability to functionally express the desired enzymes and routes becomes an important criterion. In an extreme case, the goal to achieve the theoretical yield might even result in the use of altogether different strain platforms, such as for instance the genetically less accessible acetogens (Gogerty and Bobik 2010).
Downstream processing
Assuming that a fermentation process would be performed with at least 100 g L−1 fermentable carbohydrate; then, the aforementioned isobutene yield aqueous solubility indicates that <0.5% of the produced isobutene would remain dissolved, and the rest would disappear with the fermenter off-gas. Therefore, isobutene recovery from off-gas might suffice.
In addition to about 2/3carbon dioxide and 1/3isobutene, the off-gas will be saturated by water (4,000 Pa vapor pressure at 30 °C, corresponding to 1.5% (w/w)). It will contain traces of other volatile components, such as acetic acid, at concentrations determined by the fermentation feedstock composition and the side reactions in the fermenter. The allowable concentrations of contaminants in purified isobutene will depend on its use. According to its specifications sheets, LyondellBasell sells >99.75% (w/w) isobutene with <0.05% water and <0.05% acetaldehyde (LyondellBasell 2008), to name a few potential contaminants. For simplicity, we concentrate here on the removal of CO2 and H2O from isobutene only. There are several possible ways to achieve such a gas separation (Hiller et al. 2000):
-
Stage-wise condensation to liquid
-
Pressure swing adsorption
-
Membrane permeation
-
Absorption
-
Combinations of the aforementioned methods
Stage-wise condensation such as in Fig. 6 might seem straightforward because of the large difference in boiling points (−78 °C, −7 °C, and 100 °C for CO2, isobutene, and water, respectively). This would have to be driven by electricity, which is a relatively expensive energy source. A preliminary calculation, assuming pressurizing the off-gas at 20 °C showed that condensed water might contain a certain percentage of isobutene. This stream is small, so this might be acceptable as product loss. More importantly, isobutene that would condense subsequently might contain ~2% CO2. To obtain a purer product stream, a countercurrent condensation–vaporization operation (continuous cryogenic distillation) might be required.
A benefit to this process configuration is the fact that isobutene is obtained as liquid, suitable for transportation. In the separation options treated subsequently, an extra step is necessary to accomplish liquefaction.
Pressure swing adsorption should be able to achieve the required purity because in an adsorption column, poorly adsorbed species will be pushed forward by stronger adsorbed species. Such an adsorption processes may use relatively expensive adsorbent material and will operate at high pressure, so the capital investment may be high. For membrane technology, this also holds: membranes are expensive and the process runs at moderate to high pressure. Separation of isobutene from CO2 by either adsorption or permeation might be achieved using DD3R-zeolite, for example. Isobutane molecules do not penetrate in the zeolite while carbon dioxide molecules can (van den Bergh 2010). The molecular sizes of isobutane and isobutene are almost similar.
As done for flue gases, CO2 removal can be accomplished by absorption in aqueous amine solutions. The absorbed CO2 can be liberated by heating. The water would still need to be removed from the remaining isobutene gas by one of the aforementioned methods. The disadvantage of the adsorption, permeation, and absorption configurations is that CO2 rather than isobutene is captured, while isobutene is the minor component of the two. In that respect, condensation to liquid is more favorable.
The CO2 gas emitted by the process will have to comply with legal standards. Isobutene levels in exhaust gas exceeding 0.50 kg h−1 or 50 mg m−3 are prohibited (GESTIS database on hazardous substances 2011). The liquid broth from the fermentation might be split by filtration or centrifugation in a solution and in a cell concentrate. The solution will not contain high solute concentrations and will probably be sent to wastewater treatment, although it cannot be excluded that part of it might be reused directly in the lignocellulose pretreatment process. The cell concentrate, containing the genetically modified organisms, is probably not easily recycled due to genetic instability or infection risks; then, disposal is required.
Economic considerations
The costs of fermentative isobutene production can be roughly estimated. An exchange rate of 0.7 € $−1 was taken. By analogy to other processes for base chemicals, the main costs should be due to the fermentation, in particular the carbohydrates used for fermentation (Straathof 2011). Assuming that the mass yield of isobutene on lignocellulosic hydrolysate is the aforementioned 25% and that the expenditure for lignocellulose hydrolysate is 0.16 € kg−1 (dry basis) assuming 10% (w/w) fermentable sugars (Humbird et al. 2011), these carbohydrates indeed are the largest cost contributor according to Table 1. Choosing a good volumetric productivity (1 gisobutene L−1 h−1, based on isobutanol productivity by Baez et al. (2011)) would require 5,700 m3 fermentation volume running all year round to achieve a production capacity of 50,000 metric tons per year. Suppose that 7,000 m3 fermenter volume has to be installed because of downtime and because of operating at 90% liquid per vessel. The largest available vessels might be used. Here we assume seven 1,000 m3 anaerobic stirred vessels of 0.34 M€ each (www.matche.com), thus 2.4 M€ purchase costs. The total investment associated to this will be about 5× higher (Humbird et al. 2011). If this would be depreciated linearly in 10 years, the capital charge due to fermenter investments would be ~2.4 M€ per year at a reasonable discount rate. This would translate into 0.05 € kg −1isobutene .
The mass flow of aqueous lignocellulose feed will be about 50 times the isobutene production capacity. This feed flow of 2,500,000 metric tons per year will be converted to a cell suspension of a comparable flow size, i.e., ~350 m3 h−1 which needs to be centrifuged if cell recycle is pursued to minimize costs associated with cell growth. Assuming yeast is used, cells might have a settling rate of 5 mm h−1, translating into a sedimentation area of 70,000 m2. Using 14 centrifuges with ∑ = 5,000 m2, each being purchased for 0.14 M€, economic calculations were done similar as for the fermenters. This leads to 0.05 € kg −1isobutene . These costs are relatively high (see Table 1), so alternative options such as (micro)filtration for cell retention, or no cell retention, should be pursued.
The gas purification costs will depend on the design, but it is assumed that the costs will be of the same order of magnitude as those of pure liquefied O2, N2, or CO2, i.e., ~0.07 € kg−1 according to various internet sources.
The amount of dissolved or suspended organic material to be treated as waste is estimated at 0.3 kg kg −1product . Depending on the local situation, the treatment costs can be significant, such as in Table 1.
A number of other cost sources have not been taken into account, such as minor equipment, maintenance, labor, and utility costs not linked to gas purification. It is assumed that power and cooling water costs will be low for the anaerobic fermentation. According to the estimate of other costs in Table 1, these costs contributions will not be critical. The production costs will largely depend on the price of the lignocellulose feed and on the product yield. Recycling or retaining microbial mass will be important for achieving a high product yield. Moreover, the fermenter-dependent costs and waste treatment costs will increase sharply if the microbial mass cannot be retained. Downstream costs (centrifugation, gas purification, and waste treatment) are about 20% of the total, which is relatively low (Straathof 2011).
LyondellBasell cited a price for petrochemical isobutene of 1.10 € kg−1 (personal communication, May 2011) and Global Bioenergies mentioned 1.37 € kg−1 (T. Buhl, lecture at RRB7, June 2011). These values are higher than the production costs estimation in Table 1, but the margin for profit is modest considering all uncertainties discussed. If the selling price of petrochemical isobutene will increase sharper than the purchase costs of the lignocellulose hydrolysate, the margin will increase. There is a clear incentive to try to develop the proposed strain and process.
Conclusions
Aerobic isobutene production is known since 1984. However, recent discoveries of enzyme activities for producing isobutene from isobutanol or from 3-hydroxyisovalerate have allowed the formulation of anaerobic metabolic routes for converting monosaccharides into isobutene with high yields. The development of recombinant strains has begun, using E. coli. Recovery of isobutene from fermentation off-gas is potentially simple, such that large scale fermentative production of isobutene from lignocellulose hydrolysate might become competitive with the current petrochemical production. Also, direct fermentative production seems to be more favorable than fermentative production of isobutanol followed by chemical dehydration.
References
Atsumi S, Hanai T, Liao JC (2008) Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 451:86–89
Atsumi S, Higashide W, Liao JC (2009) Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde. Nat Biotechnol 27:1177–1180
Baez A, Cho K-M, Liao J (2011) High-flux isobutanol production using engineered Escherichia coli: a bioreactor study with in situ product removal. Appl Microbiol Biotechnol 90:1681–1690
Bevers LE, Pinkse MWH, Verhaert PDEM, Hagen WR (2009) Oleate hydratase catalyzes the hydration of a non-activated carbon-carbon bond. J Bacteriol 191:5010–5012
Blombach B, Riester T, Wieschalka S, Ziert C, Youn JW, Wendisch VF, Eikmanns BJ (2011) Corynebacterium glutamicum tailored for efficient isobutanol production. Appl Environ Microbiol 77:3300–3310
Chambellon E, Rijnen L, Lorquet F, Gitton C, van Hylckama Vlieg JET, Wouters JA, Yvon M (2008) The d-2-hydroxyacid dehydrogenase incorrectly annotated PanE is the sole reduction system for branched-chain 2-keto acids in Lactococcus lactis. J Bacteriol 191:873–881
de Guzman D (2011) Rubber industry seeks bio-based chemicals potential. http://www.icis.com/Articles/2011/07/01/9481223/Rubber-industry-seeks-bio-based-chemicals-potential.html
Dickinson JR, Harrison SJ, Hewlins MJE (1998) An investigation of the metabolism of valine to isobutyl alcohol in Saccharomyces cerevisiae. J Biol Chem 273:25751–25756
Fujii T, Ogawa T, Fukuda H (1985) Production of isobutene by Rhodotorula yeasts. Agric Biol Chem 49:1541–1543
Fujii T, Ogawa T, Fukuda H (1987) Isobutene production by Rhodotorula minuta. Appl Microbiol Biotechnol 25:430–433
Fujii T, Ogawa T, Fukuda H (1988) Preparation of a cell-free, isobutene-forming system from Rhodotorula minuta. Appl Environ Microbiol 54:583–584
Fukuda H, Fujii T, Ogawa T (1984) Microbial production of C3- and C4-hydrocarbons under aerobic conditions. Agric Biol Chem 48:1679–1682
Fukuda H, Fujii T, Ogawa T (1987) Method for producing hydrocarbon mixtures. US Patent 4,698,304
Fukuda H, Fujii T, Sukita E, Tazaki M, Nagahama S, Ogawa T (1994) Reconstitution of the isobutene-forming reaction catalyzed by cytochrome P450 and P450 reductase from Rhodotorula minuta: decarboxylation with the formation of isobutene. Biochem Biophys Res Commun 201:516–522
Geddes CC, Nieves IU, Ingram LO (2011) Advances in ethanol production. Curr Opin Biotechnol 22:312–319
GESTIS database on hazardous substances (2011) http://gestis-en.itrust.de/nxt/gateway.dll?f=templates&fn=default.htm&vid=gestiseng:sdbeng. Accessed 16-09-2011
Gogerty DS, Bobik TA (2010) Isobutene formation from 3-hydroxy-3-methylbutyrate by diphosphomevalonate decarboxylase. Appl Environ Microbiol 76:8004–8010
Higashide W, Li YC, Yang YF, Liao JC (2011) Metabolic engineering of Clostridium cellulolyticum for production of isobutanol from cellulose. Appl Environ Microbiol 77:2727–2733
Hiller H, Reimert R, Marschner F, Renner H-J, Boll W, Supp E, Brejc M, Liebner W, Schaub G, Hochgesand G, Higman C, Kalteier P, Müller W-D, Kriebel M, Schlichting H, Tanz H, Stönner H-M, Klein H, Hilsebein W, Gronemann V, Zwiefelhofer U, Albrecht J, Cowper CJ, Driesen HE (2000) Gas production. In: Ullmann’s encyclopedia of industrial chemistry (electronic edition). Wiley-VCH Verlag GmbH & Co. KGaA
Humbird D, Davis R, Tao L, Kinchin C, Hsu D, Aden A (2011) Process design and economics for biochemical conversion of lignocellulosic biomass to ethanol. Technical report NREL/TP-5100-47764
Jin J, Hanefeld U (2011) The selective addition of water to C=C bonds; enzymes are the best chemists. Chem Commun 47:2502–2510
Lee JW, Kim HU, Choi S, Yi J, Lee SY (2011) Microbial production of building block chemicals and polymers. Curr Opin Biotechnol 3:1–10
LyondellBasell (2008) EUROPE SALES SPECIFICATION: Isobutylene 499156. http://www.lyondellbasell.com/techlit/techlit/SalesSpecs/2211.pdf
Marlière P (2010) Production of alkenes by enzymatic decarboxylation of 3-hydroxyalkanoic acids. WO 2010001078
Marlière P (2011a) Method for the enzymatic production of 3-hydroxy-3-methylbutyric acid from acetone and acetyl-CoA. EP 2295593
Marlière P (2011b) Method for producing an alkene comprising step of converting an alcohol by an enzymatic dehydration step. WO 2011076691
Obenaus F, Droste W, Neumeister J (2005) Butenes. In: Ullmann’s encyclopedia of industrial chemistry (electronic edition). doi:10.1002/14356007.a04_483.pub2
OECD (2003) SIDS initial assessment report: isobutylene. http://www.inchem.org/documents/sids/sids/115117.pdf
Peters M, Taylor J, Henton DE, Manzer LE (2010) Methods of preparing renewable butadiene and renewable isoprene. WO 2010099201
Roberts ES, Vaz AD, Coon MJ (1991) Catalysis by cytochrome P-450 of an oxidative reaction in xenobiotic aldehyde metabolism: deformylation with olefin formation. Proc Natl Acad Sci 88:8963–8966
Romanow-Garcia S, Hoffman HL (2007) Petroleum and its products. In: Kent JA (ed) Kent and Riegel’s handbook of industrial chemistry and biotechnology, vol 1, 11th edn. Springer, New York, pp 801–842
Sauer U, Canonaco F, Heri S, Perrenoud A, Fischer E (2004) The soluble and membrane-bound transhydrogenases UdhA and PntAB have divergent functions in NADPH metabolism of Escherichia coli. J Biol Chem 279:6613–6619
Schäfer F, Muzica L, Schuster J, Treuter N, Rosell M, Harms H, Muller RH, Rohwerder T (2011) Alkene formation from tert-alkyl ether and alcohol degradation by Aquincola tertiaricarbonis L108 and Methylibium spp. Appl Environ Microbiol 77:5981–5987
Shimaya C, Fujii T (2000) Cytochrome P450rm of Rhodotorula functions in the β-ketoadipate pathway for dissimilation of l-phenylalanine. J Biosci Bioeng 90:465–467
Shiningavamwe A, Obiero G, Albertyn J, Nicaud J-M, Smit M (2006) Heterologous expression of the benzoate para-hydroxylase encoding gene (CYP53B1) from Rhodotorula minuta by Yarrowia lipolytica. Appl Microbiol Biotechnol 72:323–329
Singh R (2010) Facts, growth, and opportunities in industrial biotechnology. Org Process Res Dev 15:175–179
Smith K, Cho K-M, Liao J (2010) Engineering Corynebacterium glutamicum for isobutanol production. Appl Microbiol Biotechnol 87:1045–1055
Straathof AJJ (2003) Auxiliary phase guidelines for microbial biotransformations of toxic substrate into toxic product. Biotechnol Prog 19:755–762
Straathof AJJ (2011) The proportion of downstream costs in fermentative production processes. In: Moo-Young M (ed) Comprehensive biotechnology, vol 2. 2nd edn. Elsevier, pp 811–814
Subramani V, Gangwal SK (2008) A review of recent literature to search for an efficient catalytic process for the conversion of syngas to ethanol. Energy Fuel 22:814–839
van den Bergh J (2010) DD3R zeolite membranes in separation and catalytic processes: modelling and application. PhD thesis, Delft University of Technology, Delft
Whited GM, Feher FJ, Benko DA, Cervin MA, Chotani GK, McAuliffe JC, LaDuca RJ, Ben-Shoshan EA, Sanford KJ (2011) Development of a gas-phase bioprocess for isoprene-monomer production using metabolic pathway engineering. Ind Biotechnol 6:152–163
Zhang C, Adesina A, Wainwright M (2002) Solubility studies of isobutene in tertiary butyl alcohol plus water mixtures. J Chem Eng Data 47:1476–1480
Acknowledgments
Thomas van Gaalen and Aljoscha Wahl have given valuable input to this review.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
van Leeuwen, B.N.M., van der Wulp, A.M., Duijnstee, I. et al. Fermentative production of isobutene. Appl Microbiol Biotechnol 93, 1377–1387 (2012). https://doi.org/10.1007/s00253-011-3853-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3853-7