Abstract
Canine atopic dermatitis (CAD) is an inflammatory and pruritic allergic skin disease with both genetic and environmental risk factors described. We performed mRNA sequencing of non-lesional axillary skin biopsies from nine German shepherd dogs. Obtained RNA sequences were mapped to the dog genome (CanFam3.1) and a high-quality skin transcriptome was generated with 23,510 expressed gene transcripts. Differentially expressed genes (DEGs) were defined by comparing three controls to five treated CAD cases. Using a leave-one-out analysis, we identified seven DEGs: five known to encode proteins with functions related to an activated immune system (CD209, CLEC4G, LOC102156842 (lipopolysaccharide-binding protein-like), LOC480601 (regakine-1-like), LOC479668 (haptoglobin-like)), one (OBP) encoding an odorant-binding protein potentially connected to rhinitis, and the last (LOC607095) encoding a novel long non-coding RNA. Furthermore, high mRNA expression of inflammatory genes was found in axillary skin from an untreated mild CAD case compared with healthy skin. In conclusion, we define genes with different expression patterns in CAD case skin helping us understand post-treatment atopic skin. Further studies in larger sample sets are warranted to confirm and to transfer these results into clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Canine atopic dermatitis (CAD) is an inflammatory and pruritic allergic skin disease with a strong genetic predisposition and is also influenced by environmental risk factors (Meury et al. 2011; Nodtvedt et al. 2007). Onset of clinical signs is typically between 6 months and 3 years of age in affected dogs. Clinical signs of CAD include eczematous skin, predominantly in the flex and friction areas of the body (Griffin and DeBoer 2001), strikingly similar to symptoms of atopic dermatitis (AD) in humans (Rhodes et al. 1987; Willemse 1988). The immune response during an atopic reaction is primarily a lymphocytic skin infiltration and plasma cell class switching to form immunoglobulin E (IgE) antibodies that recognize otherwise harmless environmental allergens. These IgE antibodies bind to IgE receptors expressed on the cell surface of mast cells and basophils and when IgE are cross-linked by the offending allergen, degranulation and release of inflammatory mediators occur. This leads to vasodilation and activation of further inflammatory responses. The overall prevalence of CAD typically ranges from 3 to 15% (Hillier and Griffin 2001; Williams 2001) and high-risk breeds include boxer, bull terrier, West Highland white terrier, German shepherd dog (GSD), and Labrador retriever (Jaeger et al. 2010; Nodtvedt et al. 2006; Sousa and Marsella 2001; Vilson et al. 2013). Heritability of CAD in Labrador and golden retrievers was estimated to be 0.47 (± 0.17) (Shaw et al. 2004).
The severity of clinical signs, the effect of different treatment protocols, and disease progression differ greatly between different CAD-affected dogs (Olivry et al. 2015). Moreover, many treatments may have adverse side effects (Bloom 2006). Anti-allergic drugs include antihistamines and immune suppressive drugs, e.g., cyclosporine A, glucocorticoids or oclacitinib. Secondary overgrowth or infections with Malassezia or Staphylococcus spp. are well-recognized flare factors; thus, infection control and prevention is important for successful disease management. Allergen-specific immunotherapy (ASIT) has been developed also for dogs and may, in some patients, be effective in inducing allergen tolerance, hence reducing or even controlling allergic symptoms (reviewed in (DeBoer 2017)). Therapies used for treating dogs with CAD are comparable to treatment of human AD patients (Werfel et al. 2014). The similarities between AD in human and dog both regarding disease presentation (Marsella and Girolomoni 2009), as well as treatment options, make the results obtained from CAD studies potentially useful also for human AD research. The variations between patients in disease progression and severity as well as response to treatment emphasize the need to develop new therapies and personalized treatment strategies in both human and dog AD patients (Cabanillas et al. 2017; Olivry et al. 2015). To further understand the mechanisms underlying CAD, including genetic risk factors, cell types, and molecular pathways, studies of skin in subclinical and active CAD stages are highly warranted.
Differentially expressed genes (DEGs) have previously been reported in a custom-designed 22K gene expression microarray study of both lesional and non-lesional skin from atopic dogs compared to skin from controls (Merryman-Simpson et al. 2008). In that study, 54 DEGs were identified and the most dysregulated gene was S100A8, encoding a calcium-binding protein involved in regulating inflammatory responses. Skin biopsies were taken from different parts of the body and various breeds were jointly analyzed. A similar study of human AD identified 217 DEGs of which the most differentially expressed genes encoded proteins involved in inflammatory responses (e.g. S100A8, -A9 and -A12, and CXCL1) and the skin barrier (e.g., keratin 16, and claudin 8) (Suarez-Farinas et al. 2015). In a meta-analysis (Ghosh et al. 2015) of five independent human AD microarray studies (Gittler et al. 2012; Guttman-Yassky et al. 2009; Olsson et al. 2006; Plager et al. 2007; Suarez-Farinas et al. 2011), 89 DEGs were identified as consistently dysregulated across all five studies. The defined genes were functionally implicated in immune responses, keratinocyte differentiation/epidermal development, inflammation, and lipid metabolism (Ghosh et al. 2015).
In this study, we collected skin biopsies from the axillary region, which is one of the typically affected body regions in the active disease stage, from CAD-affected and healthy control GSDs. We identified seven significant DEGs comparing treated CAD cases to controls. Post-study design identification of an untreated mild CAD case allowed us to compare untreated atopic versus healthy control skin and indicated inflammatory genes with high expression in the untreated mild CAD dog.
Methods
Samples
Skin biopsies were collected from 10 GSDs. The dogs were included in our previous genome-wide association study of CAD in GSDs (Tengvall et al. 2013) and recruited based on their genotypes (cases with risk alleles and healthy controls with control alleles) at the Plakophilin-2 (PKP2)-locus. One biopsy (6 mm in diameter) collected from axillary skin was fixed within 10 min in RNAlater (Ambion Inc., Austin, TX, USA) and stored at 4 °C overnight followed by storage in − 80 °C until RNA extraction.
CAD and control phenotype characterization
All six cases had been diagnosed with CAD. Clinical diagnoses were established by first ruling out other causes of pruritus such as ectoparasite infestation, staphylococcal pyoderma, and Malassezia dermatitis. Hypoallergenic dietary trials (at least 8 weeks followed by a challenge period) were conducted to evaluate the potential contribution of concurrent cutaneous adverse food reactions to the clinical signs. A CAD diagnosis was determined in dogs not adequately controlled on hypoallergenic diet and with positive reactions on intradermal allergy tests or IgE serology tests. The dogs were between 6 and 11 years old at the time of sampling. At the time point of biopsy collections, CAD cases were under treatment with ASIT (administered sub-cutaneous), methylprednisolone/medrol (cortisone), and/or cetirizine (antihistamine) (Table S1).
One dog was originally recruited as a control (control 2), but at the time of sampling, the dermatologist observed mild, non-infectious otitis externa at the examination. The clinical findings warranted an in-depth interview with the owner, which revealed that the dog had experienced summer erythema of inguinal skin and otitis externa at least twice during the last 2 years. These signs are consistent with common clinical signs of CAD (Favrot et al. 2010). An additional axillary skin biopsy from this dog was fixed in 4% PFA, paraffin embedded, and later cut and stained with hematoxylin eosin. The dermatologist observed mild perivascular infiltration of mononuclear cells in superficial dermis. That dog (control 2) was thus post-study design defined as an untreated CAD case with mild skin lesions further referred to as untreated mild CAD case. Less than 2 years after sampling, the dog was euthanized due to heart problems and never underwent a complete CAD investigation.
Healthy controls (n = 3) were recruited at ages between 9 and 11 years and had no history of either pruritus, repeated otic inflammation, or evidence of skin lesions compatible with CAD, neither prior to nor at the time of sampling. The information was based on owner statement and questionnaire, and later clinical examination at sampling by a boarded dermatologist.
RNA isolation, library preparation, and sequencing
RNA was isolated using Qiagen RNeasy Mini Kit, Quick-Start Protocol Part 1 and 2 (Jan 2011, www.qiagen.com) with a DNase I digestion step included and the optional step after step 6 in Part 1 excluded (i.e., no new collection tubes were used). Prior to RNA isolation, tissues were homogenized using a bead beater at 4 °C. Beads were washed in 99.9% EtOH for 20 min and then sprayed with RNaseZap RNase Decontamination Solution (Applied Biosystems, Foster City, CA, USA). Poly-A selected/paired-end libraries and sequencing of 100 bp paired-end reads (three lanes) using Illumina HiSeq 2000 were performed at the SNP&SEQ Technology Platform at Science for Life Laboratory, Uppsala University, Sweden. RNA concentration and quality of each sample was assessed at the SNP&SEQ Technology Platform and one sample (case 6 in Table S1) was excluded in the quality control.
Mapping procedures and quality controls
We used the tool Trimmomatic (v. 0.32) (Bolger et al. 2014) to trim the sequence ends. In total, 93–96% of the input read pairs survived trimming using the Illumina adaptors provided in the TruSeq3-PE.fa with the following settings: 2:30:10 LEADING:3 TRAILING:3 SLIDINGWINDOW:4:15 MINLEN:36. FastQC (v. 0.11.2) (Andrews 2010) was used for evaluating sequence quality and read depth per sample. Bowtie2 (v. 2.2.3) (Langmead and Salzberg 2012) and tophat (v. 2.0.12) (Trapnell et al. 2009) were used to map the reads to the canine genome version CanFam3.1 (Hoeppner et al. 2014) (Ensembl gene annotation released July 2012) and SAMtools view (v. 1.3) (Li et al. 2009) to quality filter the output bam files (−q 15). The functions cuffquant and cuffdiff from the cufflinks (v. 2.2.1) software (Trapnell et al. 2010) were used to quantify the number of aligned transcripts (measured in FPKM, i.e., fragments per kilobase of exon per million reads mapped).
Definition and visualization of differentially expressed genes
The base 2 log of the fold change for cases FPKM/controls FPKM and a test statistic were calculated using cuffdiff to compute significance of the observed change in FPKM (GitHub commit 15d2c6b). We used the default (0.05) setting for significant FDR in cuffdiff. Analyses for defining DEGs were performed by comparing all five CAD cases with three healthy controls and also by using a leave-one-out approach defining DEGs in common between eight comparisons of cases and controls, where one dog was subsequently omitted to avoid effects from individual variation in gene expression. We also performed a comparison between the untreated mild CAD case with the healthy controls. As quality control, we excluded DEGs with < 1.5 log2 fold change and DEGs with < 10 FPKM in more than 50% of the samples. In the comparison between the untreated CAD case compared to controls, we applied an additional quality control by subsequently excluding DEGs with less than double/half FPKM difference between the untreated case and any of the other individual FPKM (both controls and treated cases). R package CummeRbund (v. 2.14.0) (Goff L 2013) was used to evaluate and visualize the expression results returned by cuffdiff. R package gplots (v.3.0.3) (Warnes R G 2020) and Adobe Illustrator 2019 (v. 23.0.6) was used for creating final figures.
Results
Total mRNA expression in dog skin
In total, expression of 23,510 gene transcripts (including 6440 LOC genes), 48,265 isoforms, 36,295 transcription start sites (TSS), and 23,509 promoters were detected in the dog skin samples. All samples remaining after quality control at the sequencing platform passed the threshold of sequence quality (mean PHRED score > 31), and aligned reads per sample ranged from 36.8 to 45.6 million. Control samples showed higher within-group variation (coefficient of variation, CV2) in comparison to cases (Fig. S1A). Multi-dimensional scaling (MDS) and principal component analyses (PCA) visualizing the overall gene expression per individual showed no grouping based on cases and control status (Fig. S1B-C) and FPKM was similar across individual samples (Fig. S1D).
Differential gene expression in treated CAD cases versus controls
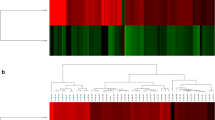
In the comparison between five CAD cases and three controls, 135 DEGs (Table S2) were identified and no expression differences between CAD cases and controls were detected for the PKP2 gene, previously reported associated with CAD (Tengvall et al. 2013). Seven DEGs were defined after two quality controls. The first quality control resulted in eight DEGs (> 1.5-fold change and with FPKM > 10 in at least 50% of the samples). The second quality control was a leave-one-out analysis resulting in 10 DEGs. The seven DEGs overlapping between both quality controls were CD209, CLEC4G, LOC102156842 (lipopolysaccharide-binding protein like), LOC480601 (regakine-1-like), LOC479668 (haptoglobin-like), LOC607095 (lysine-rich arabinogalactan protein 19-like), and OBP (Fig. 1). OBP showed lower gene expression levels whilst the other genes showed higher gene expression levels in cases compared to controls.
Differentially expressed genes in CAD cases compared to controls. Expression levels for seven DEGs from the leave-one-out analysis of CAD cases compared to controls are presented as FPKM (fragments per kilobase of exon per million reads mapped) in a heatmap, color coded based on log10-transformed values and the actual values presented as numbers in each square (a). Mean FPKM in the case and control groups are presented as staple bars (b). Description of LOC gene transcripts is referred to with superscripts a–d. OBP showed higher expression in cases whereas the others presented with lower expression levels in cases
Differential gene expression in untreated mild CAD case versus controls
One dog (control 2) was recruited as a control but later identified as an untreated mild CAD case post-study design (see “Methods” and Table S1). We compared the mRNA expression levels of this single dog to three healthy controls and identified 45 DEGs after exclusion of DEGs with log2 fold change < 1.5 and with < 10 FPKM in > 50% of samples (Table S3). Extracting DEGs with at least half/double FPKM in the untreated case compared to the other individuals (both controls and treated cases) resulted in 12 DEGs, all with higher expression in the untreated case (Fig. 2). These were S100A8, S100A9, S100A12, LOC102152183 (uncharacterized ncRNA), IL36G, LOC483068 (IL36B), DLA-79, PSMB9, IGJ, ARSF, DLA-64, and PSMB.
Differentially expressed genes in an untreated mild case compared to controls. Expression levels for 12 DEGs are presented as FPKM (fragments per kilobase of exon per million reads mapped) in a heatmap, color coded based on log10-transformed values and the actual values presented as numbers in each square (a). FPKM in the untreated mild case and mean FPKM in controls are presented as staple bars with the range from lowest to highest FPKM in the controls visualized as black strokes (b). Description of LOC gene transcripts is referred to with superscript a, b
Discussion
Dog skin transcriptome
The primary aim of this study was to analyze differential gene expression changes specific for CAD skin. By using high-quality RNA and low density of samples (n = 3) per lane in sequencing, thus yielding a high number (37–46 M) of reads per sample, a high-quality canine skin transcriptome was obtained. Gene expression data from skin of nine individual dogs of high quality and coverage can now be used to update both the current dog genome annotation version (Hoeppner et al. 2014), which has been lacking high-quality data from the canine skin transcriptome, and the recently developed database for genetic and epigenetic data from dog tissue samples (Megquier et al. 2019).
Unique study design
In contrast to previous atopic skin gene expression studies, we collected biopsies from the same skin location (axillary region) in both cases and controls from a single dog breed, to limit changes in gene expression due to body location and inter-breed variation. The treatment protocols for the five CAD cases were comparable including allergen-specific immune therapy (ASIT) aimed at inducing allergen tolerance, systemic corticosteroids, and/or antihistamines. At the time of sampling, CAD cases were clinically in remission as a result of the treatment protocol, with no active skin lesions and without pruritus or just mild pruritus; thus, the skin from these treated atopic cases was visually similar to control dog skins. Treatment protocols included methylprednisolone used in the dose range of 0.2–0.35 mg/kg every other day, and it is well known that corticosteroids have immunosuppressive properties (Bonagura 1995). Moreover, cetirizine was used in the dose 1 mg/kg BID and antihistamine blocks histamine receptors and stabilizes mast cells (Ekstrand et al. 2018). The overall gene expression profiles provided from this study showed that cases had a lower within-group variation compared to controls, which may be explained by the similar disease state in cases (i.e., CAD cases were under similar treatment protocols).
Activated immune response in subclinical atopic skin
Interestingly, non-lesional (i.e., clinically normal) skin from CAD-affected dogs is not identical to skin from healthy dogs. Non-lesional CAD skin has previously been characterized by microscopic inflammation and presence of pro-inflammatory cytokines, similar to lesional atopic skin (Nuttall et al. 2002a; Nuttall et al. 2002b; Olivry et al. 1999; Olivry et al. 1997). T-cells are known to play a crucial role in both the acute and chronic phase of AD/CAD where acute inflammatory response in AD is characterized by Th2-type cytokines (Bieber 2010). Among the seven DEGs identified in treated CAD cases versus controls, were genes encoding proteins that could account for immunological alertness and sensitivity to relapse. CD209, CLEC4G, and LOC102156842 (lipopolysaccharide-binding protein like) encode proteins involved in early immediate defense against pathogens, and LOC480601 (regakine-1-like) and LOC479668 (haptoglobin-like) encode proteins involved in monitoring inflammatory responses in the skin. LOC607095 encodes a novel long non-coding RNA, a potential new marker for skin inflammation, and OBP encodes an odorant-binding protein potentially related to the AD-connected symptom rhinitis.
CD209 (alias: CLEC4L) encodes a pattern recognition receptor, expressed on macrophages and dendritic cells, and binds to mannose-type carbohydrates commonly found on pathogens. The increased expression level of CD209 in CAD cases versus healthy controls may reflect an activated interaction between antigen-presenting cells and T-cells in CAD skin tissue. Previously, a reduction of CD209+ dendritic cells in human skin from atopic eczema patients was found associated with clinical improvement (Hassan et al. 2007). CLEC4G is positioned in the vicinity (~ 45 kb) of CD209 in the canine genome, and both genes encode proteins with similar functions as detectors of antigens. An upregulated expression of CLEC4G was also detected in a previous study of gene expression in acute lesional AD skin from dogs (Plager et al. 2012). LOC102156842 encodes a lipopolysaccharide-binding (LPB)-like protein. LPBs are acute-phase proteins that recognize lipopolysaccharide (LPS) on bacteria and provide an early inflammatory response essential for defense against invading microorganisms. High LPB concentrations in human serum were shown to reduce LPS activity (Zweigner et al. 2001). LOC480601 encodes a regakine-1-like protein. Regakine-1 is a CC chemokine that synergizes with IL-8 (CXC chemokine ligand 8) to chemoattract neutrophils and potentiate the inflammatory response in blood circulation (Gouwy et al. 2002). Higher gene expression levels of CXCL8 were detected in purified epidermal cells from AD patients compared to normal skin (Kamsteeg et al. 2010). LOC479668 encodes a Haptoglobin-like protein. Haptoglobins are acute-phase proteins shown to prevent epidermal Langerhans cells from functionally maturing in the skin, which may be important for preventing T cell–dependent inflammatory skin disease (Xie et al. 2000). Patients with skin diseases, e.g., psoriasis, had a significantly increased haptoglobin mRNA expression in epidermal keratinocytes compared to controls, and it was suggested that keratinocyte-derived haptoglobin may contribute to the downregulation of inflammatory responses in the skin (Li et al. 2005). Different haptoglobin genotypes have been reported with higher risk of disease in humans (Andersen et al. 2017) including allergic contact dermatitis (Beckman et al. 1981), bronchial asthma (Frohlander and Stjernberg 1989), and rhinitis (Piessens et al. 1984). LOC607095 corresponds to the Ensembl gene id: ENSCAFG00000041925 and is described as a lysine-rich arabinogalactan protein 19-like (Ensembl release 100, April 2020). The gene encodes nine splice variants of lncRNA where one (ENSCAFG00000079341.1) matches the position to the LOC607095 transcript (chr27:483,001-486,492, Table S2). Recently, there has been an increased focus on how long non-coding RNA species function as critical regulators of immune cell development, differentiation, and effector function, and also how they may be targeted therapeutically. Dysregulated lncRNA has been suggested in both cancer, autoimmunity, and asthma (reviewed in (Guidi et al. 2020)). OBP presented with lower expression levels in CAD cases compared to controls. OBP encodes odorant-binding proteins, which are small and abundant extracellular proteins detected in many species and specifically in the human olfactory mucus. They are participating in odor detection by carrying, deactivation, and/or selecting odorant molecules (Briand et al. 2002). Rhinitis, i.e., inflammation in the nasal passages, affects > 1/3 of human AD patients (Kapoor et al. 2008). While not as common in dogs, this clinical feature still affected around 7% of CAD-affected dogs (Favrot et al. 2010) and showed breed variations with the highest proportions reported in CAD-affected GSDs (8.8%) and West Highland white terriers (10.9%) but none of the Dalmatians (Wilhem et al. 2011). The altered expression of OBP seen in CAD cases compared to controls could potentially be a secondary effect from rhinitis.
Interestingly, the leave-one-out method defined 10 DEGs, out of which seven were included among the eight DEGs defined after the other quality control excluding DEGs with < 1.5-fold change and FPKM below 10 in > 50% of the individuals. This approach confirmed the validity of the two cutoff methods used to define DEGs.
Differentially expressed immune genes indicated in untreated mild CAD skin
The majority of DEGs with higher expression in the untreated mild CAD case compared to controls have known functions in an activated immune response and inflammation. S100A8, S100A9, and S100A12 encode proteins that are well-known markers of acute inflammation and previously implicated in several other inflammatory and autoimmune diseases including systemic lupus erythematosus, rheumatoid arthritis, and atherosclerosis (Austermann et al. 2018; Oesterle and Bowman 2015). IL-36 cytokines, including IL-36B and IL-36G, actively propagate skin inflammation by activating keratinocytes, antigen-presenting cells, and indirectly T-cells (Foster et al. 2014). IGJ encodes the immunoglobulin-joining chain of multimeric IgA and IgM, DLA-79 and DLA-64 are part of MHC class I, and PSMB8 and PSMB9 are located in the MHC class II region and encode members of the immunoproteasome that are critical for processing of MHC class I peptides (www.ncbi.nlm.nih.gov, Gene database, May 2020). ARSF belong to the family of sulfatases and has been suggested as a new marker for psoriasis in a mRNA-seq analysis of skin in humans (Li et al. 2020). LOC102152183 is an uncharacterized ncRNA and has no previously known functions but could potentially be a novel marker for skin inflammation. Due to the inclusion of only one untreated CAD case in this comparison, no certain conclusions can be drawn, but these suggestive DEGs encoding proteins with striking immunological functions and one novel ncRNA warrant further studies to confirm these findings.
No expression differences for CAD-associated Plakophilin-2 gene
The dogs in this study were recruited based on their genotypes at the PKP2-locus as this was previously found associated with CAD in GSDs (Tengvall et al. 2013; Tengvall et al. 2016). However, we detected no difference in PKP2 gene expression in the skin between the CAD cases carrying risk alleles compared to the controls with the control genotype. Two additional biopsies from axillary skin and three biopsies from the back region of each dog were included at sampling and were fixed for protein expression studies (Ardesjo-Lundgren et al. 2017), which included a few more dogs compared to the present study. In that study, T-cells and dendritic cell infiltration were identified in canine axillary skin and high PKP2 protein expression was reported in keratinocytes, T-cells, and dendritic cells, but with no differences between CAD cases and controls. The majority of the CAD cases had been under long-term systemic corticosteroid treatment (> 1 year) at the time of sampling (Ardesjo-Lundgren et al. 2017), and corticosteroids are known to inhibit activation of especially lymphocytes but also dendritic cells (Ashwell et al. 2000; Ashworth et al. 1988; Gillis et al. 1979; Leung and Bloom 2003). Since a skin biopsy consists of many cell types, a difference in PKP2 gene expression in specific cell types such as T-cells or dendritic cells may be undetectable. However, no difference in PKP2 protein expression intensity in dendritic cells was detected between CAD cases and controls (Ardesjo-Lundgren et al. 2017). Nevertheless, we cannot rule out that there is an altered PKP2 expression in CAD but the right target tissue, cell type, and/or disease state need to be defined. Overall, DEGs identified in this study may be caused by genetic alterations associated with CAD; however, in a small sample set like this, differential gene expression likely reflect the actual skin status being either untreated mild CAD, post-treatment subclinical CAD, or healthy tissue.
Future objectives
The majority of the top DEGs detected from the current analyses were highly supported by previous studies in both dog and human. We here define differential gene expression in subclinical treated CAD skin. The DEGs identified here encode proteins that may represent suitable target molecules. Further studies to detect additional DEGs and confirm the currently defined DEGs are warranted and would preferably involve skin samples from more controls and cases at different disease stages, including active lesional disease as well as treated subclinical CAD cases. Identifying specific therapeutic target molecules and biomarkers may improve the development of future diagnostic tools and therapies to become personalized to increase efficiency and reduce side effects.
Conclusion
We here generate a high-quality canine skin transcriptome from healthy controls and CAD-affected dogs representing a subclinical phenotype as an effect of treatment. We provide insights into immunological mechanisms that might account for the relapsing nature of atopic disease. Our results emphasize the striking similarities between canine and human AD also at the level of perturbed gene expression profiles in affected compared to healthy skin. These results will hopefully contribute to the foundation of future treatment strategies.
Data availability
The raw files (.fastq.gz) are uploaded on ENA (European nucleotide archive: https://www.ebi.ac.uk/ena/) with project accession number: PRJEB38104 (ERP121487).
Code availability
All software programs and versions used for mapping of reads and analyses are specified in the materials and methods section.
References
Andersen CBF, Stodkilde K, Saederup KL, Kuhlee A, Raunser S, Graversen JH, Moestrup SK (2017) Haptoglobin. Antioxid Redox Signal 26:814–831. https://doi.org/10.1089/ars.2016.6793
Andrews S (2010) FastQC: a quality control tool for high throughput sequence data. Available online at: http://www.bioinformaticsbabrahamacuk/projects/fastqc
Ardesjo-Lundgren B et al (2017) Comparison of cellular location and expression of Plakophilin-2 in epidermal cells from nonlesional atopic skin and healthy skin in German shepherd dogs. Vet Dermatol 28:e377–e388. https://doi.org/10.1111/vde.12441
Ashwell JD, Lu FW, Vacchio MS (2000) Glucocorticoids in T cell development and function*. Annu Rev Immunol 18:309–345. https://doi.org/10.1146/annurev.immunol.18.1.309
Ashworth J, Booker J, Breathnach SM (1988) Effects of topical corticosteroid therapy on Langerhans cell antigen presenting function in human skin. Br J Dermatol 118:457–469
Austermann J, Spiekermann C, Roth J (2018) S100 proteins in rheumatic diseases. Nat Rev Rheumatol 14:528–541. https://doi.org/10.1038/s41584-018-0058-9
Beckman G, Beckman L, Cedergren B, Goransson K, Liden S (1981) Blood groups, serum groups and red cell enzyme types in allergic contact dermatitis. Hum Hered 31:54–60. https://doi.org/10.1159/000153177
Bieber T (2010) Atopic dermatitis. Ann Dermatol 22:125–137. https://doi.org/10.5021/ad.2010.22.2.125
Bloom P (2006) Atopic dermatitis in dogs - hitting the moving target. Vet J 171:16–17. https://doi.org/10.1016/j.tvjl.2005.02.012
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. https://doi.org/10.1093/bioinformatics/btu170
Bonagura J (1995) Scott DW: rational use of glucocorticoids in dermatology. In: Kirks’s Current Veterinary Therapy XII. W.B. Saunders Co, pp 573–580
Briand L, Eloit C, Nespoulous C, Bézirard V, Huet JC, Henry C, Blon F, Trotier D, Pernollet JC (2002) Evidence of an odorant-binding protein in the human olfactory mucus: location, structural characterization, and odorant-binding properties. Biochemistry 41:7241–7252
Cabanillas B, Brehler AC, Novak N (2017) Atopic dermatitis phenotypes and the need for personalized medicine. Curr Opin Allergy Clin Immunol 17:309–315. https://doi.org/10.1097/ACI.0000000000000376
DeBoer DJ (2017) The future of immunotherapy for canine atopic dermatitis: a review. Vet Dermatol 28:e25–e26. https://doi.org/10.1111/vde.12416
Ekstrand C, Ingvast-Larsson C, Bondesson U, Hedeland M, Olsen L (2018) Cetirizine per os: exposure and antihistamine effect in the dog. Acta Vet Scand 60:77. https://doi.org/10.1186/s13028-018-0431-3
Favrot C, Steffan J, Seewald W, Picco F (2010) A prospective study on the clinical features of chronic canine atopic dermatitis and its diagnosis. Vet Dermatol 21:23–31. https://doi.org/10.1111/j.1365-3164.2009.00758.x
Foster AM et al (2014) IL-36 promotes myeloid cell infiltration, activation, and inflammatory activity in skin. J Immunol 192:6053–6061. https://doi.org/10.4049/jimmunol.1301481
Frohlander N, Stjernberg N (1989) Association between haptoglobin groups and hereditary predisposition for bronchial asthma. Hum Hered 39:7–11. https://doi.org/10.1159/000153824
Ghosh D, Ding L, Sivaprasad U, Geh E, Biagini Myers J, Bernstein JA, Khurana Hershey GK, Mersha TB (2015) Multiple transcriptome data analysis reveals biologically relevant atopic dermatitis signature genes and pathways. PLoS One 10:e0144316. https://doi.org/10.1371/journal.pone.0144316
Gillis S, Crabtree GR, Smith KA (1979) Glucocorticoid-induced inhibition of T cell growth factor production. I. The effect on mitogen-induced lymphocyte proliferation. J Immunol 123:1624–1631
Gittler JK et al (2012) Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol 130:1344–1354. https://doi.org/10.1016/j.jaci.2012.07.012
Goff L, Trapnell C, Kelly D (2013) cummeRbund: Analysis, exploration, manipulation, and visualization of Cufflinks high-throughput sequencing data. R package version 2.18.0
Gouwy M, Struyf S, Mahieu F, Put W, Proost P, Van Damme J (2002) The unique property of the CC chemokine regakine-1 to synergize with other plasma-derived inflammatory mediators in neutrophil chemotaxis does not reside in its NH2-terminal structure. Mol Pharmacol 62:173–180. https://doi.org/10.1124/mol.62.1.173
Griffin CE, DeBoer DJ (2001) The ACVD task force on canine atopic dermatitis (XIV): clinical manifestations of canine atopic dermatitis. Vet Immunol Immunopathol 81:255–269
Guidi R, Wedeles CJ, Wilson MS (2020) ncRNAs in type-2 immunity noncoding RNA 6. https://doi.org/10.3390/ncrna6010010
Guttman-Yassky E et al (2009) Broad defects in epidermal cornification in atopic dermatitis identified through genomic analysis. J Allergy Clin Immunol 124:1235–1244 e1258. https://doi.org/10.1016/j.jaci.2009.09.031
Hassan AS, Kaelin U, Braathen LR, Yawalkar N (2007) Clinical and immunopathologic findings during treatment of recalcitrant atopic eczema with efalizumab. J Am Acad Dermatol 56:217–221. https://doi.org/10.1016/j.jaad.2006.08.025
Hillier A, Griffin CE (2001) The ACVD task force on canine atopic dermatitis (I): incidence and prevalence. Vet Immunol Immunopathol 81:147–151
Hoeppner MP, Lundquist A, Pirun M, Meadows JRS, Zamani N, Johnson J, Sundström G, Cook A, FitzGerald MG, Swofford R, Mauceli E, Moghadam BT, Greka A, Alföldi J, Abouelleil A, Aftuck L, Bessette D, Berlin A, Brown A, Gearin G, Lui A, Macdonald JP, Priest M, Shea T, Turner-Maier J, Zimmer A, Lander ES, di Palma F, Lindblad-Toh K, Grabherr MG (2014) An improved canine genome and a comprehensive catalogue of coding genes and non-coding transcripts. PLoS One 9:e91172. https://doi.org/10.1371/journal.pone.0091172
Jaeger K, Linek M, Power HT, Bettenay SV, Zabel S, Rosychuk RA, Mueller RS (2010) Breed and site predispositions of dogs with atopic dermatitis: a comparison of five locations in three continents. Vet Dermatol 21:118–122. https://doi.org/10.1111/j.1365-3164.2009.00845.x
Kamsteeg M et al (2010) Molecular diagnostics of psoriasis, atopic dermatitis, allergic contact dermatitis and irritant contact dermatitis. Br J Dermatol 162:568–578. https://doi.org/10.1111/j.1365-2133.2009.09547.x
Kapoor R, Menon C, Hoffstad O, Bilker W, Leclerc P, Margolis DJ (2008) The prevalence of atopic triad in children with physician-confirmed atopic dermatitis. J Am Acad Dermatol 58:68–73. https://doi.org/10.1016/j.jaad.2007.06.041
Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. https://doi.org/10.1038/nmeth.1923
Leung DY, Bloom JW (2003) Update on glucocorticoid action and resistance. J Allergy Clin Immunol 111:3–22 quiz 23
Li P et al (2005) Localization of haptoglobin in normal human skin and some skin diseases. Int J Dermatol 44:280–284. https://doi.org/10.1111/j.1365-4632.2005.02088.x
Li H et al (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079. https://doi.org/10.1093/bioinformatics/btp352
Li H, Yang C, Zhang J, Zhong W, Zhu L, Chen Y (2020) Identification of potential key mRNAs and LncRNAs for psoriasis by bioinformatic analysis using weighted gene co-expression network analysis. Mol Gen Genomics. https://doi.org/10.1007/s00438-020-01654-0
Marsella R, Girolomoni G (2009) Canine models of atopic dermatitis: a useful tool with untapped potential. J Invest Dermatol 129:2351–2357. https://doi.org/10.1038/jid.2009.98
Megquier K, Genereux DP, Hekman J, Swofford R, Turner-Maier J, Johnson J, Alonso J, Li X, Morrill K, Anguish LJ, Koltookian M, Logan B, Sharp CR, Ferrer L, Lindblad-Toh K, Meyers-Wallen VN, Hoffman A, Karlsson EK (2019) BarkBase: epigenomic annotation of canine genomes. Genes (Basel) 10:10. https://doi.org/10.3390/genes10060433
Merryman-Simpson AE et al (2008) Gene (mRNA) expression in canine atopic dermatitis: microarray analysis. Vet Dermatol 19:59–66. https://doi.org/10.1111/j.1365-3164.2008.00653.x
Meury S et al (2011) Role of the environment in the development of canine atopic dermatitis in Labrador and golden retrievers. Vet Dermatol 22:327–334. https://doi.org/10.1111/j.1365-3164.2010.00950.x
Nodtvedt A, Egenvall A, Bergvall K, Hedhammar A (2006) Incidence of and risk factors for atopic dermatitis in a Swedish population of insured dogs. Vet Record 159:241–246
Nodtvedt A, Guitian J, Egenvall A, Emanuelson U, Pfeiffer DU (2007) The spatial distribution of atopic dermatitis cases in a population of insured Swedish dogs. Prevent Vet Med 78:210–222. https://doi.org/10.1016/j.prevetmed.2006.10.007
Nuttall TJ, Knight PA, McAleese SM, Lamb JR, Hill PB (2002a) Expression of Th1, Th2 and immunosuppressive cytokine gene transcripts in canine atopic dermatitis. Clin Exp Allergy 32:789–795
Nuttall TJ, Knight PA, McAleese SM, Lamb JR, Hill PB (2002b) T-helper 1, T-helper 2 and immunosuppressive cytokines in canine atopic dermatitis. Vet Immunol Immunopathol 87:379–384
Oesterle A, Bowman MA (2015) S100A12 and the S100/Calgranulins: emerging biomarkers for atherosclerosis and possibly therapeutic targets. Arterioscler Thromb Vasc Biol 35:2496–2507. https://doi.org/10.1161/ATVBAHA.115.302072
Olivry T, Naydan DK, Moore PF (1997) Characterization of the cutaneous inflammatory infiltrate in canine atopic dermatitis. Am J Dermatopathol 19:477–486
Olivry T, Dean GA, Tompkins MB, Dow JL, Moore PF (1999) Toward a canine model of atopic dermatitis: amplification of cytokine-gene transcripts in the skin of atopic dogs. Exp Dermatol 8:204–211
Olivry T et al (2015) Treatment of canine atopic dermatitis: 2015 updated guidelines from the International Committee on Allergic Diseases of Animals (ICADA). BMC Vet Res 11:210. https://doi.org/10.1186/s12917-015-0514-6
Olsson M et al (2006) Increased expression of aquaporin 3 in atopic eczema. Allergy 61:1132–1137. https://doi.org/10.1111/j.1398-9995.2006.01151.x
Piessens MF, Marien G, Stevens E (1984) Decreased haptoglobin levels in respiratory allergy. Clin Allergy 14:287–293. https://doi.org/10.1111/j.1365-2222.1984.tb02208.x
Plager DA, Leontovich AA, Henke SA, Davis MD, McEvoy MT, Sciallis GF 2nd, Pittelkow MR (2007) Early cutaneous gene transcription changes in adult atopic dermatitis and potential clinical implications. Exp Dermatol 16:28–36. https://doi.org/10.1111/j.1600-0625.2006.00504.x
Plager DA, Torres SM, Koch SN, Kita H (2012) Gene transcription abnormalities in canine atopic dermatitis and related human eosinophilic allergic diseases. Vet Immunol Immunopathol 149:136–142. https://doi.org/10.1016/j.vetimm.2012.06.003
Rhodes KH, Kerdel F, Soter NA (1987) Comparative aspects of canine and human atopic dermatitis. Semin Vet Med Surg 2:166–172
Shaw SC, Wood JL, Freeman J, Littlewood JD, Hannant D (2004) Estimation of heritability of atopic dermatitis in Labrador and Golden Retrievers. Am J Vet Res 65:1014–1020
Sousa CA, Marsella R (2001) The ACVD task force on canine atopic dermatitis (II): genetic factors. Vet Immunol Immunopathol 81:153–157
Suarez-Farinas M et al (2011) Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol 127:954–964 e951-954. https://doi.org/10.1016/j.jaci.2010.12.1124
Suarez-Farinas M et al (2015) RNA sequencing atopic dermatitis transcriptome profiling provides insights into novel disease mechanisms with potential therapeutic implications. J Allergy Clin Immunol 135:1218–1227. https://doi.org/10.1016/j.jaci.2015.03.003
Tengvall K et al (2013) Genome-wide analysis in German shepherd dogs reveals association of a locus on CFA 27 with atopic dermatitis. PLoS Genet 9:e1003475. https://doi.org/10.1371/journal.pgen.1003475
Tengvall K et al (2016) Multiple regulatory variants located in cell type-specific enhancers within the PKP2 locus form major risk and protective haplotypes for canine atopic dermatitis in German shepherd dogs. BMC Genet 17:97. https://doi.org/10.1186/s12863-016-0404-3
Trapnell C, Pachter L, Salzberg SL (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25:1105–1111. https://doi.org/10.1093/bioinformatics/btp120
Trapnell C et al (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28:511–515. https://doi.org/10.1038/nbt.1621
Vilson A, Bonnett B, Hansson-Hamlin H, Hedhammar A (2013) Disease patterns in 32,486 insured German shepherd dogs in Sweden: 1995-2006. Vet Record 173:116. https://doi.org/10.1136/vr.101577
Warnes G R, Bolker B, Bonebakker L, Gentleman R, Huber W, Liaw A, Lumley T, Maechler M, Magnusson A, Moeller S, Schwartz M, Venables B (2020) gplots: Various R Programming Tools for Plotting Data. R package version 3.0.3. https://CRAN.R-project.org/package=gplots
Werfel T, Schwerk N, Hansen G, Kapp A (2014) The diagnosis and graded therapy of atopic dermatitis. Dtsch Arztebl Int 111:509–520, i. https://doi.org/10.3238/arztebl.2014.0509
Wilhem S, Kovalik M, Favrot C (2011) Breed-associated phenotypes in canine atopic dermatitis. Vet Dermatol 22:143–149. https://doi.org/10.1111/j.1365-3164.2010.00925.x
Willemse T (1988) Comparative aspects of canine and human atopic dermatitis. Semin Vet Med Surg 3:255
Williams H (2001) Disease definition and measures of disease frequency. J Am Acad Dermatol 45:S33–S36
Xie Y, Li Y, Zhang Q, Stiller MJ, Wang CL, Streilein JW (2000) Haptoglobin is a natural regulator of Langerhans cell function in the skin. J Dermatol Sci 24:25–37. https://doi.org/10.1016/s0923-1811(00)00078-5
Zweigner J, Gramm HJ, Singer OC, Wegscheider K, Schumann RR (2001) High concentrations of lipopolysaccharide-binding protein in serum of patients with severe sepsis or septic shock inhibit the lipopolysaccharide response in human monocytes. Blood 98:3800–3808. https://doi.org/10.1182/blood.v98.13.3800
Acknowledgments
We would like to thank all the dog owners who participated with their dogs in this study as well as the veterinarians that helped with collecting biopsies at different clinics in Sweden.
Funding
Open access funding provided by Uppsala University. Sequencing was performed by the SNP&SEQ Technology Platform, Science for Life Laboratory at Uppsala University, a national infrastructure supported by the Swedish Research Council (VR-RFI) and the Knut and Alice Wallenberg Foundation. Kerstin Lindblad-Toh is a recipient of a European Research Council grant and a Swedish Research Council (distinguished professor). Grant from the Swedish Research Council 524-2012-7053 to Brita Ardesjö-Lundgren.
Author information
Authors and Affiliations
Contributions
Project design by Katarina Tengvall, Kerstin Lindblad-Toh, and Göran Andersson. Material preparation and data collection were performed by Kerstin Bergvall, Katarina Tengvall, Brita Ardesjö-Lundgren, and Fabiana H. G. Farias. Data analyses were performed by Katarina Tengvall and Marcin Kierczak. The first draft of the manuscript was written by Katarina Tengvall and Mia Olsson, and all the authors commented on the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
Owner consent was obtained for all dogs included in the study. Tissue sampling was performed with the approval of the Swedish Animal Ethical Committee (no. C138/12) and the Swedish Animal Welfare Agency (no. 31-1711/10).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tengvall, K., Bergvall, K., Olsson, M. et al. Transcriptomes from German shepherd dogs reveal differences in immune activity between atopic dermatitis affected and control skin. Immunogenetics 72, 315–323 (2020). https://doi.org/10.1007/s00251-020-01169-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-020-01169-3