Abstract
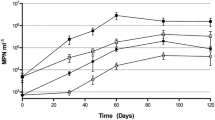
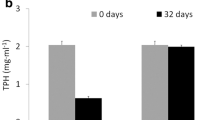
A laboratory experiment was conducted to identify key hydrocarbon degraders from a marine oil spill sample (Prestige fuel oil), to ascertain their role in the degradation of different hydrocarbons, and to assess their biodegradation potential for this complex heavy oil. After a 17-month enrichment in weathered fuel, the bacterial community, initially consisting mainly of Methylophaga species, underwent a major selective pressure in favor of obligate hydrocarbonoclastic microorganisms, such as Alcanivorax and Marinobacter spp. and other hydrocarbon-degrading taxa (Thalassospira and Alcaligenes), and showed strong biodegradation potential. This ranged from >99% for all low- and medium-molecular-weight alkanes (C15–C27) and polycyclic aromatic hydrocarbons (C0- to C2- naphthalene, anthracene, phenanthrene, dibenzothiophene, and carbazole), to 75–98% for higher molecular-weight alkanes (C28–C40) and to 55–80% for the C3 derivatives of tricyclic and tetracyclic polycyclic aromatic hydrocarbons (PAHs) (e.g., C3-chrysenes), in 60 days. The numbers of total heterotrophs and of n-alkane-, aliphatic-, and PAH degraders, as well as the structures of these populations, were monitored throughout the biodegradation process. The salinity of the counting medium affects the counts of PAH degraders, while the carbon source (n-hexadecane vs. a mixture of aliphatic hydrocarbons) is a key factor when counting aliphatic degraders. These limitations notwithstanding, some bacterial genera associated with hydrocarbon degradation (mainly belonging to α- and γ-Proteobacteria, including the hydrocarbonoclastic Alcanivorax and Marinobacter) were identified. We conclude that Thalassospira and Roseobacter contribute to the degradation of aliphatic hydrocarbons, whereas Mesorhizobium and Muricauda participate in the degradation of PAHs.







Similar content being viewed by others
References
Abraham WR, Meyer H, Yakimov M (1998) Novel glycine containing glucolipids from the alkane using bacterium Alcanivorax borkumensis. Biochim Biophys Acta 1393:57–62
Alonso-Gutiérrez J, Costa MM, Figueras A, Albaigés J, Viñas M, Solanas AM, Novoa B (2008) Alcanivorax strain detected among the cultured bacterial community from sediments affected by the 'Prestige' oil spill. Mar Ecol Prog Ser 362:25–36
Alonso-Gutiérrez J, Figueras A, Albaigés J, Jiménez N, Viñas M, Solanas AM, Novoa B (2009) Bacterial communities from shoreline environments (Costa da Morte, Northwestern Spain) affected by the Prestige oil spill. Appl Environ Microbiol 75(11):3407–3418
Altschul S, Gish W, Miller W, Myers E, Lipman J (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410
Alzaga R, Montuori P, Ortiz L, Bayona JM, Albaigés J (2004) (2003) Fast solid-phase extraction–gas chromatography–mass spectrometry procedure for oil fingerprinting application to the Prestige oil spill. J Chromatogr A 1025:133–138
Casellas M, Grifoll M, Sabaté J, Solanas AM (1998) Isolation and characterization of a 9-fluorenone-degrading bacterial strain and its role in synergistic degradation of fluorene by a consortium. Can J Microbiol 44(8):734–742
Chung W, King G (2001) Isolation, characterization, and polyaromatic hydrocarbon degradation potential of aerobic bacteria from marine macrofaunal burrow sediments and description of Lutibacterium anuloederans gen. nov., sp. nov., and Cycloclasticus spirillensus sp. nov. Appl Environ Microbiol 67(12):5585–5592
de Zwart J, Nelisse P, Kuenen J (1996) Isolation and characterization of Methylophaga sulfidovorans sp. nov.: an obligately methylotrophic, aerobic, dimethylsulfide oxidizing bacterium from a microbial mat. FEMS Microbiol Ecol 20(4):261–270
Díez S, Sabaté J, Viñas M, Bayona JM, Solanas AM, Albaigés J (2005) The Prestige oil spill I. Biodegradation of a heavy fuel oil under simulated conditions. Environ Toxicol Chem 24(9):2203–2217
Dutta T, Harayama S (2001) Biodegradation of n-alkylcycloalkanes and n-alkylbenzenes via new pathways in Alcanivorax sp. strain MBIC 4326. Appl Environ Microbiol 67(4):1970–1974
Gallego JLR, García-Martínez MJ, Llamas JF, Belloch C, Peláez AI, Sánchez J (2007) Biodegradation of oil tank bottom sludge using microbial consortia. Biodegradation 18:269–281
Gauthier MJ, Lafay B, Christen R, Fernandez L, Acquaviva M, Bonin P, Bertrand JC (1992) Marinobacter hydrocarbonoclasticus gen. nov., sp. nov., a new, extremely halotolerant, hydrocarbon-degrading marine bacterium. Int J Syst Evol Microbiol 42:568–576
Gertler C, Gerdts G, Timmis KN, Yakimov MM, Golyshin PN (2009) Populations of heavy fuel oil-degrading marine microbial community in presence of oil sorbent materials. J Appl Microbiol 107(2):590–605
Gibb S, Hatton A (2004) The occurrence and distribution of trimethylamine-N-oxide in Antarctic coastal waters. Mar Chem 91(1–4):65–75
Hall T (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hara A, Syutsubo K, Harayama S (2003) Alcanivorax which prevails in oil-contaminated seawater exhibits broad substrate specificity for alkane degradation. Environ Microbiol 5(9):746–753
Harayama S, Kishira H, Kasai Y, Shutsubo K (1999) Petroleum biodegradation in marine environments. J Molec Microbiol Biotechnol 1(1):63–70
Huang H, Bowler BFJ, Zhang Z, Oldenburg TBP, Larter SR (2003) Influence of biodegradation on carbazole and benzocarbazole distributions in oil columns from the Liaohe basin, NE China. Org Geochem 34:951–969
Jiménez N, Viñas M, Bayona JM, Albaigés J, Solanas AM (2007) The Prestige oil spill: bacterial community dynamics during a field biostimulation assay. Appl Microbiol Biotechnol 77:935–945
Jiménez N, Viñas M, Sabaté J, Díez S, Bayona JM, Solanas AM, Albaigés J (2006) The Prestige oil spill. 2. Enhanced biodegradation of a heavy fuel oil under field conditions by the use of an oleophilic fertilizer. Environ Sci Technol 40:2578–2585
Kasai Y, Kishira H, Sasaki T, Syutsubo K, Watanabe K, Harayama S (2002) Predominant growth of Alcanivorax strains in oil-contaminated and nutrient-supplemented sea water. Environ Microbiol 4(3):141–147
Kästner M, Breuer-Jammali M, Mahro B (1998) Impact of inoculation protocols, salinity, and pH on the degradation of polycyclic aromatic hydrocarbons (PAHs) and survival of PAH-degrading bacteria introduced into soil. Appl Environ Microbiol 64(1):359
Kodama Y, Sutiknowati L, Ueki A, Watanabe K (2008) Thalassospira tepidiphila sp. nov., a polycyclic aromatic hydrocarbon-degrading bacterium isolated from seawater. Int J Syst Evol Microbiol 58:711–715
Maidak BL, Cole JR, Lilburn TG, Parker CT, Saxman PR, Stredwick JM, Garrity GM, Li B, Olsen GJ, Pramanik S, Schmidt TM, Tiedje JM (2000) The RDP (ribosomal database project) continues. Nucleic Acids Res 28(1):173–174
Margesin R, Labbé D, Schinner F, Greer C, Whyte L (2003) Characterization of hydrocarbon-degrading microbial populations in contaminated and pristine alpine soils. Appl Environ Microbiol 69(6):3085–3092
McKew BA, Coulon F, Osborn A, Timmis KN, McGenity TJ (2007a) Determining the identity and roles of oil-metabolizing marine bacteria from the Thames estuary, UK. Environ Microbiol 9(1):165–176
McKew BA, Coulon F, Yakimov MM, Denaro R, Genovese M, Smith CJ, Osborn AM, Timmis KN, McGenity TJ (2007b) Efficacy of intervention strategies for bioremediation of crude oil in marine systems and effects on indigenous hydrocarbonoclastic bacteria. Environ Microbiol 9(6):1562–1571
Medina-Bellver JI, Marin P, Delgado A, Rodríguez-Sanchez A, Reyes E, Ramos JL, Marqués S (2005) Evidence for in situ crude oil biodegradation after tha Prestige oil spill Environ Microbiol 7(6):773–779
Neufeld J, Schäfer H, Cox M, Boden R, McDonald I, Murrell J (2007) Stable-isotope probing implicates Methylophaga spp and novel Gammaproteobacteria in marine methanol and methylamine metabolism. ISME J 1:480–491
Nogueira E, Pérez F, Ríos A (1997) Seasonal patterns and long-term trends in an estuarine upwelling ecosystem (Ría de Vigo, NW Spain). Estuar Coast Shelf Sci 44(3):285–300
Prince RC, Elmendorf DL, Lute JR, Hsu CS, Haith CE, Senius JD, Dechert GJ, Douglas GS, Butler EL (1994) 17α(H)-21β(H)-hopane as a conserved internal marker for estimating the biodegradation of crude oil. Environ Sci Technol 28(1):142–145
Röling WFM, Milner MG, Jones DM, Lee K, Daniel F, Swannell RPJ, Head IM (2002) Robust hydrocarbon degradation and dynamics of bacterial communities uring nutrient-enhanced oil spill bioremediation. Appl Environ Microbiol 68(11):5537–5548
Schleheck D, Tindall B, Rosselló-Mora R, Cook A (2004) Parvibaculum lavamentivorans gen. nov., sp. nov., a novel heterotroph that initiates catabolism of linear alkylbenzenesulfonate. Int J Syst Evol Microbiol 54:1489–1497
Schwermer CU, Lavik G, Abed RMM, Dunsmore B, Ferdelman TG, Stoodley P, Gieseke A, de Beer D (2008) Impact of nitrate on the structure and function of bacterial biofilm communities in pipelines used for injection of seawater into oil fields. Appl Environ Microbiol 74(9):2841–2851
Sei K, Sugimoto Y, Mori K, Maki H, Kohno T (2003) Monitoring of alkane-degrading bacteria in a sea-water microcosm during crude oil degradation by polymerase chain reaction based on alkane-catabolic genes. Environ Microbiol 5(6):517–522
Tam N, Guo C, Yau W, Wong Y (2002) Preliminary study on biodegradation of phenanthrene by bacteria isolated from mangrove sediments in Hong Kong. Mar Pollut Bull 45(1–12):316–324
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24(8):1596–1599
Thompson J, Higgins D, Gibson T (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix. Nucleic Acids Res 22:4673–4680
Tsuda H, Hagiwara A, Shibata M, Ito N (1982) Carcinogenic effect of carbazole in the liver of a (C57BL/6NxC3H/HeN)F1 mice. J Natl Cancer Inst 69:1389–1393
Vila J, Grifoll M (2009) Actions of Mycobacterium sp. strain AP1 on the saturated-and aromatic-hydrocarbon fractions of fuel oil in a marine medium. Appl Environ Microbiol 75(19):6232–6239
Viñas M, Sabaté J, Espuny M, Solanas AM (2005) Bacterial community dynamics and polycyclic aromatic hydrocarbon degradation during bioremediation of heavily creosote-contaminated soil. Appl Environ Microbiol 71(11):7008–7018
Wang B, Lai Q, Cui Z, Tan T, Shao Z (2008) A pyrene-degrading consortium from deep-sea sediment of the west pacific and its key member Cycloclasticus sp. P1. Environ Microbiol 10(8):1948–1963
Wrenn B, Venosa A (1996) Selective enumeration of aromatic and aliphatic hydrocarbon degrading bacteria by a most-probable-number procedure. Can J Microbiol 42:252–258
Yakimov MM, Timmis KN, Golyshin P (2007) Obligate oil-degrading marine bacteria. Curr Opin Biotechnol 18(3):257–266
Yu Z, Morrison M (2004) Comparisons of different hypervariable regions of rrs genes for use in fingerprinting of microbial communities by PCR-denaturing gradient gel electrophoresis. Appl Environ Microbiol 70(8):4800–4806
Acknowledgment
This research was supported by the Spanish Ministry of Education and Science (VEM2003-20068-C05 and CTM2007-61097/TECHNO). N.J. is grateful for a PhD fellowship from the Spanish Ministry of Education and Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiménez, N., Viñas, M., Guiu-Aragonés, C. et al. Polyphasic approach for assessing changes in an autochthonous marine bacterial community in the presence of Prestige fuel oil and its biodegradation potential. Appl Microbiol Biotechnol 91, 823–834 (2011). https://doi.org/10.1007/s00253-011-3321-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3321-4